Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors
Abstract
:1. Introduction
2. Results
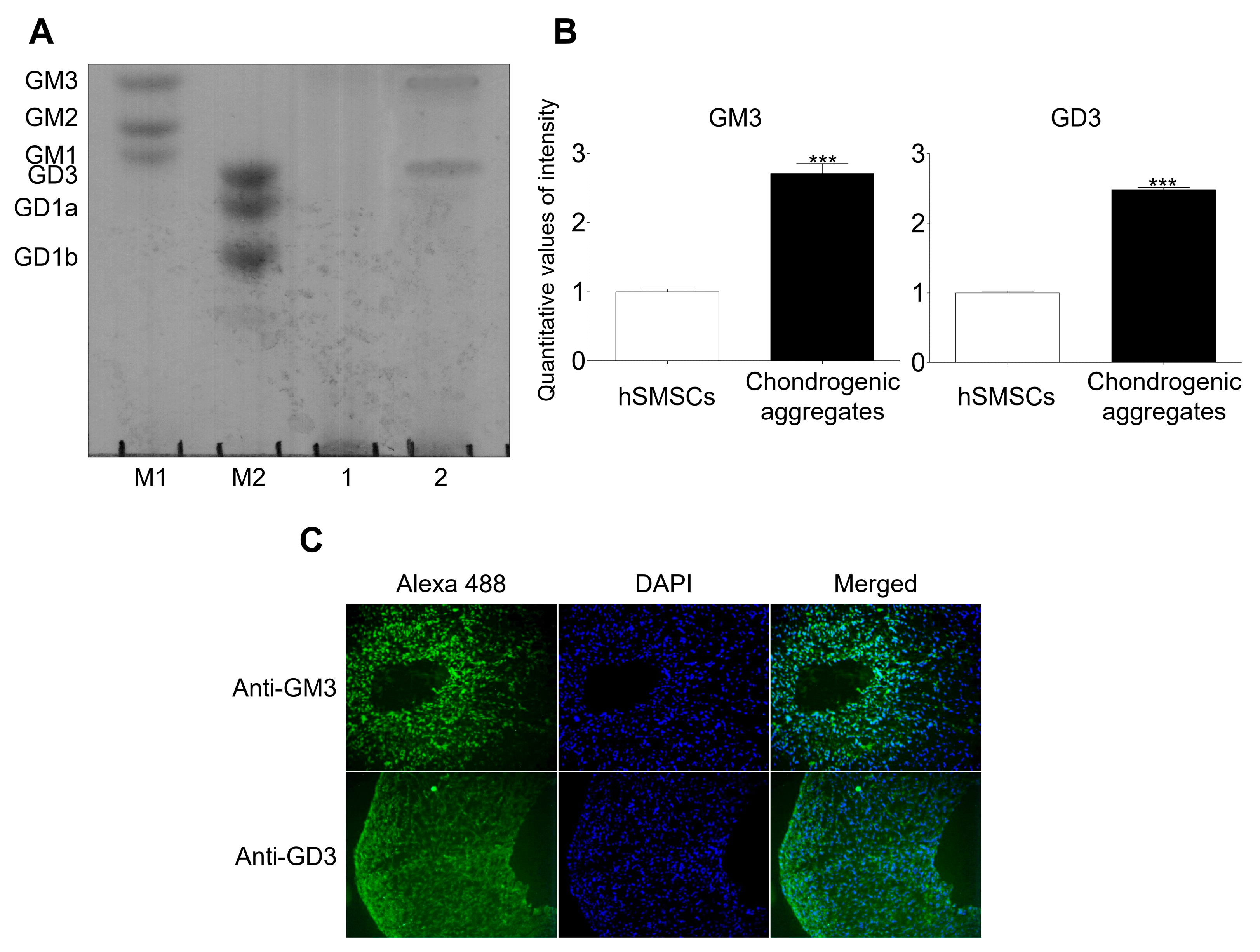
2.1. GM3 and GD3 Expression in the Chondrogenic Differentiation of hSMSC Aggregates
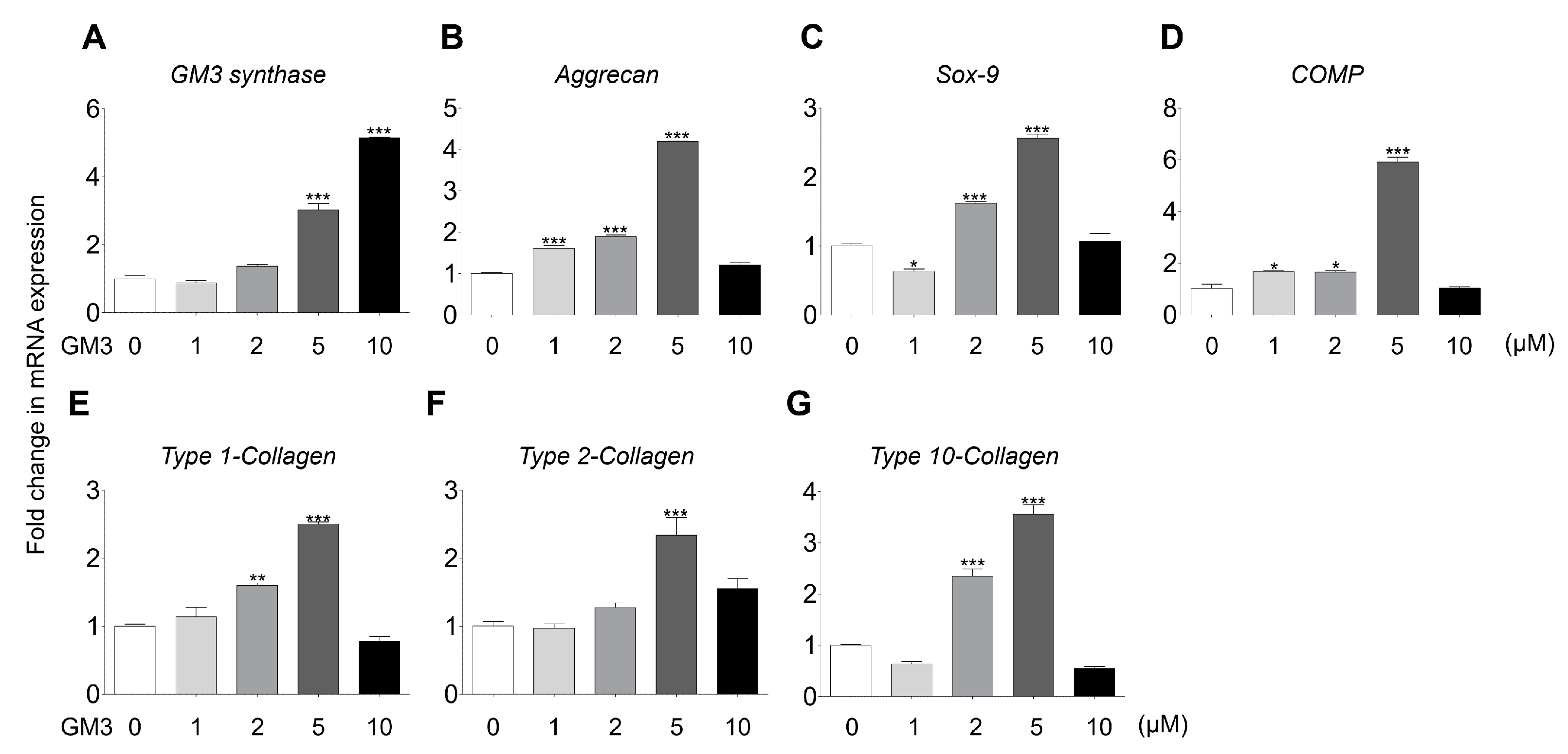
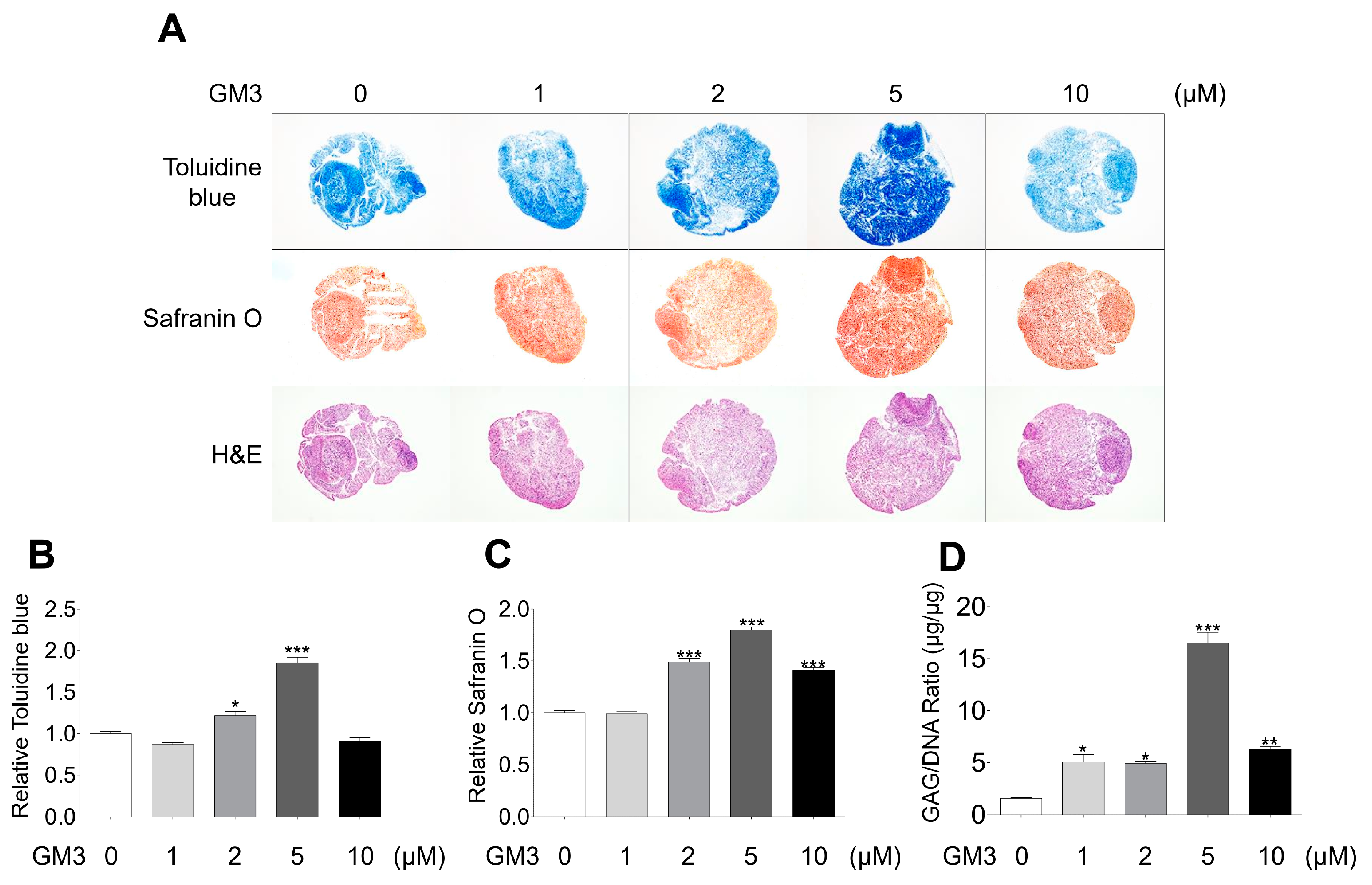
2.2. GM3 Enhanced Chondrogenic Differentiation of hSMSC Aggregates
2.3. GM3 Up-Regulated TGF-β Signaling Pathway during the Chondrogenic Differentiation of hSMSC Aggregates
3. Discussion
4. Materials and Methods
4.1. Chondrogenic Differentiation of hSMSCs in Aggregate Culture
4.2. High-Performance Thin-Layer Chromatography (HPTLC)
4.3. Immunocytochemistry
4.4. Real-Time Polymerase Chain Reaction (PCR)
4.5. Histological Analysis
4.6. Measurement of GAG Content
4.7. Western Blot
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Wilkinson, L.S.; Moore, A.R.; Pitsillides, A.A.; Willoughby, D.A.; Edwards, J.C. Comparison of surface fibroblastic cells in subcutaneous air pouch and synovial lining: differences in uridine diphosphoglucose dehydrogenase activity. Int. J. Exp. Pathol. 1993, 74, 113–115. [Google Scholar] [PubMed]
- Recklies, A.D.; Baillargeon, L.; White, C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998, 41, 997–1006. [Google Scholar] [CrossRef]
- Fife, R.S.; Caterson, B.; Myers, S.L. Identification of link proteins in canine synovial cell cultures and canine articular cartilage. J. Cell Biol. 1985, 100, 1050–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamerman, D.; Smith, C.; Keiser, H.D.; Craig, R. Glycosaminoglycans produced by human synovial cell cultures. Coll. Relat. Res. 1982, 2, 313–329. [Google Scholar] [CrossRef]
- Hakomori, S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 1990, 265, 18713–18716. [Google Scholar]
- Hakomori, S.; Yamamura, S.; Handa, A.K. Signal transduction through glyco(sphingo)lipids. Introduction and recent studies on glyco(sphingo)lipid-enriched microdomains. Ann. N. Y. Acad. Sci. 1998, 845, 1–10. [Google Scholar] [CrossRef]
