Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules
Abstract
1. Introduction
2. Results
2.1. Radiolabeling and Stability Assessment
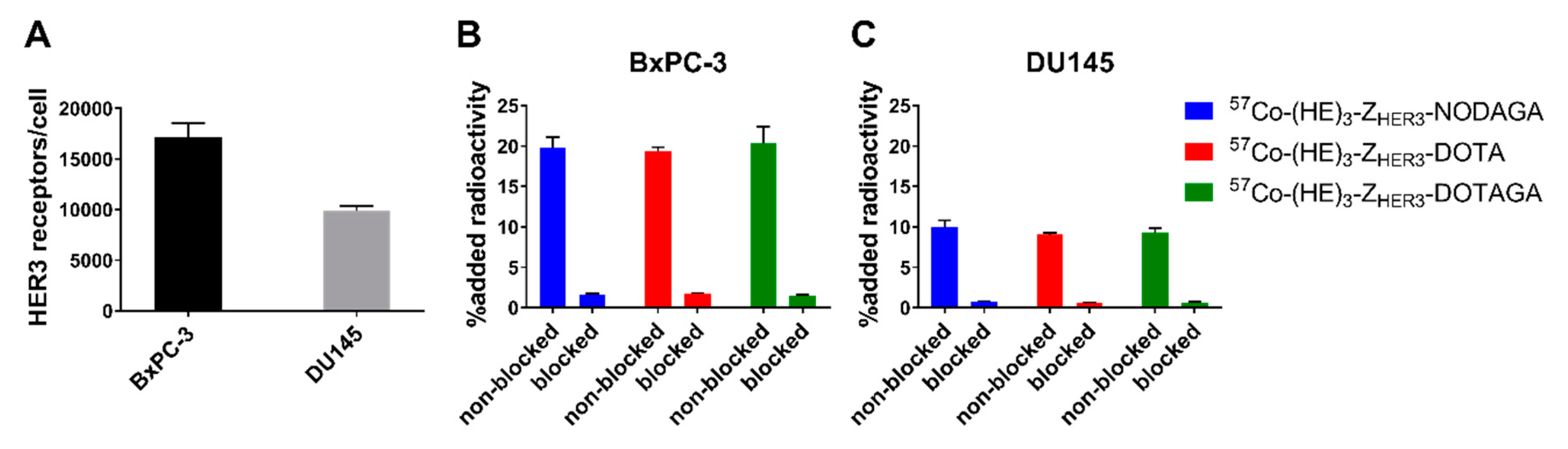
2.2. In Vitro Characterization of [57Co]Co-(HE)3-ZHER3-X
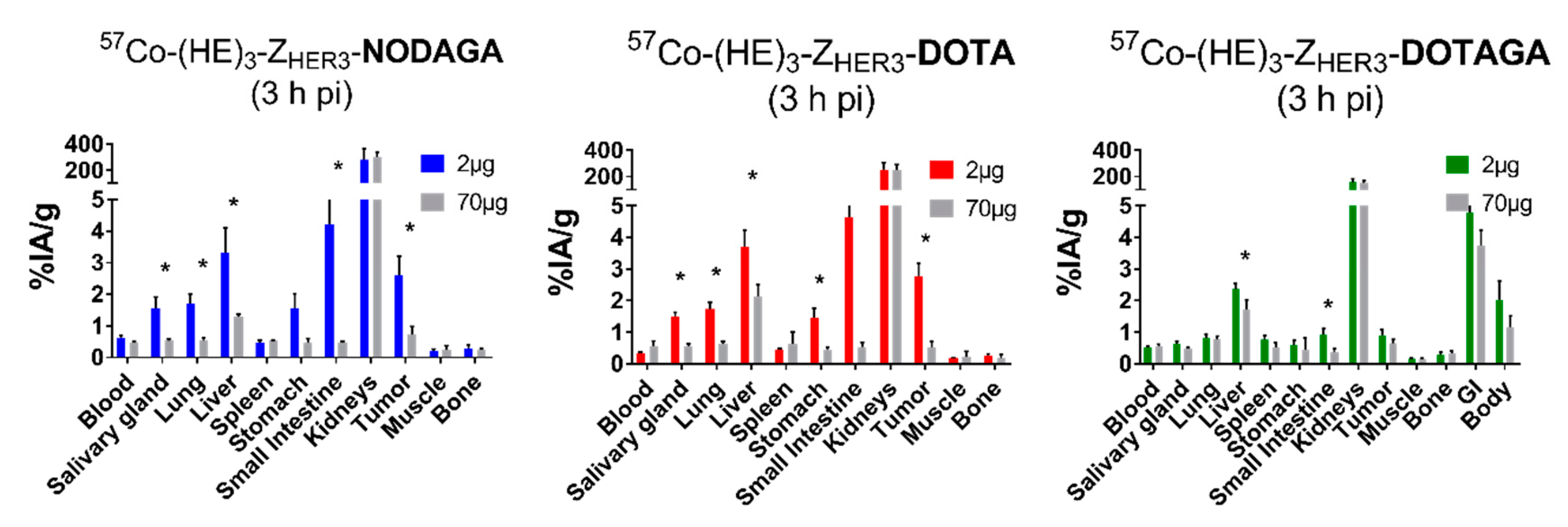
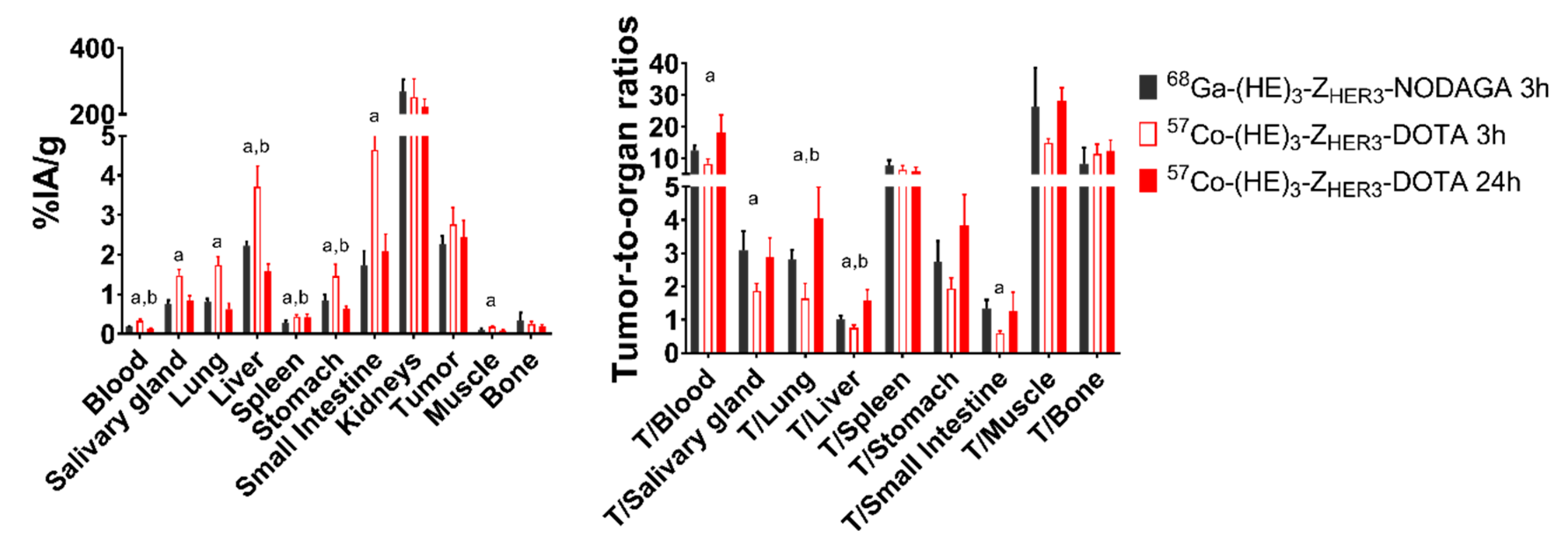
2.3. In Vivo Evaluation
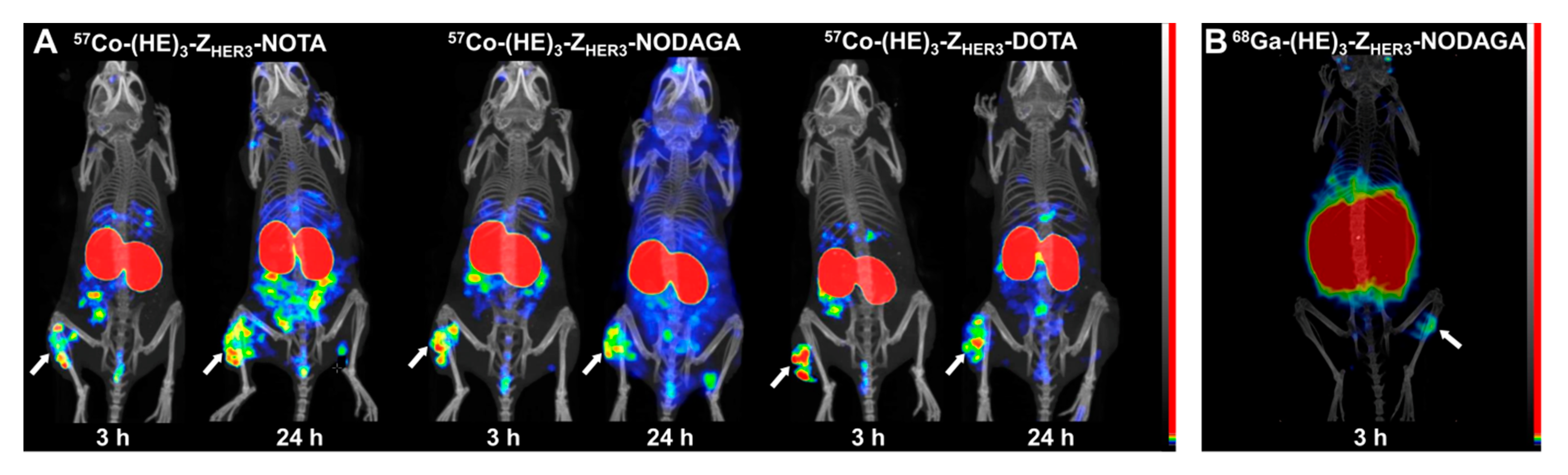
2.4. Imaging
3. Discussion
4. Materials and Methods
4.1. Radiolabeling of (HE)3-ZHER3-X and Stability of Labeled Conjugates
4.2. In Vitro Characterization of [57Co]Co-(HE)3-ZHER3-X
4.3. Measurement of HER3 Receptor Expression in BxPC-3 and DU145 Cells
4.4. Real Time Measurement of Binding Kinetics
4.5. In Vivo Specificity Test and Biodistribution of [57Co]Co-(HE)3-ZHER3-X
4.6. Imaging of HER3-Expressing Bxpc-3 Xenografted Mice
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DOTAGA | 1,4,7,10-tetraazacyclododecane,1-(glutaric acid)-4,7,10-triacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| EPR effect | Enhanced permeability and retention effect |
| ESP | Engineered scaffold protein |
| HER | Human epidermal growth factor receptor |
| ITLC | Instant thin-layered liquid chromatography |
| MIP | Maximum intensity projection |
| MRI | Magnetic resonance imaging |
| NOTA | 1-(1,3-carboxypropyl)-4,7-carboxymethyl-1,4,7-triazacyclononane |
| NODAGA | 1,4,7-triazacyclononane-N,N′,N″-triacetic acid |
| PBS | Phosphate buffer saline |
| PET | Positron emission tomography |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| SPECT | Single-photon emission tomography |
References
- Amin, D.N.; Campbell, M.R.; Moasser, M.M. The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin. Cell Dev. Biol. 2010, 21, 944–950. [Google Scholar] [CrossRef]
- Gala, K.; Chandarlapaty, S. Molecular pathways: HER3 targeted therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Ocana, A.; Vera-Badillo, F.; Seruga, B.; Templeton, A.; Pandiella, A.; Amir, E. HER3 overexpression and survival in solid tumors: A meta-analysis. J. Natl. Cancer Inst. 2013, 105, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lyu, H.; Huang, J.; Liu, B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol. Cancer 2014, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Garrett, J.T.; Olivares, M.G.; Rinehart, C.; Granja-Ingram, N.D.; Sánchez, V.; Chakrabarty, A.; Dave, B.; Cook, R.S.; Pao, W.; McKinely, E.; et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl. Acad. Sci. USA 2011, 108, 5021–5026. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Yuan, L.; Garrett, J.T. HER3 signaling and targeted therapy in cancer. Oncol. Rev. 2018, 12, 355. [Google Scholar] [CrossRef]
