Abstract
Ras homolog protein enriched in brain (Rheb) is a key activator of mammalian target of rapamycin complex 1 (mTORC1). The activation of mTORC1 by Rheb is associated with various processes such as protein synthesis, neuronal growth, differentiation, axonal regeneration, energy homeostasis, autophagy, and amino acid uptake. In addition, Rheb–mTORC1 signaling plays a crucial role in preventing the neurodegeneration of hippocampal neurons in the adult brain. Increasing evidence suggests that the constitutive activation of Rheb has beneficial effects against neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Our recent studies revealed that adeno-associated virus serotype 1 (AAV1) transduction with Rheb(S16H), a constitutively active form of Rheb, exhibits neuroprotective properties through the induction of various neurotrophic factors, promoting neurotrophic interactions between neurons and astrocytes in the hippocampus of the adult brain. This review provides compelling evidence for the therapeutic potential of AAV1–Rheb(S16H) transduction in the hippocampus of the adult brain by exploring its neuroprotective effects and mechanisms.
1. Introduction
Neurodegenerative diseases, which are becoming increasingly prevalent, are characterized by the physical decay and eventual loss of neurons. Neurodegenerative changes within the hippocampus and an extended neuronal network involving the medial temporal and medial parietal lobes result in the memory impairment observed in Alzheimer’s disease (AD) [1,2]. AD is the result of slow neuron degeneration, which starts in the hippocampus and spreads to the rest of the brain. The hippocampus plays a strategic role in the neurobiology of memory, being involved in the hierarchical spread of amyloid and neurofibrillary tangles [3,4]. The World Health Organization has identified dementia caused by AD as a public health priority, which will affect an estimated 115 million people worldwide by 2050 if there is no effective preventative strategy. Despite progress in elucidating the underlying mechanisms and genes involved in certain hallmarks of AD, including neuronal degeneration, extracellular neuritic plaques, and intracellular neurofibrillary tangles, the mechanisms underlying the degeneration and functional disruption that occur in AD remain unknown, and treatments to prevent the progression of the disease are limited [1,5,6]. Although the incomplete understanding of the etiology of AD hinders the development of knowledge-based targeted therapeutics, various studies have demonstrated that alterations in the levels of specific neurotrophic factors (NTFs), such as brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF), are associated with the pathogenesis of neurodegenerative diseases such as AD and Parkinson’s disease (PD) [5,7,8,9,10,11,12,13]. These observations suggest that the sustained expression of neurotrophic factors may be useful for protecting neurons in the adult brain, and the regulatory system of a key molecule that can produce neurotrophic factors is a potential therapeutic target against neurodegenerative diseases.
Ras homolog protein enriched in brain (Rheb) is a member of the Ras superfamily containing Rheb1 and Rheb2 [14]. Rheb is expressed at high basal levels in the hippocampus, cerebral cortex, occipital pole, frontal lobe, and temporal lobe [15]. Rheb is biochemically activated by growth factors such as epithelial growth factor (EGF), fibroblast growth factor (FGF), glial cell line-derived neurotrophic factor (GDNF), CNTF, and BDNF and it is associated with neuronal growth, differentiation, aging, axonal regeneration, energy homeostasis, autophagy, and amino acid uptake [16,17]. It is well known that Rheb functions as a key activator of mammalian target of rapamycin complex 1 (mTORC1), and the deletion of Rheb could lead to reduced cortical thickness and defective myelination in the brain [18]. Rheb could negatively regulate autophagy [19], resulting in protein aggregation, which can be associated with the progression and severity of neurodegenerative diseases such as AD, PD, and Huntington disease (HD) [20,21,22,23]. However, there has been accumulating evidence of the neuroprotective effects of enhanced Rheb expression against neurodegenerative diseases including PD [11,24,25], AD [26,27], and spinal cord injury [28]. Interestingly, the hyperactivation of Rheb could protect hippocampal neurons via neurotrophic interactions between neurons and astrocytes against neurotoxic conditions in the hippocampus of the adult brain [5,26,29,30].
A cure for neurodegenerative diseases has not been developed; however, accumulating evidence suggests that Rheb may be considered as one of the possible treatment targets for neurodegenerative diseases due to its pleiotropic role in the production of various NTFs. Therefore, this study provides insight into the role of Rheb signaling pathways in neurodegenerative diseases and the beneficial effects of Rheb as a potential therapeutic agent against hippocampal neurodegeneration in the adult brain.
2. Characteristics of Rheb
Rheb proteins, monomeric proteins of approximately 21 kDa, are highly conserved during evolution, and their expression is found in yeast and humans [14]. Rheb consists of 184 amino acids; the N-terminal 169 amino acids make up the GTPase domain, and the 15 remaining C-terminal residues make up a highly variable region ending in a CAAX motif. The crystal structures of Rheb bound with GTP, GppNHp, or GDP have been determined [14]. The structure of Rheb is highly similar to that of other small GTPases, with a closer resemblance to those of Ras and Rap than to those of Rab5A and RhoA [14]. In addition, due to the unique structure of Rheb, Gln64 (corresponding to Gln61 in Ras) is buried in a hydrophobic core and cannot interact with either GTP or the catalytic active site. Another difference compared with Ras is that a conserved tyrosine residue (Tyr35) in Rheb (corresponding to Tyr32 in Ras) shields the phosphate moiety of GTP. These two unique structural features of Rheb suggest that the mechanism of GTPase in Rheb differs from that in Ras. Recent findings have clarified the role of Rheb in activating mTORC1 signaling at the lysosomal membrane [31,32]. Two different complexes interact with each other on the lysosomal membrane. One of the complexes contains mTORC1, which is localized in lysosomes by the action of a heterodimeric GTPase (Rag) [33,34]. Rag consists of RagA/RagC or RagB/RagD. The binding of Rag to the lysosomal membrane requires Ragulator, which consists of multiple proteins including p18, p14, and MP1. The other complex is tuberous sclerosis complex (TSC)/Rheb, which is localized in lysosomes via a farnesyl group on Rheb. The co-localization of Rheb and mTORC1 facilitates the activation of mTORC1 by Rheb. Further insights into the regulation of these lysosomal events can be gained from recent studies that investigated the signals affecting the localization of these proteins [35,36]. Insulin causes the dissociation of TSC and the release of TSC from lysosomes, resulting in the activation of Rheb [35]. On the other hand, amino acid starvation does not affect the lysosomal localization of TSC2. Since amino acid starvation affects mTORC1 localization, it is suggested that insulin signals through TSC/Rheb, while amino acids signal through Rag and mTORC1. However, Rag GTPase binds to TSC2, and this binding is stimulated by amino acid starvation [36]. Thus, further studies should reveal the interplay between two different signals that converge on the lysosomal membrane.
3. Rheb–mTOR Signaling in the Brain
Tuberous sclerosis (TS) disease, also called TSC, is a genetic disorder characterized by the growth of numerous noncancerous tumors in many parts of the body [37]. TSC1 and TSC2, intracellular molecules named after TSC, are associated with the activation of Rheb/mTOR signaling pathway [24,25]. The activity of Rheb is regulated by TSC2, which acts as a guanosine triphosphatase-activating protein (GAP) that enhances the hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP) by Rheb [24,38,39,40,41,42]. The heterodimer of the two proteins is a critical negative regulator of Rheb, resulting in the inhibition of mTOR [43], which is controlled by insulin. Insulin triggers the activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway. Activated Akt then increases TSC2 phosphorylation at serine 939 and 981, leading to the dissociation of TSC1/2 [44].