- Hakomori, S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 1981, 50, 733–764. [Google Scholar] [CrossRef]
- Hakomori, S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.K.; Macala, L.J.; Taki, T.; Weinfield, H.M.; Yu, F.S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 1988, 50, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K. Development regulation of ganglioside metabolism. Prog. Brain Res. 1994, 101, 31–44. [Google Scholar] [PubMed]
- Ryu, J.S.; Ko, K.; Lee, J.W.; Park, S.B.; Byun, S.J.; Jeong, E.J.; Ko, K.; Choo, Y.K. Gangliosides are involved in neural differentiation of human dental pulp-derived stem cells. Biochem. Biophys. Res. Commun. 2009, 387, 266–271. [Google Scholar] [CrossRef]
- Yang, H.J.; Jung, K.Y.; Kwak, D.H.; Lee, S.H.; Ryu, J.S.; Kim, J.S.; Chang, K.T.; Lee, J.W.; Choo, Y.K. Inhibition of ganglioside GD1a synthesis suppresses the differentiation of human mesenchymal stem cells into osteoblasts. Dev. Growth Differ. 2011, 53, 323–332. [Google Scholar] [CrossRef]
- Lee, D.H.; Koo, D.B.; Ko, K.; Ko, K.; Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Do, S.I.; et al. Effects of daunorubicin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2007, 362, 313–318. [Google Scholar] [CrossRef]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: direct interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Kabayama, K.; Sato, T.; Saito, K.; Loberto, N.; Prinetti, A.; Sonnino, S.; Kinjo, M.; Igarashi, Y.; Inokuchi, J. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 13678–13683. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, N.; Yoon, S.J.; Itoh, K.; Nakayama, K. Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J. Biol. Chem. 2009, 284, 6147–6155. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I.; Ryu, J.S.; Yeo, J.E.; Choi, Y.J.; Kim, Y.S.; Ko, K.; Koh, Y.G. Overexpression of TGF-beta1 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1593–1599. [Google Scholar] [CrossRef]
