IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity
Abstract
1. Introduction
2. Results
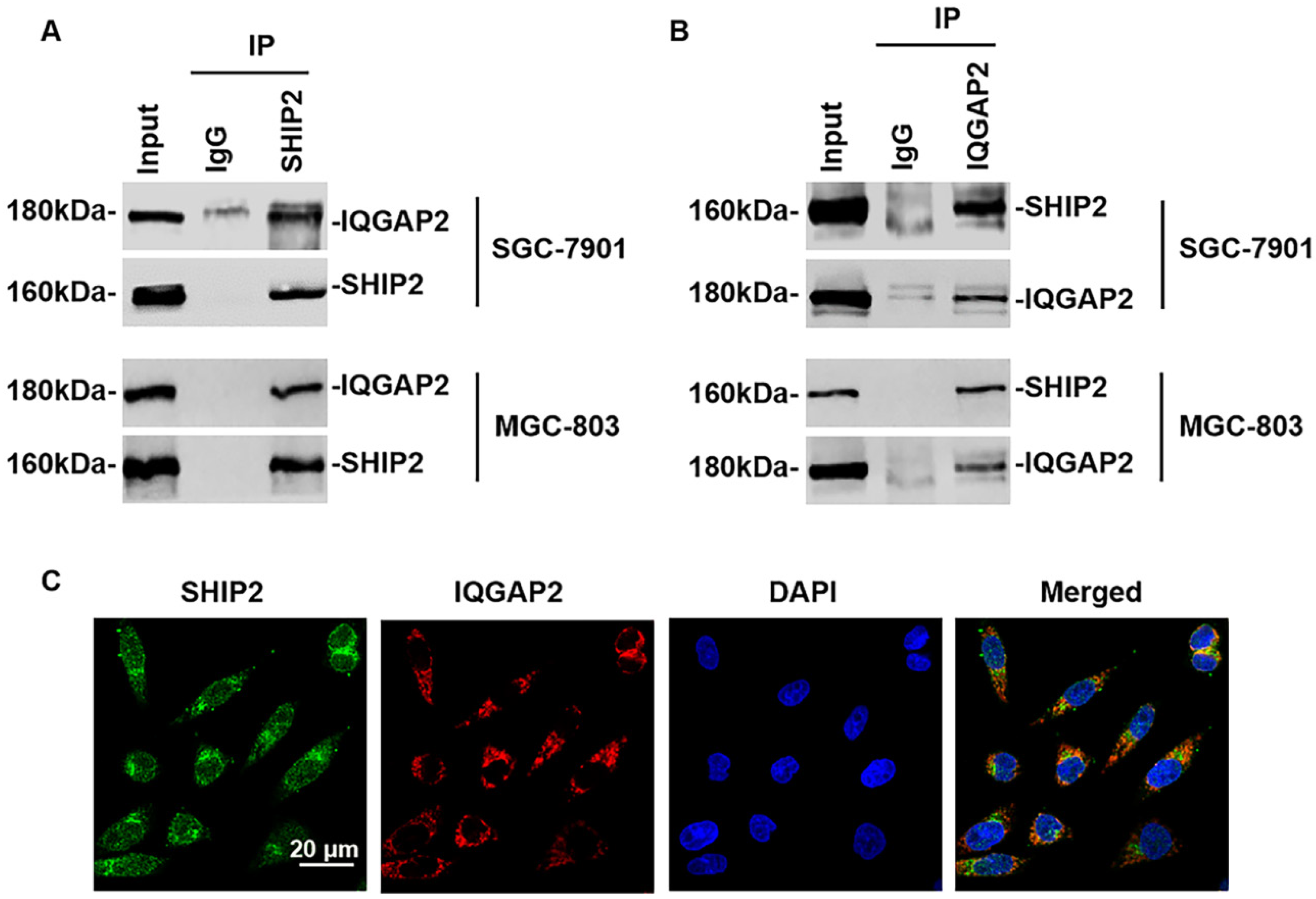
2.1. Expression of IQGAP2 and SHIP2 in Human GC
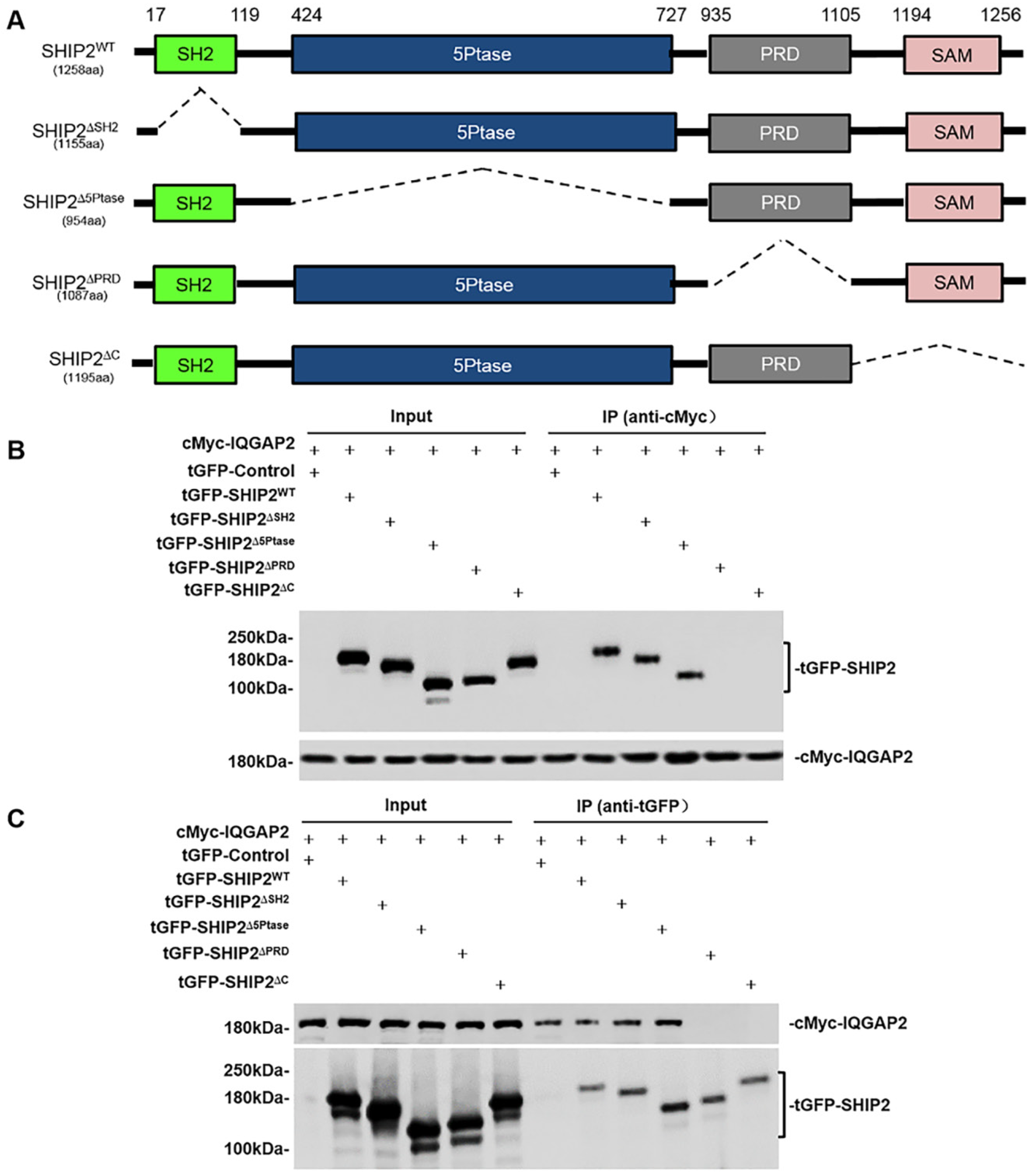
2.2. SHIP2 Physically Interacts with IQGAP2 through PRD and SAM Domains
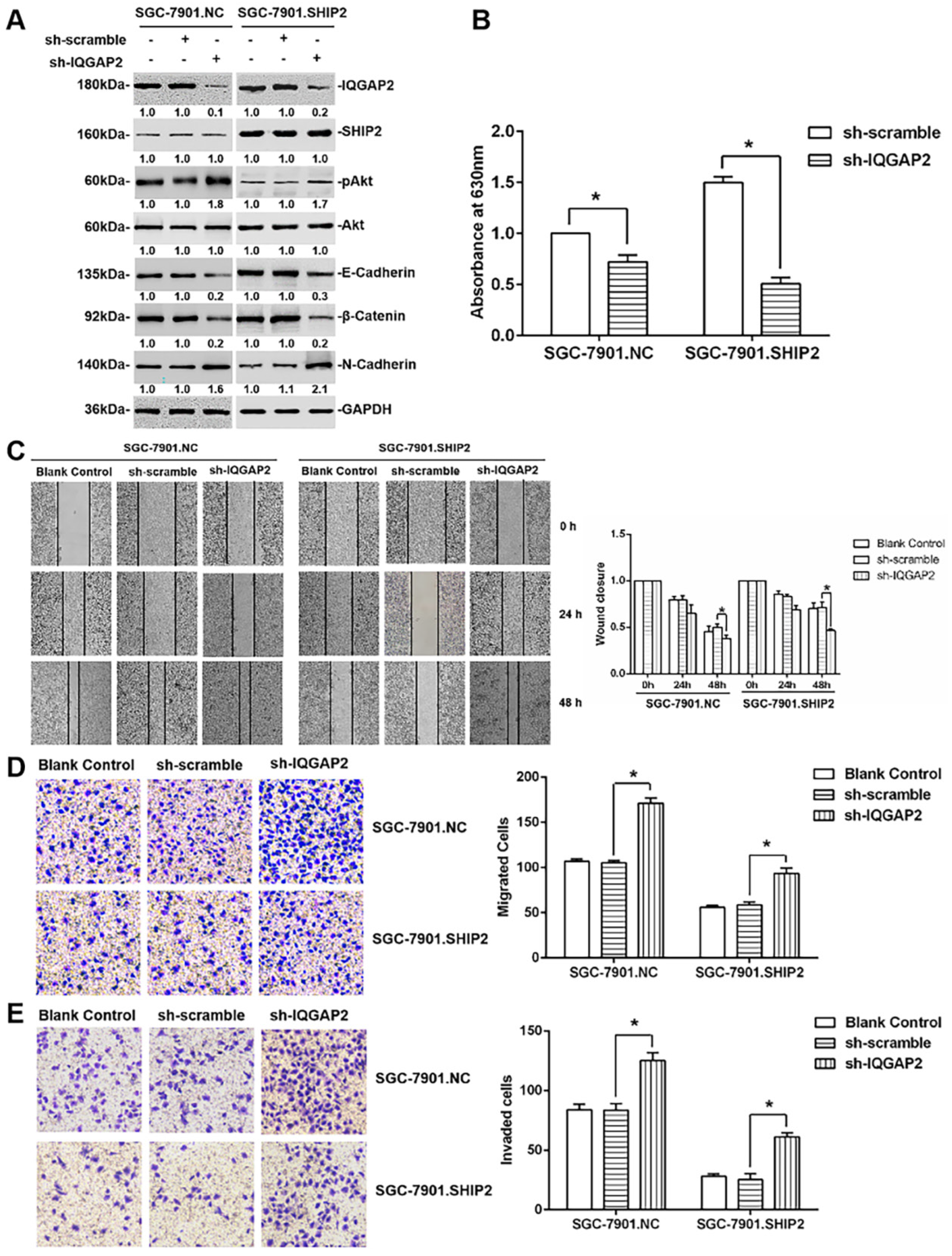
2.3. IQGAP2 Inhibits Migration and Invasion of GC Cells Correlated with SHIP2 Enzyme Activity
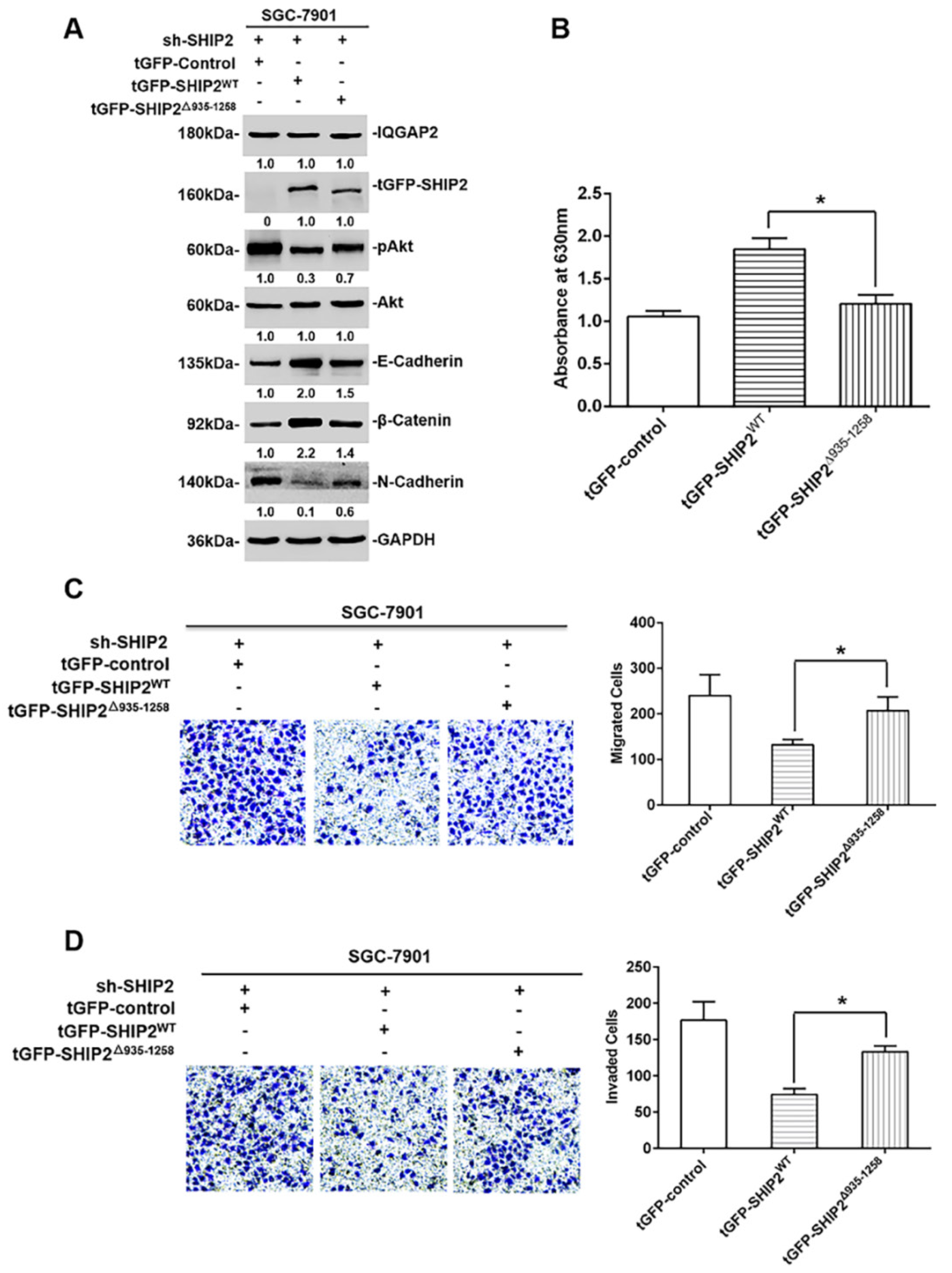
2.4. The Inhibition of IQGAP2 on Migration and Invasion of GC Cells Is Partially Due to Elevating SHIP2 Phosphatase Activity
3. Discussion
4. Materials and Methods
4.1. Cell lines and Cell Culture
4.2. Plasmids and Lentiviruses
4.3. Immunoprecipitation Assay
4.4. Western Blot Analysis
4.5. Immunofluorescence Staining
4.6. Wound Healing and Transwell Assays
4.7. RNA Isolation and Real-Time Quantitative PCR Analysis (qRT-PCR)
4.8. Malachite Green Phosphatase Assays
4.9. Quantitation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fruman, D.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Eramo, M.; Mitchell, C.A. Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem. Soc. Trans. 2016, 44, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kuniyasu, H. Significance of AKT in gastric cancer (Review). Int. J. Oncol. 2014, 45, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.C.; Blumenthal, G.; Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 2011, 11, 289–301. [Google Scholar] [CrossRef]
- Wang, H.; Loerke, D.; Bruns, C.; Müller, R.; Koch, P.A.; Puchkov, D.; Schultz, C.; Haucke, V. Phosphatidylinositol 3,4-bisphosphate synthesis and turnover are spatially segregated in the endocytic pathway. J. Biol. Chem. 2019, 295, 1091–1104. [Google Scholar] [CrossRef]