- Lyu, H.; Han, A.; Polsdofer, E.; Liu, S.; Liu, B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm. Sin. B 2018, 8, 503–510. [Google Scholar] [CrossRef]
- Pool, M.; Kol, A.; de Jong, S.; de Vries, E.G.E.; Lub-de Hooge, M.N.; Terwisscha van Scheltinga, A.G.T. 89Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. mAbs 2017, 9, 1370–1378. [Google Scholar] [CrossRef][Green Version]
- Terwisscha van Scheltinga, A.G.T.; Lub-de Hooge, M.N.; Abiraj, K.; Schröder, C.P.; Pot, L.; Bossenmaier, B.; Thomas, M.; Hölzlwimmer, G.; Friess, T.; Kosterink, J.G.W.; et al. ImmunoPET and biodistribution with human epidermal growth factor receptor 3 targeting antibody 89Zr-RG7116. mAbs 2014, 6, 1051–1058. [Google Scholar] [CrossRef]
- Wehrenberg-Klee, E.; Turker, N.S.; Heidari, P.; Larimer, B.; Juric, D.; Baselga, J.; Scaltriti, M.; Mahmood, U. Differential Receptor Tyrosine Kinase PET Imaging for Therapeutic Guidance. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 1413–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bensch, F.; Lamberts, L.E.; Smeenk, M.M.; Jorritsma-Smit, A.; Lub-de Hooge, M.N.; Terwisscha van Scheltinga, A.G.T.; de Jong, J.R.; Gietema, J.A.; Schröder, C.P.; Thomas, M.; et al. (89)Zr-Lumretuzumab PET Imaging before and during HER3 Antibody Lumretuzumab Treatment in Patients with Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6128–6137. [Google Scholar] [CrossRef] [PubMed]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Malm, M.; Rosestedt, M.; Varasteh, Z.; Andersson, K.; Selvaraju, R.K.; Altai, M.; Honarvar, H.; Strand, J.; Ståhl, S.; et al. Imaging of HER3-expressing xenografts in mice using a (99m)Tc(CO) 3-HEHEHE-Z HER3:08699 affibody molecule. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Rosestedt, M.; Andersson, K.G.; Mitran, B.; Tolmachev, V.; Löfblom, J.; Orlova, A.; Ståhl, S. Affibody-mediated PET imaging of HER3 expression in malignant tumours. Sci. Rep. 2015, 5, 15226. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.; D’Huyvetter, M.; Devoogdt, N.; Frejd, F.Y.; Sörensen, J.; Orlova, A.; Keyaerts, M.; Tolmachev, V. Same-Day Imaging Using Small Proteins: Clinical Experience and Translational Prospects in Oncology. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 885–891. [Google Scholar] [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef]