It is well known that mTOR, a serine/threonine kinase that belongs to the family of PI3K-related kinases, acts as a central protein that controls cell growth and proliferation through transcriptional and translational mechanisms in response to various extracellular stimuli such as amino acids and growth factors [45]. Recently, a study demonstrated the requirement of intact F-actin dynamics for proper mTORC1 activation in response to netrin-1 in the axonal growth cones of tectal neurons [46]. mTORC1 is rapamycin-sensitive and contains regulatory-associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein 8 (mLST8), and the 40 kDa proline-rich Akt substrate (PRAS40) [47,48]. The downstream targets of mTOR include two independent targets, i.e., ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), both of which play important roles in mRNA translation [49]. mTOR stimulates translational initiation through direct phosphorylation and activation of S6K1 [50] and 4E-BP1 [51]. mTOR has also emerged as an important regulator of various neurological processes including neuronal differentiation, morphogenesis, synaptic plasticity, learning, and memory [52,53]. The mTOR pathway has been found to adversely affect neural circuit formation. Furthermore, the mTOR pathway regulates axon length [54] and axon guidance in response to environmental signals [55]. Among neurons, local protein synthesis in synapses distant from the soma is mediated by mTOR and is critical for the formation of the neural circuit. The expression levels of synaptic proteins, such as Arc and synapsin, are increased by mTOR activation [56]. The hyperactivation of the mTOR pathway via the loss of Pten has been shown to increase glutamatergic and GABAergic signals [57]. In addition to neurons, the mTOR pathway has been found to regulate glial cells during neural circuit formation. Deletion of the core component of mTORC1 or mTORC2 (Raptor or Rictor) was reported to result in defective myelination and oligodendrocyte maturation [58]. mTORC1 activity is important for regulating the organism size and survival during embryonic development. The deficiency of Raptor leads to a reduced brain size in developing embryos due to impaired cell cycle progression and increased apoptosis [59]. Rheb knockout has been observed to cause embryonic lethality in mice. Moreover, mTOR has been reported to be critical for long-lasting forms of neuronal plasticity, such as long-term potentiation (LTP), long-term depression (LTD), and learning and memory, which require protein synthesis [53,60,61,62]. For example, learning transiently increases p-mTOR in the hippocampus [62,63]. The blockade of mTOR signaling with rapamycin impairs hippocampus-dependent learning in tasks such as inhibitory avoidance [64], and both voluntary [65,66] and forced exercise enhance learning in the same task [67]. A recent study showed that Rheb overexpression-mediated synaptic growth was morphologically and functionally different from that in the presence of the TSC mutant, indicating that TSC may be able to regulate the neuromuscular junction synapse independent of the Rheb–mTORC1 pathway [68]. In addition, Rheb augments the expression of NTFs such as BDNF, CNTF, and GDNF through the activation of mTORC1 in the brain [11,69]. NTFs support the cell growth, survival, synaptic plasticity, and differentiation of both developing and mature neurons [70]. On the other hand, Rheb activation inhibits the non-selective degradation of protein aggregation mediated by autophagy, resulting in cell death due to the accumulation of misfolded proteins [19]. Autophagy dysfunction can be implicated in the pathogenesis of neurodegenerative disorders including PD and AD [20,21,22,23]. However, it is still unclear whether the alteration of autophagic regulation by neuronal Rheb upregulation is involved in neurotoxicity or neuroprotection in the adult brain with neurodegenerative diseases. Therefore, further studies are needed to determine the connection between neuroprotection and Rheb-mediated autophagy in neurodegenerative diseases such as PD and AD.
4. Role of Rheb in Neurodegeneration in the Hippocampus
As mentioned previously, Rheb augments the expression of NTFs such as BDNF, CNTF, and GDNF through the activation of mTORC1 in the brain. NTFs regulate the development, maintenance, function, and plasticity of mature neurons. BDNF is essential for a basal level of adult hippocampal neurogenesis and the survival and integration of new-born neurons into the hippocampal circuitry [9,71,72]. BDNF also plays a crucial role in the early and late phases of LTP, the cellular substrate for learning and memory [9,71,72,73,74,75,76]. Altered BDNF functionality has been observed in different neurodegenerative diseases [77,78]. Several studies showed that the mRNA and protein expression levels of BDNF and its full-length receptor tropomyosin receptor kinase B (TrkB) are decreased in the hippocampus and neocortex of AD postmortem brains and in the substantia nigra of PD patients [7,79,80,81]. BDNF administration has been shown to improve learning and memory in demented animals [82]. In addition, BDNF has been observed to have neuroprotective effects against β-amyloid (Aβ) and tau toxicity in AD models [83,84].
GDNF binds to its co-receptor alpha1 (GFRα1) and can promote the survival of different neuronal populations in the central and peripheral nervous systems [85]. The Ras/MAPK and PI3K/Akt cascades can be activated when GDNF binds to its main receptor, which consists of the ligand-binding component GFRα and the tyrosine kinase RET [86,87]. Several studies on the ability of GDNF to maintain mitochondrial activity have been conducted. By using a mouse model of PD, Meka et al. showed that GDNF could improve impaired mitochondrial function by activating the NF-κB transcription factor, mediated by the RET kinase through the PI3K pathway [88]. The GDNF–GFRα1 complex is essential for proper hippocampal circuit development. GDNF–GFRα1 signaling contributes to synapse development and the maturation of hippocampal neurons [89]. The GDNF–GFRα1 complex was observed to promote dendritic growth and postsynaptic differentiation in cultured hippocampal neurons through neural cell adhesion (NCAM) signaling [89]. The overexpression of GDNF in hippocampal astrocytes induced the recovery of spatial cognitive abilities in aged impaired rats. Several studies have shown that GDNF can enhance motor functions in aged rats and non-human primates, which may be associated with dopaminergic induction and regeneration of the nigrostriatal pathway [90,91,92,93,94]. Furthermore, GDNF is known to promote neuronal health and has neural regenerative properties [95]. Low GDNF levels were reported in patients with AD and in 3xTg AD mice [96,97]. In addition, GFRα1 deficiency has been observed in the brain of AD patients [98]. GDNF overexpression in astrocytes was found to exhibit neuroprotective effects through the upregulation of BDNF production, resulting in the preservation of learning and memory in 3xTg AD mice [99]. In addition, the upregulation of GDNF in activated astrocytes after brain injury is thought to play an active role in neuronal survival and plasticity [100]. Activated astrocytes have been observed to upregulate NTFs, antioxidants, and other key molecules, all of which support neuronal and oligodendrocyte survival as well as tissue repair [101]. These results suggest that the crosstalk between neurons and astrocytes is a potential neuroprotective mechanism of NTFs in neurodegenerative diseases.