- Malek, M.; Kielkowska, A.; Chessa, T.; Anderson, K.; Barneda, D.; Pir, P.; Nakanishi, H.; Eguchi, S.; Koizumi, A.; Sasaki, J.; et al. PTEN Regulates PI(3,4)P2 Signaling Downstream of Class I PI3K. Mol. Cell 2017, 68, 566–580.e10. [Google Scholar] [CrossRef]
- Deneubourg, L.; Vanderwinden, J.-M.; Erneux, C. Regulation of SHIP2 function through plasma membrane interaction. Adv. Enzym. Regul. 2010, 50, 262–271. [Google Scholar] [CrossRef]
- Hibbs, M.L.; Raftery, A.L.; Tsantikos, E. Regulation of hematopoietic cell signaling by SHIP-1 inositol phosphatase: Growth factors and beyond. Growth Factors 2018, 36, 213–231. [Google Scholar] [CrossRef]
- Ooms, L.M.; Fedele, C.G.; Astle, M.V.; Ivetac, I.; Cheung, V.; Pearson, R.B.; Layton, M.J.; Forrai, A.; Nandurkar, H.; Mitchell, C.A. The Inositol Polyphosphate 5-Phosphatase, PIPP, Is a Novel Regulator of Phosphoinositide 3-Kinase-dependent Neurite Elongation. Mol. Biol. Cell 2006, 17, 607–622. [Google Scholar] [CrossRef][Green Version]
- Ye, Y.; Jin, L.; Wilmott, J.; Hu, W.L.; Yosufi, B.; Thorne, R.F.; Liu, T.; Rizos, H.; Yan, X.G.; Dong, L.; et al. PI(4,5)P2 5-phosphatase A regulates PI3K/Akt signalling and has a tumour suppressive role in human melanoma. Nat. Commun. 2013, 4, 1508. [Google Scholar] [CrossRef]