- Ahlgren, S.; Tolmachev, V. Radionuclide molecular imaging using Affibody molecules. Curr. Pharm. Biotechnol. 2010, 11, 581–589. [Google Scholar] [CrossRef]
- Andersson, K.G.; Rosestedt, M.; Varasteh, Z.; Malm, M.; Sandström, M.; Tolmachev, V.; Löfblom, J.; Ståhl, S.; Orlova, A. Comparative evaluation of 111In-labeled NOTA-conjugated affibody molecules for visualization of HER3 expression in malignant tumors. Oncol. Rep. 2015, 34, 1042–1048. [Google Scholar] [CrossRef]
- Da Pieve, C.; Allott, L.; Martins, C.D.; Vardon, A.; Ciobota, D.M.; Kramer-Marek, G.; Smith, G. Efficient [18F]AlF Radiolabeling of ZHER3:8698 Affibody Molecule for Imaging of HER3 Positive Tumors. Bioconjug. Chem. 2016, 27, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.; Da Pieve, C.; Burley, T.A.; Smith, R.; Ciobota, D.M.; Allott, L.; Harrington, K.J.; Oyen, W.J.G.; Smith, G.; Kramer-Marek, G. HER3-Mediated Resistance to Hsp90 Inhibition Detected in Breast Cancer Xenografts by Affibody-Based PET Imaging. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Martiniova, L.; Palatis, L.D.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Robinson, M.K.; Hodge, K.M.; Horak, E.; Sundberg, Å.L.; Russeva, M.; Shaller, C.C.; von Mehren, M.; Shchaveleva, I.; Simmons, H.H.; Marks, J.D.; et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br. J. Cancer 2008, 99, 1415–1425. [Google Scholar] [CrossRef]
- Rinne, S.S.; Leitao, C.D.; Mitran, B.; Bass, T.Z.; Andersson, K.G.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Optimization of HER3 expression imaging using affibody molecules: Influence of chelator for labeling with indium-111. Sci. Rep. 2019, 9, 655. [Google Scholar] [CrossRef]
- Rosestedt, M.; Andersson, K.G.; Mitran, B.; Rinne, S.S.; Tolmachev, V.; Löfblom, J.; Orlova, A.; Ståhl, S. Evaluation of a radiocobalt-labelled affibody molecule for imaging of human epidermal growth factor receptor 3 expression. Int. J. Oncol. 2017, 51, 1765–1774. [Google Scholar] [CrossRef]
- Andersson, K.G.; Oroujeni, M.; Garousi, J.; Mitran, B.; Ståhl, S.; Orlova, A.; Löfblom, J.; Tolmachev, V. Feasibility of imaging of epidermal growth factor receptor expression with ZEGFR:2377 affibody molecule labeled with 99mTc using a peptide-based cysteine-containing chelator. Int. J. Oncol. 2016, 49, 2285–2293. [Google Scholar] [CrossRef]