CNTF belongs to the interleukin-6 family of cytokines and is expressed mainly in astrocytes, whereas CNTF receptor α subunit (CNTFRα) is expressed predominantly in neural progenitor cells and hippocampal neurons [102]. CNTF has potent effects on the development and maintenance of the nervous system, inducing neuronal survival and differentiation by stimulating gene expression in sensory, sympathetic, and motor neurons [103]. In addition, the neuroprotective properties of CNTF demonstrate that it is a survival factor for sympathetic, sensory, and hippocampal neurons. CNTF could promote neural stem cell division directly through its receptor [104]. It has been reported that CNTF protects neurons from degeneration arising from multiple etiologies [105,106]. In a previous study, CNTF contributed to the full recovery of cognitive functions associated with the stabilization of synaptic protein levels in the Tg2576 AD mouse model [107]. These results indicate the potential of CNTF as a therapeutic agent for treating neurodegenerative diseases. Taken together, increasing evidence suggests that a lack of trophic support may contribute significantly to neurodegeneration, and NTFs have emerged as promising therapeutic agents for neurodegenerative diseases [108]. Nevertheless, the clinical utility of systemic BDNF, GDNF, and CNTF is limited by poor blood–brain barrier (BBB) permeability, a short half-life, and off-target effects [109,110].
Rheb has been reported to increase the levels of acetylcholine and total choline in the adult rat brain, which are important for maintaining cognitive functions in AD brains [5]. Axon degeneration and synapse and dendritic spine loss have been observed in brains as a result of aging and neurodegenerative diseases [111,112,113]. A decrease in synaptic connectivity is considered to be a major cause of altered mood and impaired perception and cognition in neurodegenerative diseases. Several studies have demonstrated the role of Rheb–mTOR signaling in axon elongation and synaptic morphogenesis [114,115]. Akt–Rheb–mTORC1 signaling can enhance not only the axon length but also the axon number per neuron and the number of neurons having multiple axons [116]. In addition, Rheb activation can enhance dendritic morphogenesis and synaptic integration in the subventricular zone [117]. TSC2–Rheb–mTOR signaling regulates the ephrin–Eph receptor system to mediate axon guidance in the visual system [118]. Furthermore, long-term synaptic plasticity, learning, and memory rely on de novo protein synthesis. Rheb–mTOR signaling is considered an important mechanism for the maintenance of LTP and learning and memory functions [60,61]. Rheb has been reported to interact with beta-secretase 1 (BACE1) in the mouse brain, resulting in the inhibition of BACE1 activity and the reduction of Aβ generation [119]. Furthermore, the deletion of Rheb could lead to deficits in spatial memory functions, a behavioral hallmark of AD progression [119]. In the brain of patients with AD, the levels of Rheb are significantly downregulated compared with its levels in a normal healthy brain [23].
On the other hand, Rheb can be an issue in neurodegenerative disease progression. Several studies indicated that TSC–Rheb–mTOR signaling could regulate aggresome formation, contributing to the disposal of misfolded proteins via the proteasome and autophagy system. Autophagy failure can trigger neuronal cell death in several ways, depending on where the defect is along the pathway. When proteolytic clearance steps are compromised, autolysosomes/lysosomes accumulate mutant and oxidized proteins, protein oligomers and aggregates, damaged organelles, and other incompletely digested products, some of which increase the permeability of lysosomal membranes and cause hydrolases to be released into the cytoplasm, in some cases even from otherwise intact lysosomes [112]. Autophagy-related pathology has been noted in late-onset neurodegenerative diseases including AD, PD, amyotrophic lateral sclerosis (ALS), and HD [23]. mTORC1 negatively controls the initiation of autophagy, contributing to the formation of pathological protein aggregates. Previous studies [120,121] showed that mTOR activation could downregulate Aβ clearance by inhibiting autophagy functions with the suppression of several signaling pathways including PI3K/Akt, glycogen synthase kinase 3 (GSK-3), AMP-activated protein kinase (AMPK), and insulin/insulin-like growth factor 1 (IGF-1) [122]. In addition, the activation of mTOR contributes to aberrant hyperphosphorylated tau [123]. The alteration of mTOR activity may be correlated with neurological symptoms such as epilepsy, mental retardation, autism, and brain tumors [61,124,125,126]. These findings suggest that Rheb–mTORC1 signaling may be associated with the onset and progression of neurodegenerative diseases.
Although the Rheb–mTOR signaling pathway may act as a double-edged sword in neurodegeneration, it is a central regulator of NTFs such as BDNF, CNTF, and GDNF and thus is a potential therapeutic target for neurodegenerative diseases. Therefore, studies that elucidate the role of the Rheb–mTOR signaling pathway in neurodegenerative diseases are of great importance.
5. Mutation of Rheb Leading to Constitutive Activation
The expression levels of growth-associated proteins are lower in adulthood than in the process of development. Consequently, the regenerative capacity of mature neurons is limited in the aging brain [127]. As described previously, Rheb–mTOR signaling is a key regulator of various mechanisms associated with neuronal survival and regeneration. Accordingly, several studies have identified Rheb mutations that lead to constitutive activation (Table 1). The mutation of serine to histidine at position 16 of Rheb [Rheb(S16H)] has been found to exhibit gain-of-function properties, resulting in highly activated mTOR signaling in TSC1/2-overexpressing cells [128]. Our previous studies showed that the overexpression of Rheb(S16H) could significantly increase the levels of NTFs such as GDNF, CNTF, and BDNF in the adult brain [5,11,26,29,69]. The mutation of tyrosine to asparagine at position 35 of Rheb [Rheb(Y35N)] in renal cell carcinoma has been reported to cause mTORC1 hyperactivation by increasing the resistance to TSC2 GAP activity, resulting in the hyperproliferation of tumor cells [129]. Another study identified the mutation of glycine to alanine at position 63 of Rheb [Rheb(G35A)], which could stimulate the phosphorylation of S6 kinase more strongly compared wild-type Rheb in HeLa cells [130]. The mutation of glutamine to leucine at position 64 of Rheb [Rheb(Q64L)] was found to strongly induce oncogenic transformation in chicken embryonic fibroblast cultures. Cells with Rheb(Q64L) overexpression could promote the constitutive phosphorylation of the ribosomal proteins S6K and 4E-BP1 [131] and exhibit increased GTP loading and partial resistance to TSC–GAP [132]. Another study identified specific Rheb hyperactive mutations in four different residues: (a) V17G and V17A, (b) S21G and S21I, (c) K120R, (d) N153S and N153T [133]. In comparison with wild-type Rheb, these hyperactive mutations of Rheb conferred resistance to canavanine toxicity. The GTP-binding affinity of these mutations was closely similar to that of wild-type Rheb, whereas the GDP-binding affinity was greatly reduced. In our previous study, we demonstrated the neuroprotective potential of the constitutively active forms of Rheb with mutations such as Rheb(S16H) and Rheb(N153T) in a neurotoxin-induced animal model of PD [24]. In particular, Rheb(S16H) upregulation by adeno-associated virus serotype 1 (AAV1) administration in nigral dopaminergic neurons significantly increased the activation of mTORC1, resulting in greater neuroprotective effects compared with Rheb(N153T) upregulation in the animal model of PD [24]. In addition, our studies demonstrated that the upregulation of neuronal Rheb(S16H) could protect neurons against neurodegeneration and promote axonal regrowth in the nigrostriatal dopamine system and hippocampus of the adult brain [11,24,25,30,69,134]. The activation of mTORC1 induced by Rheb(S16H) transduction of hippocampal neurons was found to result in BDNF production, which protected against thrombin-induced neurotoxicity in the rat hippocampus [5]. In the same manner, AAV1–Rheb(S16H) transduction in the hippocampus of transgenic AD mice prevented cognitive function impairment [26,29].