- Taylor, V.; Wong, M.; Brandts, C.H.; Reilly, L.; Dean, N.M.; Cowsert, L.M.; Moodie, S.; Stokoe, D. 5′ Phospholipid Phosphatase SHIP-2 Causes Protein Kinase B Inactivation and Cell Cycle Arrest in Glioblastoma Cells. Mol. Cell. Biol. 2000, 20, 6860–6871. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ge, Y.M.; Xiao, M.M.; Guo, L.M.; Li, Q.; Hao, J.Q.; Da, J.; Hu, W.L.; Zhang, X.D.; Xu, J.; et al. Suppression of SHIP2 contributes to tumorigenesis and proliferation of gastric cancer cells via activation of Akt. J. Gastroenterol. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Dumont, V.; Van, M.; Wang, H.; Dash, S.; Berg, M.; Naams, J.-B.; Hautala, L.C.; et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2018, 33, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.; Gaiteri, C.; Sullivan, S.E.; White, C.C.; Tasaki, S.; Xu, J.; Taga, M.; Klein, H.-U.; Patrick, E.; Komashko, V.; et al. A molecular network of the aging human brain provides insights into the pathology and cognitive decline of Alzheimer’s disease. Nat. Neurosci. 2018, 21, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Catsyne, C.-A.V.; Sayyed, S.A.; Molina-Ortiz, P.; Moes, B.; Communi, D.; Muller, J.; Heusschen, R.; Caers, J.; Azzi, A.; Erneux, C.; et al. Altered chondrocyte differentiation, matrix mineralization and MEK-Erk1/2 signaling in an INPPL1 catalytic knock-out mouse model of opsismodysplasia. Adv. Biol. Regul. 2019, 100651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Di, M.; Han, H. Upregulation of SHIP2 participates in the development of breast cancer via promoting Wnt/β-catenin signaling. OncoTargets Ther. 2019, 12, 7067–7077. [Google Scholar] [CrossRef]
- Fu, M.; Gu, X.; Ni, H.; Zhang, W.; Chang, F.; Gong, L.; Chen, X.; Li, J.; Qiu, L.; Shi, C.; et al. High expression of inositol polyphosphate phosphatase-like 1 associates with unfavorable survival in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2515–2522. [Google Scholar]
