1. Introduction
Cellulite is the most common modification of skin appearance in women, causing them to seek aesthetic treatments [
1]. The scientific literature about cellulite consists of a large number of papers, in which different hypotheses about the origin of all skin modifications were formulated [
2,
3,
4]. Despite the numerous efforts of researchers and aesthetic surgeons, the initial phenomenon that leads to the cascade of events causing the orange peel characteristic and the modification of the subcutaneous compartment, is still matter of debate in the scientific community [
5]. There are three principal theories about cellulite’s etiology. The first theory, formulated by Nürnberger and Muller, is based on the different architectural structure of subcutaneous tissues in male and female [
6,
7]. Using TC, they described the female subcutaneous and dermal compartment as constituted by collagen fibers organized in rectangular lobules surrounded by branches of collagen disposed perpendicularly to the skin surface, while male subcutaneous and dermal compartments are characterized by the deposition of collagen fibers randomly disposed in the tissue, in order to form small polygonal lobules [
7]. Nürnberger and Muller confirmed, in females, the thickening of collagen fibers that surround the lobules, affecting its protrusion, which is composed by mature adipocytes compressed inside each lobule. This mechanism is influenced by estrogen causing the protrusion of these
papillae adiposae visible only in women [
2,
7].
The second theory, formulated by Merlen and Curri, is based on the hypothesis of vascular changes. The authors described a different pattern of lymphatic drainage and blood circulation in cellulite-affected tissue that leads to the development of fibrosis [
8,
9]. The third theory, formulated by Gruber and Huber and Draelos attributes the development of cellulite to the chronic inflammation subsequent to the estrogens’ action and to the deposition of glycosaminoglycans (GAGs) by dermal fibroblasts [
10,
11].
Our paper aims to improve the knowledge about cellulite insurgence and development of studying the morphology of adipose tissue affected by cellulite; the novel aspects could influence the surgical procedures finalized to the reduction of the affected area. We studied cellulite affected tissues with a multimodal approach: magnetic resonance imaging (MRI), ultrastructural analysis (Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)) and proteomics both of cellulite tissue and MUSE (multi-lineage differentiating stress enduring) cells, a subpopulation of mesenchymal stem cells that are stress-tolerant and pluripotent, with special regenerative capability [
12,
13,
14]. We identified these cells in cellulite-affected tissue and these findings pave the way for further studies aimed to investigate how these stem cell subpopulations play a role in the cellulite etiology. These cells are characterized by a high regenerative capability and could have a role in the dermis adipose tissue modification during the early phases of cellulite development. In fact, we observed MUSE cells closed to mature unilocular adipocytes and to sweat glands. Their quantity in cellulite affected tissue suggest a pivotal role of MUSE cells in this pathology. To our knowledge, this is the first study of cellulite proteome and it allowed to characterize the first step of the cascade of events implicated in cellulite development. In the present study, the authors investigated samples of tissue affected by cellulite excised from cadaver and biopsies of women subjected to surgical treatments to remove orange peel characteristics on the skin. The samples of tissue excised from cadavers were examined by magnetic resonance imaging to verify the structure of subcutaneous and dermal compartment, while biopsies collected from patients were employed for the isolation of mesenchymal stem cells and for proteome analysis.
3. Discussion
Many surgeons and numerous papers defined cellulite as an aesthetic problem that requires cosmetic treatments in order to obtain a smooth skin [
2,
4,
6]. Most surgeons and researchers affirmed that cellulite represents the most frequent event in post pubertal women. In fact, all the studies concerning cellulite revealed that about 85–90% of women are affected by an “orange peel” skin characteristic [
15,
16]. During the last 10 years, published papers focused on the different possible modifications of the subcutaneous adipose tissue that could represent the initial events of cellulite [
17,
18]. They described disorders in blood capillaries organization, different distribution of collagen fibers in the subcutaneous compartment, impairment of the lymphatic drainage, and, because cellulite is typical only of women, they attributed all these phenomena to estrogen activity [
3]. The present study, based on a multimodal approach, aimed to describe cellulite starting from the skin appearance and delving into the description of deeper compartments, in order to identify the possible causes leading to the deep modification observed in the dermis.