Table 1.
List of mutations associated with the constitutive activation of Rheb.
6. Mechanisms of Rheb(S16H)-Induced Neuroprotection in the Hippocampus of the Adult Brain
To date, the upregulation of neuronal Rheb(S16H) has demonstrated some beneficial effects in the hippocampus of the adult brain under neurotoxic conditions. Our previous studies demonstrated that neuronal transduction with AAV1–Rheb(S16H) prevented thrombin-induced neuronal death in the hippocampus of the adult rat brain [5,29]. Similarly, previous studies reported that neuronal transduction of Rheb(S16H) could protect dopaminergic neurons in the substantia nigra of mice against neurotoxin-induced dopamine neuronal death [11,24,25,69] and prothrombin kringle-2 (pKr-2)-induced neurotoxic inflammation [30]. AAV1–Rheb(S16H) transduction was found to have preventive effects on LTP impairment and cognitive decline in 5XFAD mice, which represent a transgenic mouse model of AD carrying five mutations associated with early-onset familial Alzheimer’s disease (FAD) [26]. These neuroprotective effects could be mediated by the stimulation of mTORC1-related neuronal BDNF production following AAV1–Rheb(S16H) administration, which might occur regardless of the levels of neuroinflammatory molecules. In addition, we recently found that neuronal transduction with AAV1–Rheb(S16H) could increase the expression of GDNF in both hippocampal neurons and astrocytes via autocrine and paracrine BDNF–TrkB signaling [29,135]. Similarly, previous studies reported that the expression of neuronal Rheb(S16H) could protect dopaminergic neurons through the elevation of GDNF and BDNF expression in the substantia nigra against neurotoxin-induced dopamine neuronal death [69] and pKr-2-induced neurotoxic inflammation [30]. Furthermore, Rheb(S16H) upregulation could induce the upregulation of cAMP response element-binding protein (CREB) phosphorylation in dopamine neurons, which may be associated with the synthesis of GDNF and BDNF. In the hippocampus of the adult brain, AAV1–Rheb(S16H) transduction could induce sustained increases in the levels of full-length TrkB and CNTFRα in reactive astrocytes and hippocampal neurons, respectively [29]. In addition, we demonstrated that neuronal BDNF produced by the transduction of hippocampal neurons with Rheb(S16H) could induce reactive astrocytes, which could lead to CNTF production through the activation of astrocytic TrkB and the upregulation of neuronal BDNF and astrocytic CNTF, resulting in synergistic effects on the survival of hippocampal neurons in vivo [29]. In 5XFAD mice, AAV1–Rheb(S16H) transduction was found to have preventive effects on LTP impairment and cognitive decline [26]. These beneficial effects may be attributed to the interaction between neurons and astrocytes promoted by multiple NTFs induced by Rheb(S16H) transduction of hippocampal neurons, which result in neuroprotection in the hippocampus of 5XFAD mice.
Astrocytes are the most abundant cells in the brain and play an important role in the homeostatic maintenance of physiological environments, such as ion concentration and pH in the central nervous system [136,137]. Although previous studies indicated that astrocyte activation is involved in neurodegeneration through inflammatory responses in the adult brain [138,139], reactive astrocytes may have beneficial effects such as increased neuronal survival, growth, and activity through barrier function to restrict tissue damage and neuroinflammation [137,140,141]. In addition, they have been found to produce NTFs such as GDNF and CNTF in animal models of neurodegenerative diseases [142,143,144]. A study reported that the loss of astrocytes in the brain of GFAP-knockout mice resulted in a larger infarct area induced by ischemic brain injury following middle cerebral artery occlusion [145]. Overall, these findings suggest that astrocytes are important for maintaining a normal neural system in the adult brain. Furthermore, the induction of reactive astrocytes, which can protect against neurodegeneration in patients with neurodegenerative diseases such as AD and PD, was observed to support neuronal survival and functional maintenance in vivo. In previous studies, morphological changes in astrocytes and microglia were clearly observed in the Rheb (S16H)-treated hippocampus of the adult brain [26,29,135]. Moreover, the levels of Aβ were significantly reduced after AAV1–Rheb(S16H) transduction in the hippocampus of 5XFAD mice. These observations suggest that AAV1–Rheb(S16H) transduction of hippocampal neurons could strengthen the neuroprotective system through the stimulation of mTORC1-mediated neurotrophic interactions involving BDNF, CNTF, and GDNF between neurons and astrocytes in the hippocampus of the adult brain, resulting in neuroprotection in the hippocampus in vivo under various neurodegenerative conditions (Figure 1).
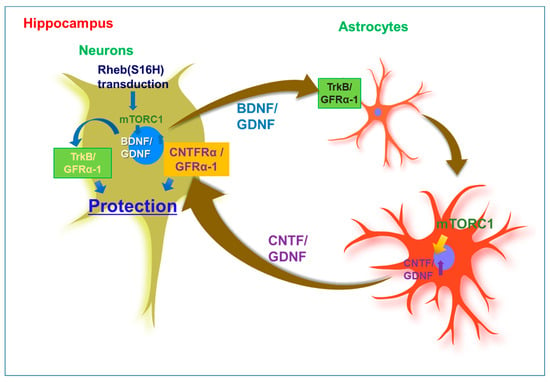
Figure 1.
Mechanisms of neuroprotection through neuronal upregulation of mutated ras homolog protein enriched in brain [Rheb(S16H)] in the hippocampus of the adult brain. Upregulation of Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) by neuronal transduction with adeno-associated virus serotype 1 (AAV1)–Rheb(S16H) can additionally contribute to the activation of BDNF/TrkB and GDNF/GFRα-1 signaling pathways, respectively, in astrocytes via the interactions between neurons and astrocytes in the adult hippocampus, resulting in the production of astrocytic ciliary neurotrophic factor (CNTF) and GDNF for hippocampal neuroprotection. These observations suggest that Rheb(S16H) transduction of hippocampal neurons may be a good strategy to protect adult neurons in the hippocampus in vivo.
7. Conclusion
The current therapies for AD and PD are initially effective, alleviating the main symptoms of these diseases. However, disease progression is not prevented. In contrast to the current treatments, Rheb(S16H) transduction of adult neurons may provide a novel therapeutic option. Our recent studies suggest that Rheb(S16H) transduction of hippocampal neurons could stimulate NTFs production, strengthening the neuroprotective system in the hippocampus of the adult brain and reducing neurodegeneration. Although further studies are needed to determine the clinical relevance of the AAV1–Rheb(S16H) transduction approach for neurodegenerative diseases, this approach may be a useful strategy for protecting hippocampal neurons in the lesioned brain, and its effects may be beneficial for patients with neurodegenerative diseases such as AD.
Author Contributions
G.J.M. and S.R.K. designed and co-wrote the first manuscript. G.J.M and M.S. provided the references for the table. M.S. and S.R.K. edited the revised manuscript. S.R.K. supervised the whole manuscript. All authors contributed to the preparation of the manuscript and approved the final manuscript.