- Fu, M.; Fan, W.; Pu, X.; Ni, H.; Zhang, W.; Chang, F.; Gong, L.; Xiong, L.; Wang, J.; Gu, X. Elevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2013, 6, 2185–2191. [Google Scholar]
- Yang, J.; Fu, M.; Ding, Y.; Weng, Y.; Fan, W.; Pu, X.; Ge, Z.; Zhan, F.; Ni, H.; Zhang, W.; et al. High SHIP2 Expression Indicates Poor Survival in Colorectal Cancer. Dis. Markers 2014, 2014, 218968. [Google Scholar] [CrossRef]
- Yu, J.; Ryan, D.G.; Getsios, S.; Oliveira-Fernandes, M.; Fatima, A.; Lavker, R.M. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc. Natl. Acad. Sci. USA 2008, 105, 19300–19305. [Google Scholar] [CrossRef]
- Edimo, W.E.; Janssens, V.; Waelkens, E.; Erneux, C. Reversible Ser/Thr SHIP phosphorylation: A new paradigm in phosphoinositide signalling? BioEssays 2012, 34, 634–642. [Google Scholar] [CrossRef]
- Xie, J.; Erneux, C.; Pirson, I. How does SHIP1/2 balance PtdIns(3,4)P 2 and does it signal independently of its phosphatase activity? BioEssays 2013, 35, 733–743. [Google Scholar] [CrossRef]
- Awad, A.; Gassama-Diagne, A. PI3K/SHIP2/PTEN pathway in cell polarity and hepatitis C virus pathogenesis. World J. Hepatol. 2017, 9, 18–29. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Bialkowska, A.B.; Snider, A.J.; Gnatenko, D.V.; Hannun, Y.A.; Yang, V.W.; Schmidt, V.A. IQ Motif-Containing GTPase-Activating Protein 2 (IQGAP2) Is a Novel Regulator of Colonic Inflammation in Mice. PLoS ONE 2015, 10, e0129314. [Google Scholar] [CrossRef]