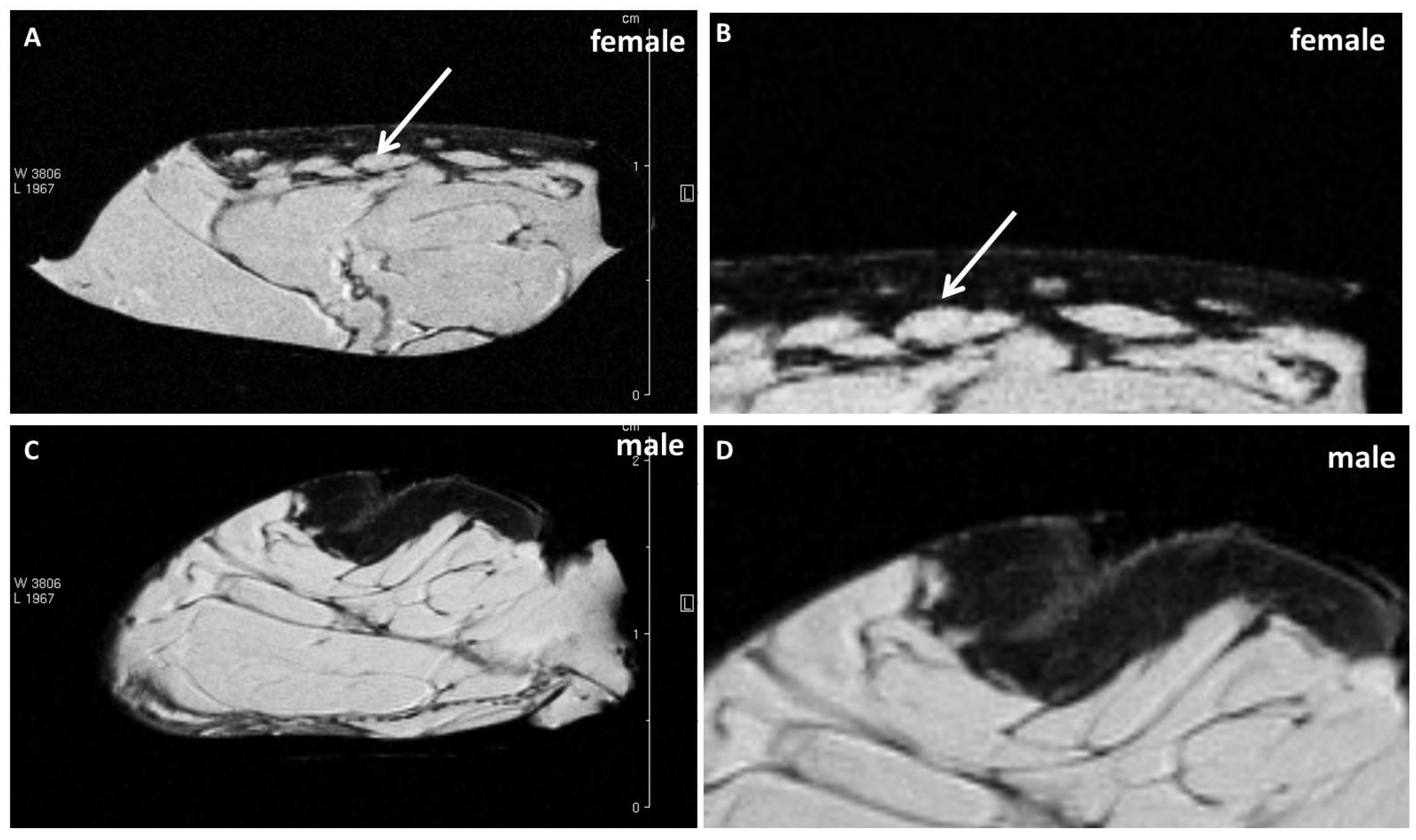
Examining the cellulite specimens, collected from different women, it was possible to observe the dimpling on the skin and the organization of subcutaneous and dermal compartments. First of all, using magnetic resonance imaging, it was possible to reveal the presence of small adipose papillae, located in the dermis, that are not present in men. MRI acquisitions allow to determine the area occupied by collagen in each patient, tracking a region of interest on 10 different MRI slices for each patient. These values collected from men and women and examined by MRI confirmed the higher deposition of collagen fiber in women subjected to cellulite, if compared with men. It is not possible to identify the type of collagen incremented in the specimens with cellulite. A specific staining is necessary and will need to be the focus of another paper. Men were characterized by different distribution of collagen fibers that occupied a smaller area of the dermis that the area determined in cellulite samples excised from women. This finding confirms the role of fibroblasts involved in the deposition of a higher amount of collagen in adipose tissue affected by cellulite than in men.