Funding
This research was supported by a grant from the National Research Foundation of Korea (NRF-2020R1A2C2007954).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75 e62. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991, 1, 213–216. [Google Scholar] [CrossRef]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.T.; Nam, J.H.; Shin, W.H.; Leem, E.; Jeong, K.H.; Jung, U.J.; Bae, Y.S.; Jin, Y.H.; Kholodilov, N.; Burke, R.E.; et al. In vivo AAV1 transduction with hRheb(S16H) protects hippocampal neurons by BDNF production. Mol. Ther. 2015, 23, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Ji, X.; Mao, X.; Xie, L.; Jia, J.; Galvan, V.; Greenberg, D.A.; Jin, K. Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural. Regen. Res. 2017, 12, 549–557. [Google Scholar] [CrossRef]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef]
- Chauhan, N.B.; Siegel, G.J.; Lee, J.M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J. Chem. Neuroanat. 2001, 21, 277–288. [Google Scholar] [CrossRef]
- Jeong, K.H.; Nam, J.H.; Jin, B.K.; Kim, S.R. Activation of CNTF/CNTFRalpha signaling pathway by hRheb(S16H) transduction of dopaminergic neurons in vivo. PLoS ONE 2015, 10, e0121803. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.S.; Hains, J.M.; Armanini, M.; Laramee, G.R.; Johnson, S.A.; Winslow, J.W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991, 7, 695–702. [Google Scholar] [CrossRef]
- Sopova, K.; Gatsiou, K.; Stellos, K.; Laske, C. Dysregulation of neurotrophic and haematopoietic growth factors in Alzheimer’s disease: From pathophysiology to novel treatment strategies. Curr. Alzheimer. Res. 2014, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, S.; Xu, X.; Li, Y.; Guan, K.; Arnold, E.; Ding, J. Structural basis for the unique biological function of small GTPase RHEB. J. Biol. Chem. 2005, 280, 17093–17100. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Araki, Y.; Kontani, K.; Nishina, H.; Katada, T. Novel role of the small GTPase Rheb: Its implication in endocytic pathway independent of the activation of mammalian target of rapamycin. J. Biochem. 2005, 137, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.H.; Thapar, N.; Guo, L.; Martinez, M.; Maris, J.; Gau, C.L.; Lengyel, J.A.; Tamanoi, F. Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J. Cell Sci. 2003, 116, 3601–3610. [Google Scholar] [CrossRef]
- Aspuria, P.J.; Tamanoi, F. The Rheb family of GTP-binding proteins. Cell Signal. 2004, 16, 1105–1112. [Google Scholar] [CrossRef]
- Lee, J.H.; Tecedor, L.; Chen, Y.H.; Monteys, A.M.; Sowada, M.J.; Thompson, L.M.; Davidson, B.L. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron 2015, 85, 303–315. [Google Scholar] [CrossRef]
- Zhou, X.; Ikenoue, T.; Chen, X.; Li, L.; Inoki, K.; Guan, K.L. Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 8923–8928. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 17, 802–815. [Google Scholar] [CrossRef]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Yang, D.S. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Beal, M.F.; Thomas, B. Autophagy in neurodegenerative disorders: Pathogenic roles and therapeutic implications. Trends Neurosci. 2010, 33, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kareva, T.; Yarygina, O.; Kholodilov, N.; Burke, R.E. AAV transduction of dopamine neurons with constitutively active Rheb protects from neurodegeneration and mediates axon regrowth. Mol. Ther. 2012, 20, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Chen, X.; Oo, T.F.; Kareva, T.; Yarygina, O.; Wang, C.; During, M.; Kholodilov, N.; Burke, R.E. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann. Neurol. 2011, 70, 110–120. [Google Scholar] [CrossRef]
- Moon, G.J.; Kim, S.; Jeon, M.T.; Lee, K.J.; Jang, I.S.; Nakamura, M.; Kim, S.R. Therapeutic Potential of AAV1-Rheb(S16H) Transduction Against Alzheimer’s Disease. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Shahani, N.; Pryor, W.; Swarnkar, S.; Kholodilov, N.; Thinakaran, G.; Burke, R.E.; Subramaniam, S. Rheb GTPase regulates beta-secretase levels and amyloid beta generation. J. Biol. Chem. 2014, 289, 5799–5808. [Google Scholar] [CrossRef]
- Wu, D.; Klaw, M.C.; Connors, T.; Kholodilov, N.; Burke, R.E.; Tom, V.J. Expressing Constitutively Active Rheb in Adult Neurons after a Complete Spinal Cord Injury Enhances Axonal Regeneration beyond a Chondroitinase-Treated Glial Scar. J. Neurosci. 2015, 35, 11068–11080. [Google Scholar] [CrossRef]
- Jeon, M.T.; Moon, G.J.; Kim, S.; Choi, M.; Oh, Y.S.; Kim, D.W.; Kim, H.J.; Lee, K.J.; Choe, Y.; Ha, C.M.; et al. Neurotrophic interactions between neurons and astrocytes following AAV1-Rheb(S16H) transduction in the hippocampus in vivo. Br. J. Pharmacol. 2020, 177, 668–686. [Google Scholar] [CrossRef]
- Kim, S.; Moon, G.J.; Oh, Y.S.; Park, J.; Shin, W.H.; Jeong, J.Y.; Choi, K.S.; Jin, B.K.; Kholodilov, N.; Burke, R.E.; et al. Protection of nigral dopaminergic neurons by AAV1 transduction with Rheb(S16H) against neurotoxic inflammation in vivo. Exp. Mol. Med. 2018, 50, e440. [Google Scholar] [CrossRef]
- Groenewoud, M.J.; Zwartkruis, F.J. Rheb and Rags come together at the lysosome to activate mTORC1. Biochem. Soc. Trans. 2013, 41, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell. Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Goraksha-Hicks, P.; Li, L.; Neufeld, T.P.; Guan, K.L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 2008, 10, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef]
- Demetriades, C.; Doumpas, N.; Teleman, A.A. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 2014, 156, 786–799. [Google Scholar] [CrossRef]
- Northrup, H.; Wheless, J.W.; Bertin, T.K.; Lewis, R.A. Variability of expression in tuberous sclerosis. J. Med. Genet. 1993, 30, 41–43. [Google Scholar] [CrossRef]
- Castro, A.F.; Rebhun, J.F.; Clark, G.J.; Quilliam, L.A. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J. Biol. Chem. 2003, 278, 32493–32496. [Google Scholar] [CrossRef]
- Garami, A.; Zwartkruis, F.J.; Nobukuni, T.; Joaquin, M.; Roccio, M.; Stocker, H.; Kozma, S.C.; Hafen, E.; Bos, J.L.; Thomas, G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol. Cell 2003, 11, 1457–1466. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Xu, T.; Guan, K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003, 17, 1829–1834. [Google Scholar] [CrossRef]
- Tee, A.R.; Manning, B.D.; Roux, P.P.; Cantley, L.C.; Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 2003, 13, 1259–1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Saucedo, L.J.; Ru, B.; Edgar, B.A.; Pan, D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 2003, 5, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Corradetti, M.N.; Guan, K.L. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.L.; Tee, A.R.; Short, J.D.; Bergeron, J.M.; Kim, J.; Shen, J.; Guo, R.; Johnson, C.L.; Kiguchi, K.; Walker, C.L. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J. Cell Biol. 2006, 173, 279–289. [Google Scholar] [CrossRef]
- Gingras, A.C.; Raught, B.; Sonenberg, N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001, 15, 807–826. [Google Scholar] [CrossRef]
- Piper, M.; Lee, A.C.; van Horck, F.P.; McNeilly, H.; Lu, T.B.; Harris, W.A.; Holt, C.E. Differential requirement of F-actin and microtubule cytoskeleton in cue-induced local protein synthesis in axonal growth cones. Neural Dev. 2015, 10, 3. [Google Scholar] [CrossRef]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Shibuya, N.; Inoue, K.-i.; Kubota, K. Metabolic Shunt Pathways, Carcinoma, and mTOR. In Molecules to Medicine with mTOR. Academic Press: Cambridge, MA, USA, 2016; pp. 429–438.