- White, C.D.; Khurana, H.; Gnatenko, D.V.; Li, Z.; Odze, R.D.; Sacks, D.B.; Schmidt, V.A. IQGAP1 and IQGAP2 are Reciprocally Altered in Hepatocellular Carcinoma. BMC Gastroenterol. 2010, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yan, J.; Cutz, J.-C.; Rybak, A.; He, L.; Wei, F.; Kapoor, A.; Schmidt, V.A.; Tao, L.; Tang, D. IQGAP2, A candidate tumour suppressor of prostate tumorigenesis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, L.; Hou, H.; Zhou, J.; Li, X. Epigenetic regulation of IQGAP2 promotes ovarian cancer progression via activating Wnt/?-catenin signaling. Int. J. Oncol. 2015, 48, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-H.; Akiyama, Y.; Fukamachi, H.; Yanagihara, K.; Akashi, T.; Yuasa, Y. IQGAP2 inactivation through aberrant promoter methylation and promotion of invasion in gastric cancer cells. Int. J. Cancer 2007, 122, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- White, C.D.; Brown, M.D.; Sacks, D.B. IQGAPs in cancer: A family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009, 583, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.W.; Sacks, D.B. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003, 542, 7–11. [Google Scholar] [CrossRef]
- Mateer, S.C.; Wang, N.; Bloom, G.S. IQGAPs: Integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell Motil. Cytoskelet. 2003, 55, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kaibuchi, K.; Kuroda, S.; Amano, M. Regulation of the Cytoskeleton and Cell Adhesion by the Rho Family GTPases in Mammalian Cells. Annu. Rev. Biochem. 1999, 68, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A. An Emerging Role for IQGAP1 in Regulating Protein Traffic. Sci. World J. 2010, 10, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Sharma, M.; Henderson, B.R. IQGAP1 regulation and roles in cancer. Cell. Signal. 2009, 21, 1471–1478. [Google Scholar] [CrossRef]