- Garousi, J.; Andersson, K.G.; Dam, J.H.; Olsen, B.B.; Mitran, B.; Orlova, A.; Buijs, J.; Ståhl, S.; Löfblom, J.; Thisgaard, H.; et al. The use of radiocobalt as a label improves imaging of EGFR using DOTA-conjugated Affibody molecule. Sci. Rep. 2017, 7, 5961. [Google Scholar] [CrossRef]
- Summer, D.; Garousi, J.; Oroujeni, M.; Mitran, B.; Andersson, K.G.; Vorobyeva, A.; Löfblom, J.; Orlova, A.; Tolmachev, V.; Decristoforo, C. Cyclic versus Noncyclic Chelating Scaffold for 89Zr-Labeled ZEGFR:2377 Affibody Bioconjugates Targeting Epidermal Growth Factor Receptor Overexpression. Mol. Pharm. 2018, 15, 175–185. [Google Scholar] [CrossRef]
- Dahlsson Leitao, C.; Rinne, S.S.; Mitran, B.; Vorobyeva, A.; Andersson, K.G.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Molecular Design of HER3-Targeting Affibody Molecules: Influence of Chelator and Presence of HEHEHE-Tag on Biodistribution of 68Ga-Labeled Tracers. Int. J. Mol. Sci. 2019, 20, 1080. [Google Scholar] [CrossRef] [PubMed]
- Warnders, F.J.; Terwisscha van Scheltinga, A.G.T.; Knuehl, C.; van Roy, M.; de Vries, E.F.J.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub-de Hooge, M.N. Human Epidermal Growth Factor Receptor 3-Specific Tumor Uptake and Biodistribution of 89Zr-MSB0010853 Visualized by Real-Time and Noninvasive PET Imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 1210–1215. [Google Scholar]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.F.; Thisgaard, H. A PSMA Ligand Labeled with Cobalt-55 for PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2017, 19, 915–922. [Google Scholar] [CrossRef] [PubMed]
- De Reuck, J.; Stevens, H.; Jansen, H.; Keppens, J.; Strijckmans, K.; Goethals, P.; Lemahieu, I.; Santens, P.; Korf, J. The significance of cobalt-55 positron emission tomography in ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 1999, 8, 17–21. [Google Scholar] [CrossRef]
- Mitran, B.; Thisgaard, H.; Rosenström, U.; Dam, J.H.; Larhed, M.; Tolmachev, V.; Orlova, A. High Contrast PET Imaging of GRPR Expression in Prostate Cancer Using Cobalt-Labeled Bombesin Antagonist RM26. Contrast Media Mol. Imaging 2017, 2017, 6873684. [Google Scholar] [CrossRef]