- Isotani, S.; Hara, K.; Tokunaga, C.; Inoue, H.; Avruch, J.; Yonezawa, K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J. Biol. Chem. 1999, 274, 34493–34498. [Google Scholar] [CrossRef]
- Gingras, A.C.; Raught, B.; Sonenberg, N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999, 68, 913–963. [Google Scholar] [CrossRef]
- Gipson, T.T.; Johnston, M.V. Plasticity and mTOR: Towards restoration of impaired synaptic plasticity in mTOR-related neurogenetic disorders. Neural. Plast. 2012, 2012, 486402. [Google Scholar] [CrossRef] [PubMed]
- Graber, T.E.; McCamphill, P.K.; Sossin, W.S. A recollection of mTOR signaling in learning and memory. Learn. Mem 2013, 20, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, L.; Huang, T.; Lin, T.V.; Miyares, L.; Wen, J.; Hsieh, L.; Bordey, A. Activating the translational repressor 4E-BP or reducing S6K-GSK3beta activity prevents accelerated axon growth induced by hyperactive mTOR in vivo. Hum. Mol. Genet. 2015, 24, 5746–5758. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.C.; Zivraj, K.H.; Holt, C.E. Local translation and mRNA trafficking in axon pathfinding. Results Probl Cell Differ. 2009, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Raab-Graham, K.F.; Haddick, P.C.; Jan, Y.N.; Jan, L.Y. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science 2006, 314, 144–148. [Google Scholar] [CrossRef]
- Weston, M.C.; Chen, H.; Swann, J.W. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J. Neurosci. 2012, 32, 11441–11452. [Google Scholar] [CrossRef]
- Wahl, S.E.; McLane, L.E.; Bercury, K.K.; Macklin, W.B.; Wood, T.L. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J. Neurosci. 2014, 34, 4453–4465. [Google Scholar] [CrossRef] [PubMed]
- Cloetta, D.; Thomanetz, V.; Baranek, C.; Lustenberger, R.M.; Lin, S.; Oliveri, F.; Atanasoski, S.; Ruegg, M.A. Inactivation of mTORC1 in the developing brain causes microcephaly and affects gliogenesis. J. Neurosci. 2013, 33, 7799–7810. [Google Scholar] [CrossRef]
- Jaworski, J.; Sheng, M. The growing role of mTOR in neuronal development and plasticity. Mol. Neurobiol. 2006, 34, 205–219. [Google Scholar] [CrossRef]
- Swiech, L.; Perycz, M.; Malik, A.; Jaworski, J. Role of mTOR in physiology and pathology of the nervous system. Biochim. Biophys. Acta. 2008, 1784, 116–132. [Google Scholar] [CrossRef]
- Santini, E.; Huynh, T.N.; Klann, E. Mechanisms of translation control underlying long-lasting synaptic plasticity and the consolidation of long-term memory. Prog. Mol. Biol. Transl. Sci. 2014, 122, 131–167. [Google Scholar] [CrossRef] [PubMed]
- Slipczuk, L.; Bekinschtein, P.; Katche, C.; Cammarota, M.; Izquierdo, I.; Medina, J.H. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Jobim, P.F.; Pedroso, T.R.; Christoff, R.R.; Werenicz, A.; Maurmann, N.; Reolon, G.K.; Roesler, R. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol. Learn. Mem. 2012, 97, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Baliego, L.G.; Peixinho-Pena, L.F.; de Almeida, A.A.; Venancio, D.P.; Scorza, F.A.; de Mello, M.T.; Arida, R.M. Aerobic exercise attenuates inhibitory avoidance memory deficit induced by paradoxical sleep deprivation in rats. Brain Res. 2013, 1529, 66–73. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Foster, T.C.; Ormerod, B.K. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav. Immun. 2013, 28, 25–43. [Google Scholar] [CrossRef]
- Lovatel, G.A.; Elsner, V.R.; Bertoldi, K.; Vanzella, C.; Moyses Fdos, S.; Vizuete, A.; Spindler, C.; Cechinel, L.R.; Netto, C.A.; Muotri, A.R.; et al. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol. Learn. Mem. 2013, 101, 94–102. [Google Scholar] [CrossRef]
- Natarajan, R.; Trivedi-Vyas, D.; Wairkar, Y.P. Tuberous sclerosis complex regulates Drosophila neuromuscular junction growth via the TORC2/Akt pathway. Hum. Mol. Genet. 2013, 22, 2010–2023. [Google Scholar] [CrossRef]
- Nam, J.H.; Leem, E.; Jeon, M.T.; Jeong, K.H.; Park, J.W.; Jung, U.J.; Kholodilov, N.; Burke, R.E.; Jin, B.K.; Kim, S.R. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: Neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson’s disease. Mol. Neurobiol. 2015, 51, 487–499. [Google Scholar] [CrossRef]
- Platholi, J.; Lee, F.S. Neurotrophic Factors. In Handbook of Developmental Neurotoxicology; Academic Press: Cambridge, MA, USA,, 2018; pp. 55–64. [Google Scholar]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Wang, L.; Chang, X.; She, L.; Xu, D.; Huang, W.; Poo, M.M. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J. Neurosci. 2015, 35, 8384–8393. [Google Scholar] [CrossRef]
- Ji, Y.; Lu, Y.; Yang, F.; Shen, W.; Tang, T.T.; Feng, L.; Duan, S.; Lu, B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010, 13, 302–309. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Panja, D.; Bramham, C.R. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology 2014, 76, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Diniz, B.S.; Teixeira, A.L. Brain-derived neurotrophic factor and Alzheimer’s disease: Physiopathology and beyond. Neuromolecular Med. 2011, 13, 217–222. [Google Scholar] [CrossRef]
- Schindowski, K.; Belarbi, K.; Buee, L. Neurotrophic factors in Alzheimer’s disease: Role of axonal transport. Genes Brain Behav. 2008, 7, 43–56. [Google Scholar] [CrossRef]
- Lee, J.; Fukumoto, H.; Orne, J.; Klucken, J.; Raju, S.; Vanderburg, C.R.; Irizarry, M.C.; Hyman, B.T.; Ingelsson, M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005, 194, 91–96. [Google Scholar] [CrossRef]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Ando, S.; Kobayashi, S.; Waki, H.; Kon, K.; Fukui, F.; Tadenuma, T.; Iwamoto, M.; Takeda, Y.; Izumiyama, N.; Watanabe, K.; et al. Animal model of dementia induced by entorhinal synaptic damage and partial restoration of cognitive deficits by BDNF and carnitine. J. Neurosci. Res. 2002, 70, 519–527. [Google Scholar] [CrossRef]