- Watanabe, T.; Wang, S.; Kaibuchi, K. IQGAPs as key regulators of actin-cytoskeleton dynamics. Cell Struct. Funct. 2015, 40, 69–77. [Google Scholar]
- Gnatenko, D.V.; Xu, X.; Zhu, W.; Schmidt, V.A. Transcript Profiling Identifies Iqgap2−/− Mouse as a Model for Advanced Human Hepatocellular Carcinoma. PLoS ONE 2013, 8, e71826. [Google Scholar] [CrossRef]
- Kumar, D.; Hassan, K.; Pattnaik, N.; Mohapatra, N.; Dixit, M. Reduced expression of IQGAP2 and higher expression of IQGAP3 correlates with poor prognosis in cancers. PLoS ONE 2017, 12, e0186977. [Google Scholar] [CrossRef]
- Ohmachi, T.; Tanaka, F.; Mimori, K.; Inoue, H.; Yanaga, K.; Mori, M. Clinical Significance of TROP2 Expression in Colorectal Cancer. Clin. Cancer Res. 2006, 12, 3057–3063. [Google Scholar] [CrossRef]
- Ernst, T.; Hergenhahn, M.; Kenzelmann, M.; Cohen, C.D.; Bonrouhi, M.; Weninger, A.; Klären, R.; Gröne, E.F.; Wiesel, M.; Güdemann, C.; et al. Decrease and Gain of Gene Expression Are Equally Discriminatory Markers for Prostate Carcinoma. Am. J. Pathol. 2002, 160, 2169–2180. [Google Scholar] [CrossRef]
- Dyson, J.M.; O’Malley, C.; Becanovic, J.; Munday, A.D.; Berndt, M.C.; Coghill, I.D.; Nandurkar, H.; Ooms, L.M.; Mitchell, C.A. The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin. J. Cell Biol. 2001, 155, 1065–1080. [Google Scholar] [CrossRef]