- Hao, J.; Varshney, R.R.; Wang, D.A. Engineering osteogenesis and chondrogenesis with gene-enhanced therapeutic cells. Curr. Opin. Mol. Ther. 2009, 11, 404–410. [Google Scholar] [PubMed]
- Park, J.S.; Woo, D.G.; Yang, H.N.; Lim, H.J.; Chung, H.M.; Park, K.H. Heparin-bound transforming growth factor-beta3 enhances neocartilage formation by rabbit mesenchymal stem cells. Transplantation 2008, 85, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Monroy, J.; Diaz de Leon, L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. Int. J. Dev. Biol. 1999, 43, 59–67. [Google Scholar] [PubMed]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Saika, S.; Kono-Saika, S.; Ohnishi, Y.; Sato, M.; Muragaki, Y.; Ooshima, A.; Flanders, K.C.; Yoo, J.; Anzano, M.; Liu, C.Y.; et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 2004, 164, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Sekiya, I.; Vuoristo, J.T.; Larson, B.L.; Prockop, D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 4397–4402. [Google Scholar] [CrossRef] [Green Version]
- Sekiya, I.; Larson, B.L.; Vuoristo, J.T.; Cui, J.G.; Prockop, D.J. Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J. Bone Miner. Res. 2004, 19, 256–264. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Prockop, D.J.; Fitzpatrick, L.A.; Koo, W.W.; Gordon, P.L.; Neel, M.; Sussman, M.; Orchard, P.; Marx, J.C.; Pyeritz, R.E.; et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 1999, 5, 309–313. [Google Scholar] [CrossRef]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Muneta, T.; Kondo, S.; Mizuno, M.; Takakuda, K.; Ichinose, S.; Tabuchi, T.; Koga, H.; Tsuji, K.; Sekiya, I. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthr. Cartil. 2015, 23, 1007–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatsushika, D.; Muneta, T.; Nakamura, T.; Horie, M.; Koga, H.; Nakagawa, Y.; Tsuji, K.; Hishikawa, S.; Kobayashi, E.; Sekiya, I. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr. Cartil. 2014, 22, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, H.; Muneta, T.; Tsuji, K.; Horie, M.; Koga, H.; Ozeki, N.; Kobayashi, E.; Sekiya, I. Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem. Biophys. Res. Commun. 2013, 435, 603–609. [Google Scholar] [CrossRef]
- Yanagisawa, M. Stem cell glycolipids. Neurochem. Res. 2011, 36, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Ladisch, S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J. Biol. Chem. 2004, 279, 36481–36489. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Ko, K.; You, H.K.; Jung, K.Y.; Choo, Y.K. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem. Biophys. Res. Commun. 2008, 371, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; De Marzo, A.; Vetrini, F.; Marigo, V. Fibroblast growth factor and epidermal growth factor differently affect differentiation of murine retinal stem cells in vitro. Mol. Vis. 2007, 13, 1842–1850. [Google Scholar] [PubMed]
- Vrijens, P.; Noppen, S.; Boogaerts, T.; Vanstreels, E.; Ronca, R.; Chiodelli, P.; Laporte, M.; Vanderlinden, E.; Liekens, S.; Stevaert, A.; et al. Influenza virus entry via the GM3 ganglioside-mediated platelet-derived growth factor receptor beta signalling pathway. J. Gen. Virol. 2019, 100, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Dam, D.H.M.; Wang, X.Q.; Sheu, S.; Vijay, M.; Shipp, D.; Miller, L.; Paller, A.S. Ganglioside GM3 Mediates Glucose-Induced Suppression of IGF-1 Receptor-Rac1 Activation to Inhibit Keratinocyte Motility. J. Investig. Dermatol. 2017, 137, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, X.; Wang, C.; Li, Y.; Luan, M.; Ma, K. Ganglioside GM3 exerts opposite effects on motility via epidermal growth factor receptor and hepatocyte growth factor receptor-mediated migration signaling. Mol. Med. Rep. 2015, 11, 2959–2966. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Sun, P.; Go, L.; Koti, V.; Fliman, M.; Paller, A.S. Ganglioside GM3 promotes carcinoma cell proliferation via urokinase plasminogen activator-induced extracellular signal-regulated kinase-independent p70S6 kinase signaling. J. Investig. Dermatol. 2006, 126, 2687–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Ryu, J.S.; Lee, J.W.; Kwak, D.H.; Ko, K.; Choo, Y.K. Comparison of ganglioside expression between human adipose- and dental pulp-derived stem cell differentiation into osteoblasts. Arch. Pharm. Res. 2010, 33, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Chung, T.W.; Song, K.H.; Kwak, C.H.; Choi, H.J.; Ha, K.T.; Chang, Y.C.; Lee, Y.C.; Kim, C.H. Ganglioside GM3 is required for caffeic acid phenethyl ester-induced megakaryocytic differentiation of human chronic myelogenous leukemia K562 cells. Biochem. Cell Biol. 2014, 92, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Nagafuku, M.; Okuyama, K.; Onimaru, Y.; Suzuki, A.; Odagiri, Y.; Yamashita, T.; Iwasaki, K.; Fujiwara, M.; Takayanagi, M.; Ohno, I.; et al. CD4 and CD8 T cells require different membrane gangliosides for activation. Proc. Natl. Acad. Sci. USA 2012, 109, E336–E342. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.H.; Lee, S.; Kim, S.J.; Ahn, S.H.; Song, J.H.; Choo, Y.K.; Choi, B.K.; Jung, K.Y. Ganglioside GM3 inhibits the high glucose- and TGF-beta1-induced proliferation of rat glomerular mesangial cells. Life Sci. 2005, 77, 2540–2551. [Google Scholar] [CrossRef]
- Guan, F.; Handa, K.; Hakomori, S.I. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. USA 2009, 106, 7461–7466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Chung, T.W.; Choi, H.J.; Kwak, C.H.; Song, K.H.; Suh, S.J.; Kwon, K.M.; Chang, Y.C.; Park, Y.G.; Chang, H.W.; et al. Ganglioside GM3 participates in the TGF-beta1-induced epithelial-mesenchymal transition of human lens epithelial cells. Biochem. J. 2013, 449, 241–251. [Google Scholar] [CrossRef]