- Arancibia, S.; Silhol, M.; Mouliere, F.; Meffre, J.; Hollinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.S.; Shen, L.L.; Zhu, C.; Bu, X.L.; Liu, Y.H.; Liu, C.H.; Yao, X.Q.; Zhang, L.L.; Zhou, H.D.; Walker, D.G.; et al. Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl. Psychiatry 2016, 6, e907. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, T.V.; Mishchenko, T.A.; Mitroshina, E.V.; Shirokova, O.M.; Pimashkin, A.S.; Kastalskiy, I.A.; Mukhina, I.V.; Kazantsev, V.B.; Vedunova, M.V. Glial cell line-derived neurotrophic factor (GDNF) counteracts hypoxic damage to hippocampal neural network function in vitro. Brain Res. 2018, 1678, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochim. Biophys. Acta 2013, 1834, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Meka, D.P.; Muller-Rischart, A.K.; Nidadavolu, P.; Mohammadi, B.; Motori, E.; Ponna, S.K.; Aboutalebi, H.; Bassal, M.; Annamneedi, A.; Finckh, B.; et al. Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J. Clin. Invest. 2015, 125, 1873–1885. [Google Scholar] [CrossRef]
- Irala, D.; Bonafina, A.; Fontanet, P.A.; Alsina, F.C.; Paratcha, G.; Ledda, F. The GDNF-GFRalpha1 complex promotes the development of hippocampal dendritic arbors and spines via NCAM. Development 2016, 143, 4224–4235. [Google Scholar] [CrossRef]
- Bowenkamp, K.E.; Lapchak, P.A.; Hoffer, B.J.; Bickford, P.C. Glial cell line-derived neurotrophic factor reverses motor impairment in 16–17 month old rats. Neurosci.. Lett. 1996, 211, 81–84. [Google Scholar] [CrossRef]
- Emerich, D.F.; Plone, M.; Francis, J.; Frydel, B.R.; Winn, S.R.; Lindner, M.D. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996, 736, 99–110. [Google Scholar] [CrossRef]
- Grondin, R.; Cass, W.A.; Zhang, Z.; Stanford, J.A.; Gash, D.M.; Gerhardt, G.A. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003, 23, 1974–1980. [Google Scholar] [CrossRef]
- Hebert, M.A.; Gerhardt, G.A. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J. Pharmacol. Exp. Ther. 1997, 282, 760–768. [Google Scholar] [PubMed]
- Matlik, K.; Voikar, V.; Vilenius, C.; Kulesskaya, N.; Andressoo, J.O. Two-fold elevation of endogenous GDNF levels in mice improves motor coordination without causing side-effects. Sci. Rep. 2018, 8, 11861. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Straten, G.; Eschweiler, G.W.; Maetzler, W.; Laske, C.; Leyhe, T. Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer’s disease and normal controls. J. Alzheimers Dis. 2009, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Revilla, S.; Sunol, C.; Garcia-Mesa, Y.; Gimenez-Llort, L.; Sanfeliu, C.; Cristofol, R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014, 81, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Yang, L.B.; He, P.; Lindholm, K.; Lu, B.; Li, R.; Shen, Y. Deficiency of GDNF Receptor GFRalpha1 in Alzheimer’s Neurons Results in Neuronal Death. J. Neurosci. 2014, 34, 13127–13138. [Google Scholar] [CrossRef]
- Revilla, S.; Ursulet, S.; Alvarez-Lopez, M.J.; Castro-Freire, M.; Perpina, U.; Garcia-Mesa, Y.; Bortolozzi, A.; Gimenez-Llort, L.; Kaliman, P.; Cristofol, R.; et al. Lenti-GDNF gene therapy protects against Alzheimer’s disease-like neuropathology in 3xTg-AD mice and MC65 cells. CNS Neurosci. Ther. 2014, 20, 961–972. [Google Scholar] [CrossRef]
- Marco, S.; Canudas, A.M.; Canals, J.M.; Gavalda, N.; Perez-Navarro, E.; Alberch, J. Excitatory amino acids differentially regulate the expression of GDNF, neurturin, and their receptors in the adult rat striatum. Exp. Neurol. 2002, 174, 243–252. [Google Scholar] [CrossRef]
- Liberto, C.M.; Albrecht, P.J.; Herx, L.M.; Yong, V.W.; Levison, S.W. Pro-regenerative properties of cytokine-activated astrocytes. J. Neurochem. 2004, 89, 1092–1100. [Google Scholar] [CrossRef]
- Sleeman, M.W.; Anderson, K.D.; Lambert, P.D.; Yancopoulos, G.D.; Wiegand, S.J. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv. 2000, 74, 265–272. [Google Scholar] [CrossRef]
- Blanchard, J.; Chohan, M.O.; Li, B.; Liu, F.; Iqbal, K.; Grundke-Iqbal, I. Beneficial effect of a CNTF tetrapeptide on adult hippocampal neurogenesis, neuronal plasticity, and spatial memory in mice. J. Alzheimers Dis. 2010, 21, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Duff, E.; Baile, C.A. Ciliary neurotrophic factor: A role in obesity? Nutr. Rev. 2003, 61, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Sieving, P.A.; Caruso, R.C.; Tao, W.; Coleman, H.R.; Thompson, D.J.; Fullmer, K.R.; Bush, R.A. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 3896–3901. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.; Youssef, I.; Utvik, J.K.; Florent-Bechard, S.; Barthelemy, V.; Malaplate-Armand, C.; Kriem, B.; Stenger, C.; Koziel, V.; Olivier, J.L.; et al. Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer’s disease. J. Neurosci. 2010, 30, 7516–7527. [Google Scholar] [CrossRef]
- Longo, F.M.; Yang, T.; Knowles, J.K.; Xie, Y.; Moore, L.A.; Massa, S.M. Small molecule neurotrophin receptor ligands: Novel strategies for targeting Alzheimer’s disease mechanisms. Curr. Alzheimer. Res. 2007, 4, 503–506. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Curran, G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- 110 Dittrich, F.; Thoenen, H.; Sendtner, M. Ciliary neurotrophic factor: Pharmacokinetics and acute-phase response in rat. Ann. Neurol 1994, 35, 151–163. [Google Scholar] [CrossRef]
- Petralia, R.S.; Mattson, M.P.; Yao, P.J. Communication breakdown: The impact of ageing on synapse structure. Ageing Res. Rev. 2014, 14, 31–42. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Pigino, G.F.; Brady, S.T.; Lazarov, O.; Binder, L.I.; Morfini, G.A. Axonal degeneration in Alzheimer’s disease: When signaling abnormalities meet the axonal transport system. Exp. Neurol. 2013, 246, 44–53. [Google Scholar] [CrossRef]