- Westerlund, K.; Honarvar, H.; Norrström, E.; Strand, J.; Mitran, B.; Orlova, A.; Eriksson Karlström, A.; Tolmachev, V. Increasing the Net Negative Charge by Replacement of DOTA Chelator with DOTAGA Improves the Biodistribution of Radiolabeled Second-Generation Synthetic Affibody Molecules. Mol. Pharm. 2016, 13, 1668–1678. [Google Scholar] [CrossRef]
- Mitran, B.; Thisgaard, H.; Rinne, S.; Dam, J.H.; Azami, F.; Tolmachev, V.; Orlova, A.; Rosenström, U. Selection of an optimal macrocyclic chelator improves the imaging of prostate cancer using cobalt-labeled GRPR antagonist RM26. Sci. Rep. 2019, 9, 17086. [Google Scholar] [CrossRef]
- Gourni, E.; Del Pozzo, L.; Kheirallah, E.; Smerling, C.; Waser, B.; Reubi, J.-C.; Paterson, B.M.; Donnelly, P.S.; Meyer, P.T.; Maecke, H.R. Copper-64 Labeled Macrobicyclic Sarcophagine Coupled to a GRP Receptor Antagonist Shows Great Promise for PET Imaging of Prostate Cancer. Mol. Pharm. 2015, 12, 2781–2790. [Google Scholar] [CrossRef]
- Andersen, T.L.; Baun, C.; Olsen, B.B.; Dam, J.H.; Thisgaard, H. Improving Contrast and Detectability: Imaging with [55Co]Co-DOTATATE in Comparison with [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 228–233. [Google Scholar] [CrossRef]
- Cicone, F.; Gnesin, S.; Denoël, T.; Stora, T.; van der Meulen, N.P.; Müller, C.; Vermeulen, C.; Benešová, M.; Köster, U.; Johnston, K.; et al. Internal radiation dosimetry of a 152Tb-labeled antibody in tumor-bearing mice. Ejnmmi Res. 2019, 9, 53. [Google Scholar] [CrossRef]
- Tolmachev, V.; Grönroos, T.J.; Yim, C.-B.; Garousi, J.; Yue, Y.; Grimm, S.; Rajander, J.; Perols, A.; Haaparanta-Solin, M.; Solin, O.; et al. Molecular design of radiocopper-labelled Affibody molecules. Sci. Rep. 2018, 8, 6542. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Xu, T.; Dahlsson Leitao, C.; Ståhl, S.; Löfblom, J.; Orlova, A.; Tolmachev, V.; Vorobyeva, A. Influence of Residualizing Properties of the Radiolabel on Radionuclide Molecular Imaging of HER3 Using Affibody Molecules. Int. J. Mol. Sci. 2020, 21, 1312. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Dahlsson Leitao, C.; Gentry, J.; Mitran, B.; Abouzayed, A.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Increase in negative charge of 68Ga/chelator complex reduces unspecific hepatic uptake but does not improve imaging properties of HER3-targeting affibody molecules. Sci. Rep. 2019, 9, 17710. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Eltis, L.D. The biological occurrence and trafficking of cobalt. Metallomics 2011, 3, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent.-Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Bass, T.Z.; Rosestedt, M.; Mitran, B.; Frejd, F.Y.; Löfblom, J.; Tolmachev, V.; Ståhl, S.; Orlova, A. In vivo evaluation of a novel format of a bivalent HER3-targeting and albumin-binding therapeutic affibody construct. Sci. Rep. 2017, 7, 43118. [Google Scholar] [CrossRef]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, É.; Lukeš, I. Gallium(III) Complexes of DOTA and DOTA–Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.-F.; Thisgaard, H. In Vivo Evaluation of a Bombesin Analogue Labeled with Ga-68 and Co-55/57. Mol. Imaging Biol. 2016, 18, 368–376. [Google Scholar] [CrossRef]
- Wållberg, H.; Orlova, A. Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: Implications for development of labeled tracers. Cancer Biother. Radiopharm. 2008, 23, 435–442. [Google Scholar] [CrossRef]
- Orlova, A.; Hofström, C.; Strand, J.; Varasteh, Z.; Sandstrom, M.; Andersson, K.; Tolmachev, V.; Gräslund, T. [99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a new Affibody conjugate for visualization of insulin-like growth factor-1 receptor expression in malignant tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Orlova, A.; Andersson, K. Methods for radiolabelling of monoclonal antibodies. Methods Mol. Biol. Clifton NJ 2014, 1060, 309–330. [Google Scholar]
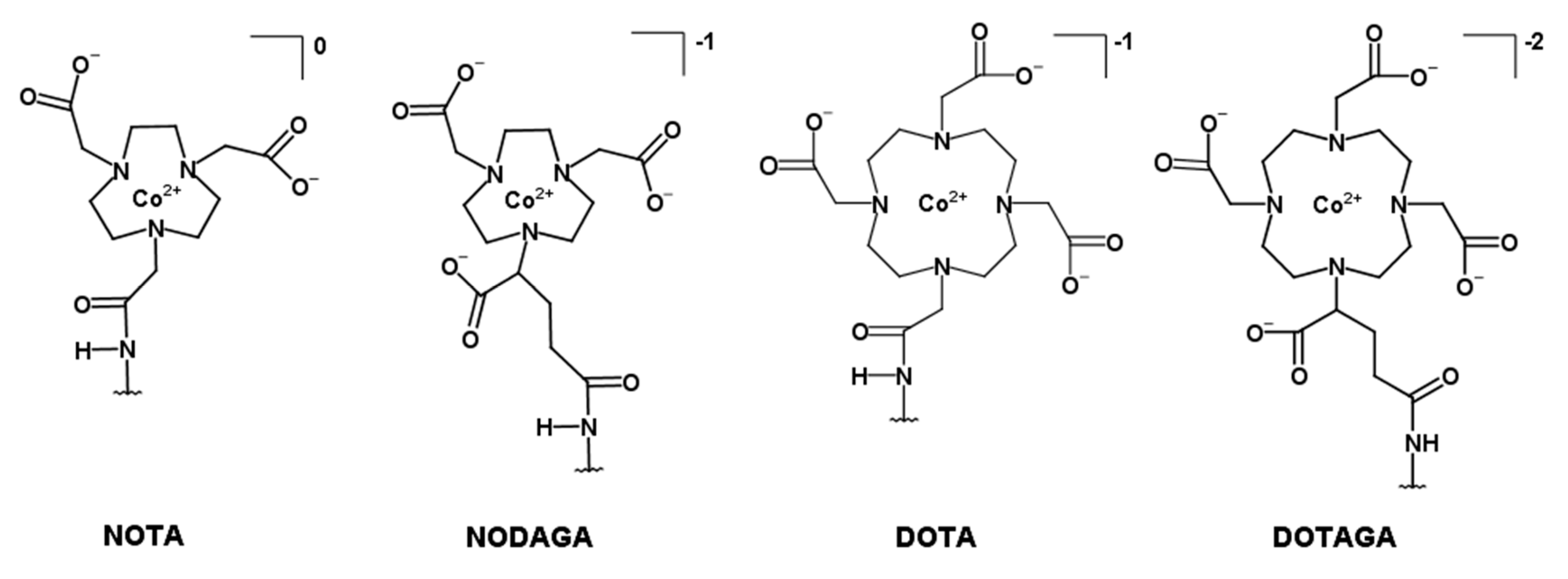

| NOTA * | NODAGA | DOTA | DOTAGA | |
|---|---|---|---|---|
| Radiochemical yield (%) | 81 ± 11% (n = 6) | 99.7 ± 0.2 (n = 2) | 99.7 ± 0.4 (n = 2) | 99.3 ± 0.7 (n = 2) |
| % Release in PBS, 24 h, RT | stable | 0 ± 0 | 0 ± 0 | 0.2 ± 0.3 |
| % Release in human serum, 24 h, 37 °C | 0 ± 0 | 0.4 ± 0.8 | 0.03 ± 0.05 |
| NOTA (n = 3) | NODAGA (n = 3) | DOTA (n = 4) | DOTAGA (n = 3) | |
|---|---|---|---|---|
| ka (1/Ms) | 2 × 105 ± 2 × 105 | 1.19 × 105 ± 0.09 × 105 | 1.0 × 105 ± 0.3 × 105 | 1.2 × 105 ± 0.8 × 105 |
| kd (1/s) | 1.28 × 10−5 ± 0.10 × 10−5 | 1.0 × 10−5 ± 0.7 × 10−5 | 2 × 10−5 ± 1 × 10−5 | 1.4 × 10−5 ± 0.4 × 10−5 |
| KD (nM) | 0.1 ± 0.1 | 0.09 ± 0.07 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Organ | NOTA | NODAGA | DOTA | DOTAGA | ||||
|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |
| Blood | 1.0 ± 0.1 a,b,c,* | 0.31 ± 0.04 a,b,c,* | 0.61 ± 0.09 a,d,* | 0.18 ± 0.02 a,* | 0.33 ± 0.04 b,d,f,* | 0.14 ±0.02 b,f,* | 0.52 ±0.04 c,f,* | 0.22 ±0.02 c,* |
| Salivary Gland | 0.9 ± 0.2 a,b | 0.6 ± 0.2 | 1.6 ± 0.4 a,e | 1 ± 1 | 1.5 ± 0.1 b,f,* | 0.9 ± 0.1 * | 0.64 ±0.09 e,f | 0.4 ±0.1 |