- Paternotte, N.; Zhang, J.; Vandenbroere, I.; Backers, K.; Blero, D.; Kioka, N.; Vanderwinden, J.-M.; Pirson, I.; Erneux, C. SHIP2 interaction with the cytoskeletal protein Vinexin. FEBS J. 2005, 272, 6052–6066. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroere, I.; Paternotte, N.; Dumont, J.E.; Erneux, C.; Pirson, I. The c-Cbl-associated protein and c-Cbl are two new partners of the SH2-containing inositol polyphosphate 5-phosphatase SHIP2. Biochem. Biophys. Res. Commun. 2003, 300, 494–500. [Google Scholar] [CrossRef]
- Raaijmakers, J.H.; Deneubourg, L.; Rehmann, H.; De Koning, J.; Zhang, Z.; Krugmann, S.; Erneux, C.; Bos, J.L. The PI3K effector Arap3 interacts with the PI(3,4,5)P3 phosphatase SHIP2 in a SAM domain-dependent manner. Cell. Signal. 2007, 19, 1249–1257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, N.; Topping, R.S.; Decker, S.J. SH2-Containing Inositol 5′-Phosphatase SHIP2 Associates with the p130Cas Adapter Protein and Regulates Cellular Adhesion and Spreading. Mol. Cell. Biol. 2001, 21, 1416–1428. [Google Scholar] [CrossRef]
- Kato, K.; Yazawa, T.; Taki, K.; Mori, K.; Wang, S.; Nishioka, T.; Hamaguchi, T.; Itoh, T.; Takenawa, T.; Kataoka, C.; et al. The inositol 5-phosphatase SHIP2 is an effector of RhoA and is involved in cell polarity and migration. Mol. Biol. Cell 2012, 23, 2593–2604. [Google Scholar] [CrossRef]
- Wu, C.; Cui, X.; Huang, L.; Shang, X.; Wu, B.; Wang, N.; He, K.; Han, Z.-G. IRTKS Promotes Insulin Signaling Transduction through Inhibiting SHIP2 Phosphatase Activity. Int. J. Mol. Sci. 2019, 20, 2834. [Google Scholar] [CrossRef]