- David, M.J.; Portoukalian, J.; Rebbaa, A.; Vignon, E.; Carret, J.P.; Richard, M. Characterization of gangliosides from normal and osteoarthritic human articular cartilage. Arthritis Rheum. 1993, 36, 938–942. [Google Scholar] [CrossRef]
- Sasazawa, F.; Onodera, T.; Yamashita, T.; Seito, N.; Tsukuda, Y.; Fujitani, N.; Shinohara, Y.; Iwasaki, N. Depletion of gangliosides enhances cartilage degradation in mice. Osteoarthr. Cartil. 2014, 22, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.F. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, M.; He, F.; Vunjak-Novakovic, G. Synovium-derived stem cell-based chondrogenesis. Differentiation 2008, 76, 1044–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulyk, W.M.; Franklin, J.L.; Hoffman, L.M. Sox9 expression during chondrogenesis in micromass cultures of embryonic limb mesenchyme. Exp. Cell Res. 2000, 255, 327–332. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.-S.; Seo, S.Y.; Jeong, E.-J.; Kim, J.-Y.; Koh, Y.-G.; Kim, Y.I.; Choo, Y.-K. Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors. Int. J. Mol. Sci. 2020, 21, 1967. https://doi.org/10.3390/ijms21061967
Ryu J-S, Seo SY, Jeong E-J, Kim J-Y, Koh Y-G, Kim YI, Choo Y-K. Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors. International Journal of Molecular Sciences. 2020; 21(6):1967. https://doi.org/10.3390/ijms21061967
Chicago/Turabian StyleRyu, Jae-Sung, Sang Young Seo, Eun-Jeong Jeong, Jong-Yeup Kim, Yong-Gon Koh, Yong Il Kim, and Young-Kug Choo. 2020. "Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors" International Journal of Molecular Sciences 21, no. 6: 1967. https://doi.org/10.3390/ijms21061967
APA StyleRyu, J.-S., Seo, S. Y., Jeong, E.-J., Kim, J.-Y., Koh, Y.-G., Kim, Y. I., & Choo, Y.-K. (2020). Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors. International Journal of Molecular Sciences, 21(6), 1967. https://doi.org/10.3390/ijms21061967