| Lung | 1.0 ± 0.1 a,b,* | 0.41 ± 0.07 * | 1.7 ± 0.3 a,e,* | 0.47 ± 0.04 * | 1.7 ± 0.2 b,f,* | 0.6 ± 0.2 e,* | 0.8 ± 0.1 e,f,* | 0.33 ± 0.04 e,* |
| Liver | 1.56 ± 0.52 a,b | 1.1 ± 0.2 | 3.3 ± 0.8 a,* | 1.4 ± 0.2 * | 3.7 ± 0.5 b,f,* | 1.6 ± 0.2 * | 2.4 ± 0.2 e,f,* | 1.5 ± 0.2 * |
| Spleen | 0.57 ± 0.04 c | 0.6 ± 0.2 | 0.47 ±0.09 e | 0.35 ±0.05 e | 0.43 ± 0.05 f | 0.42 ± 0.08 | 0.8 ± 0.1 c,e,f | 0.7 ± 0.1 e |
| Stomach | 0.78 ± 0.06 a,b,* | 0.35 ± 0.04 a,b,* | 1.5 ±0.5 a,e,* | 0.65 ± 0.06 a,e,* | 1.5 ± 0.3 b,f,* | 0.65 ± 0.05 b,f,* | 0.6 ± 0.1 e,f,* | 0.31 ± 0.05 b,f,* |
| Small intestine | 1.6 ±0.3 a,b,* | 0.7 ± 0.1 a,b,* | 4 ± 1 a,e,* | 1.6 ±0.3 a,e,* | 4.6 ± 0.8 b,f,* | 2.1 ± 0.04 b,f,* | 0.9 ± 0.2 e,f | 0.6 ± 0.10 e,f |
| Kidneys | 194 ± 17 * | 106 ± 67 a,b,* | 279 ± 84 | 213 ± 7 a | 253 ± 54 | 223 ± 23 b,f | 156 ± 27 c | 131 ±13 f |
| Tumor | 1.55 ± 0.26 a,b | 1.1 ± 0.3 b | 2.6 ± 0.6 a,e,* | 1.4 ± 0.4 d,* | 2.8 ± 0.4 b,f | 2.4 ± 0.4 b,d,f | 0.9 ± 0.2 e,f | 0.8 ± 0.1 f |
| Muscle | 0.19 ± 0.04 | 0.13 ± 0.04 | 0.20 ± 0.06 * | 0.09 ± 0.02 * | 0.19 ± 0.01 * | 0.09 ± 0.02 * | 0.16 ± 0.02 | 0.11 ± 0.03 |
| Bone | 0.3 ± 0.1 | 0.25 ± 0.05 | 0.3 ± 0.1 | 0.15 ±0.05 | 0.25 ± 0.06 | 0.20 ± 0.03 | 0.28 ± 0.09 | 0.19 ± 0.07 |
| GI (%ID) | 2.4 ± 0.5 a,b,* | 1.3 ± 0.2 * | 5.0 ± 0.4 a,* | 2.8 ± 0.7 * | 5.7 ± 0.9 b,f,* | 2.7 ± 0.7 * | 4.78 ± 0.9f | 1.13 ±0.2 |
| Body (%ID) | 5.9 ± 0.4 a,b | 3 ± 2 a,b | 10 ± 2 a,e,* | 4.5 ± 0.7 a,e,* | 9.0 ± 1.0 b,f,* | 4.9 ± 0.56 b,f,* | 2 ± 0.6 e,f,* | 3.1 ±0.3 e,f,* |
| Tumor/Organ Ratios | NOTA | NODAGA | DOTA | DOTAGA | ||||
|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |
| T/Blood | 1.5 ± 0.2 a,b,* | 3.4 ± 0.6 b | 4 ± 1a,d,e | 8 ± 3d | 8 ± 1 b,d,f,* | 18 ± 5 b,d,f | 1.8 ±0.3 e,f,* | 3.4 ± 0.5 f |
| T/Salivary gland | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.3 ± 0.7 d | 1.9 ± 0.2* | 2.9 ± 0.6 d | 1.5 ± 0.3 | 2.1 ± 0.9 |
| T/Lung | 1.6 ±0.3 * | 2.6 ± 0.4 | 1.6 ± 0.6 | 3 ± 1 | 1.6 ± 0.4* | 4.1 ± 0.9 e | 1.1 ±0.1* | 2.3 ± 0.4 e |
| T/Liver | 1.06 ± 0.31 c | 1.0 ± 0.2 b | 0.78 ± 0.09 e | 1.0 ± 0.3 | 0.74 ± 0.08 f,* | 1.6 ± 0.3 b,f | 0.44 ± 0.09 c,e,f | 0.50 ± 0.07 f |
| T/Spleen | 2.7 ± 0.3 a,b,* | 1.89 ±0.08 b | 6 ± 2 a,e | 4 ± 2 e | 6 ± 1 b,f | 6 ± 1 f | 1.2 ± 0.2 e,f | 1.2 ± 0.3 e,f |
| T/Stomach | 2.0 ± 0.2 * | 3.1 ± 0.8 | 1.7 ±0.4 | 2.1 ± 0.7 | 2 ± 0.2 * | 3.8 ± 0.9 | 1.6 ± 0.5 * | 2.5 ± 0.4 |