- Brill, S.; Li, S.; Lyman, C.W.; Church, D.M.; Wasmuth, J.J.; Weissbach, L.; Bernards, A.; Snijders, A.J. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol. Cell. Biol. 1996, 16, 4869–4878. [Google Scholar] [CrossRef]
- Chiariello, C.S.; LaComb, J.F.; Bahou, W.F.; Schmidt, V.A. Ablation of Iqgap2 protects from diet-induced hepatic steatosis due to impaired fatty acid uptake. Regul. Pept. 2011, 173, 36–46. [Google Scholar] [CrossRef][Green Version]
- Watt, N.T.; Gage, M.C.; Patel, P.; Viswambharan, H.; Sukumar, P.; Galloway, S.; Yuldasheva, N.; Imrie, H.; Walker, A.; Griffin, K.J.; et al. Endothelial SHIP2 Suppresses Nox2 NADPH Oxidase–Dependent Vascular Oxidative Stress, Endothelial Dysfunction, and Systemic Insulin Resistance. Diabetes 2017, 66, 2808–2821. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Shao, Y.; Ren, L.; Liu, X.; Li, Y.; Xu, J.; Ye, Y. IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity. Int. J. Mol. Sci. 2020, 21, 1968. https://doi.org/10.3390/ijms21061968
Xu L, Shao Y, Ren L, Liu X, Li Y, Xu J, Ye Y. IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity. International Journal of Molecular Sciences. 2020; 21(6):1968. https://doi.org/10.3390/ijms21061968
Chicago/Turabian StyleXu, Liang, Yuling Shao, Lin Ren, Xiansheng Liu, Yunyun Li, Jiegou Xu, and Yan Ye. 2020. "IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity" International Journal of Molecular Sciences 21, no. 6: 1968. https://doi.org/10.3390/ijms21061968
APA StyleXu, L., Shao, Y., Ren, L., Liu, X., Li, Y., Xu, J., & Ye, Y. (2020). IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity. International Journal of Molecular Sciences, 21(6), 1968. https://doi.org/10.3390/ijms21061968