- Salvadores, N.; Sanhueza, M.; Manque, P.; Court, F.A. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front. Neurosci. 2017, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Werner, H.; Puschel, A.W. Rheb and mTOR regulate neuronal polarity through Rap1B. J. Biol. Chem. 2008, 283, 33784–33792. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, S.F.; Alvarez, V.A.; Ridenour, D.A.; Kwiatkowski, D.J.; Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005, 8, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Di Nardo, A.; Kramvis, I.; Meikle, L.; Kwiatkowski, D.J.; Sahin, M.; He, X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008, 22, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, C.A.; Lin, T.V.; Feliciano, D.M.; Zhang, L.; Hsieh, L.S.; Bordey, A. Rheb activation in subventricular zone progenitors leads to heterotopia, ectopic neuronal differentiation, and rapamycin-sensitive olfactory micronodules and dendrite hypertrophy of newborn neurons. J. Neurosci. 2013, 33, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Di Nardo, A.; Han, J.M.; Baharanyi, H.; Kramvis, I.; Huynh, T.; Dabora, S.; Codeluppi, S.; Pandolfi, P.P.; Pasquale, E.B.; et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat. Neurosci. 2010, 13, 163–172. [Google Scholar] [CrossRef]
- Shahani, N.; Huang, W.C.; Varnum, M.; Page, D.T.; Subramaniam, S. Forebrain depletion of Rheb GTPase elicits spatial memory deficits in mice. Neurobiol. Aging 2017, 50, 134–143. [Google Scholar] [CrossRef][Green Version]
- Tang, Z.; Ioja, E.; Bereczki, E.; Hultenby, K.; Li, C.; Guan, Z.; Winblad, B.; Pei, J.J. mTor mediates tau localization and secretion: Implication for Alzheimer’s disease. Biochim. Biophys. Acta. 2015, 1853, 1646–1657. [Google Scholar] [CrossRef]
- Tang, Z.; Bereczki, E.; Zhang, H.; Wang, S.; Li, C.; Ji, X.; Branca, R.M.; Lehtio, J.; Guan, Z.; Filipcik, P.; et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: Implication for Alzheimer disease. J. Biol. Chem. 2013, 288, 15556–15570. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, G.; He, W.; Xiao, M.; Yan, L.J. Activation of mTOR: A culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015, 11, 1015–1030. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Wu, D.K.; Liu, J.K. mTOR and tau phosphorylated proteins in the hippocampal tissue of rats with type 2 diabetes and Alzheimer’s disease. Mol. Med. Rep. 2013, 7, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Garelick, M.G.; Kennedy, B.K. TOR on the brain. Exp. Gerontol. 2011, 46, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.O.; Sahin, M. The neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, M.G.; Moore, D.L.; Smith, R.P.; Goldberg, J.L.; Bixby, J.L.; Lemmon, V.P. High content screening of cortical neurons identifies novel regulators of axon growth. Mol. Cell Neurosci. 2010, 44, 43–54. [Google Scholar] [CrossRef]
- Yan, L.; Findlay, G.M.; Jones, R.; Procter, J.; Cao, Y.; Lamb, R.F. Hyperactivation of mammalian target of rapamycin (mTOR) signaling by a gain-of-function mutant of the Rheb GTPase. J. Biol. Chem. 2006, 281, 19793–19797. [Google Scholar] [CrossRef]
- Ghosh, A.P.; Marshall, C.B.; Coric, T.; Shim, E.H.; Kirkman, R.; Ballestas, M.E.; Ikura, M.; Bjornsti, M.A.; Sudarshan, S. Point mutations of the mTOR-RHEB pathway in renal cell carcinoma. Oncotarget 2015, 6, 17895–17910. [Google Scholar] [CrossRef]
- Mazhab-Jafari, M.T.; Marshall, C.B.; Ho, J.; Ishiyama, N.; Stambolic, V.; Ikura, M. Structure-guided mutation of the conserved G3-box glycine in Rheb generates a constitutively activated regulator of mammalian target of rapamycin (mTOR). J. Biol Chem 2014, 289, 12195–12201. [Google Scholar] [CrossRef]
- Jiang, H.; Vogt, P.K. Constitutively active Rheb induces oncogenic transformation. Oncogene 2008, 27, 5729–5740. [Google Scholar] [CrossRef]
- Li, Y.; Inoki, K.; Guan, K.L. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol. Cell Biol. 2004, 24, 7965–7975. [Google Scholar] [CrossRef]
- Urano, J.; Comiso, M.J.; Guo, L.; Aspuria, P.J.; Deniskin, R.; Tabancay, A.P., Jr.; Kato-Stankiewicz, J.; Tamanoi, F. Identification of novel single amino acid changes that result in hyperactivation of the unique GTPase, Rheb, in fission yeast. Mol. Microbiol. 2005, 58, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.T.; Kim, S.R. Roles of Rheb(S16H) in substantia nigra pars compacta dopaminergic neurons in vivo. Biomed. Rep. 2015, 3, 137–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeon, M.-T.; Kim, S.R. Induction of GDNF and GFRα-1 following to AAV1-Rheb(S16H) administration in the hippocampus in vivo. IBRO Reports 2019, 6, S347. [Google Scholar] [CrossRef]
- Mulligan, S.J.; MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Kim, J.H.; Lee, S.; Kim, J.H.; Seo, J.W.; Jin, M.; Lee, M.G.; Jang, I.S.; Lee, W.H.; Suk, K. Phenotypic polarization of activated astrocytes: The critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. J. Immunol. 2013, 191, 5204–5219. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.H.; Seo, M.; Kim, J.H.; Jin, M.; Jeon, S.; Seo, J.W.; Lee, W.H.; Bing, S.J.; Jee, Y.; et al. Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: The pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues. J. Biol. Chem. 2014, 289, 16773–16789. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Dietrich, H.K.; Axtell, R.C.; Williams, A.M.; Egusquiza, R.; Wai, K.M.; Koshy, A.A.; Buckwalter, M.S. Astrocytic TGF-beta signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J. Immunol. 2014, 193, 139–149. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Fathali, N.; Doyle, K.P.; Williams, A.M.; Han, J.; Buckwalter, M.S. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 2014, 62, 1227–1240. [Google Scholar] [CrossRef]
- Harada, C.; Guo, X.; Namekata, K.; Kimura, A.; Nakamura, K.; Tanaka, K.; Parada, L.F.; Harada, T. Glia- and neuron-specific functions of TrkB signalling during retinal degeneration and regeneration. Nat. Commun. 2011, 2, 189. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Kohsaka, S.; Wada, E.; Yoshida, K.; Ohno, S.; Mamada, H.; Tanaka, K.; Parada, L.F.; Wada, K. Microglia-Muller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J. Neurosci. 2002, 22, 9228–9236. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Park, E.S.; Won, S.Y.; Lee, Y.A.; Kim, K.I.; Jeong, J.Y.; Baek, J.Y.; Cho, E.J.; Jin, M.; Chung, Y.C.; et al. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 2015, 138, 3610–3622. [Google Scholar] [CrossRef] [PubMed]
- Nawashiro, H.; Brenner, M.; Fukui, S.; Shima, K.; Hallenbeck, J.M. High susceptibility to cerebral ischemia in GFAP-null mice. J. Cereb. Blood Flow Metab. 2000, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).