| T/Small intestine | 1.0 ± 0.2 b,* | 1.6 ± 0.2 | 0.6 ± 0.2 | 0.9 ± 0.4 | 0.60 ± 0.05 b,f | 1.3 ± 0.6 | 1.0 ± 0.1 e,f | 1.3 ± 0.2 |
| T/Muscle | 8 ± 2 a,b | 8 ± 1 b | 14 ± 4 a,e | 17 ± 7 d,e | 15 ± 1 b,f,* | 28 ± 4 b,d,f | 6 ± 1 e,f | 7 ± 2 e,f |
| T/Bone | 6 ± 3 | 4.3 ± 0.4 b | 10 ± 3 | 10 ±7 | 11 ± 2 f | 12 ± 3 e | 5 ± 3 f | 4 ± 1 e |
| Nuclide | Half-Life (Hour) | Mode of Decay | Mean Positron Energy (keV) | Principal Photon Emissions |
|---|---|---|---|---|
| 55Co | 17.5 | β+ 76% EC 24% | 570 | 511 (152%), 477 (20.2%), 931 (75%), 1317 (7.1%), 1408 (16.9%) |
| 64Cu | 12.7 | β+ 17.6% β− 37% EC 24% | 278 | 511 (35.2%), 1346 (0.5%) |
| 66Ga | 9.49 | β+ 57% EC 43% | 1750 | 511 (114%), 834 (5.9%), 1039 (37.0%), 2752 (22.7%) |
| 86Y | 14.7 | β+ 31.9 %EC 67% | 660 | 511 (66%), 443 (16.9%), 628 (32.6%), 646 (9.2%), 703 (15.4%), 777 (22.4%), 1077 (82.5%), 1153 (30.5%), 1854 (17.2%), 1920 (20.8%) |
| 90Nb | 14.6 | β+ 51.2% EC 49.8% | 660 | 511 (102%), 141 (66.8%), 1129 (92.7%), 2186 (18%), 2318 (82%) |
| 152Tb | 17.5 | β+ 20.3% EC 79.7% | 1140 | 511 (40.6%), 271 (9.5%), 344 (63.5%), 586 (9.2%), 779 (5.5%) |
| 76Br | 16.2 | β+ 55% EC 45% | 1188 | 511 (110%), 657 (16%), 1853 (14.7%), 2792 (5.6%), 2950 (7.4%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinne, S.S.; Dahlsson Leitao, C.; Saleh-nihad, Z.; Mitran, B.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules. Int. J. Mol. Sci. 2020, 21, 1972. https://doi.org/10.3390/ijms21061972
Rinne SS, Dahlsson Leitao C, Saleh-nihad Z, Mitran B, Tolmachev V, Ståhl S, Löfblom J, Orlova A. Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules. International Journal of Molecular Sciences. 2020; 21(6):1972. https://doi.org/10.3390/ijms21061972
Chicago/Turabian StyleRinne, Sara S., Charles Dahlsson Leitao, Zahra Saleh-nihad, Bogdan Mitran, Vladimir Tolmachev, Stefan Ståhl, John Löfblom, and Anna Orlova. 2020. "Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules" International Journal of Molecular Sciences 21, no. 6: 1972. https://doi.org/10.3390/ijms21061972
APA StyleRinne, S. S., Dahlsson Leitao, C., Saleh-nihad, Z., Mitran, B., Tolmachev, V., Ståhl, S., Löfblom, J., & Orlova, A. (2020). Benefit of Later-Time-Point PET Imaging of HER3 Expression Using Optimized Radiocobalt-Labeled Affibody Molecules. International Journal of Molecular Sciences, 21(6), 1972. https://doi.org/10.3390/ijms21061972