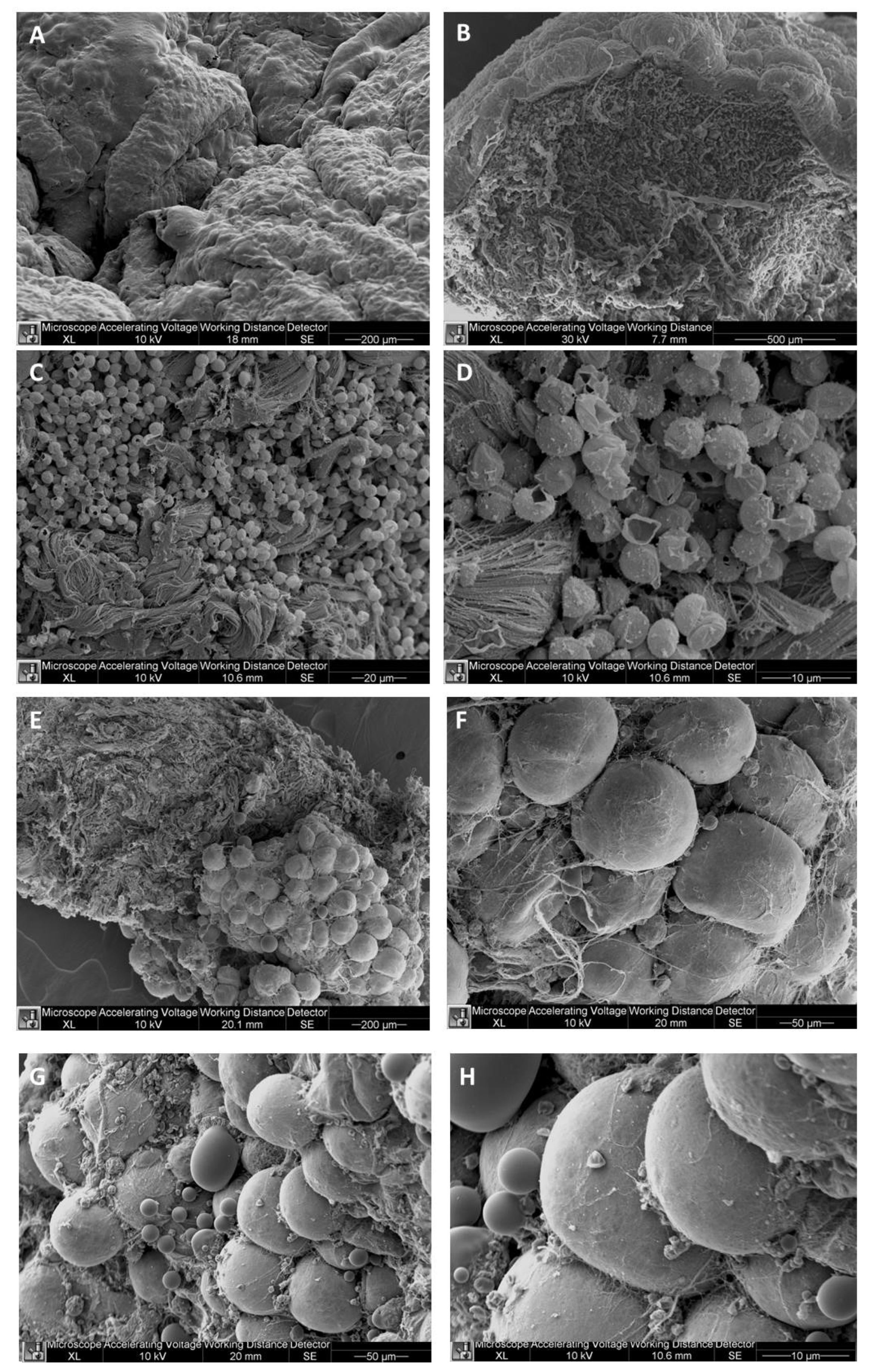
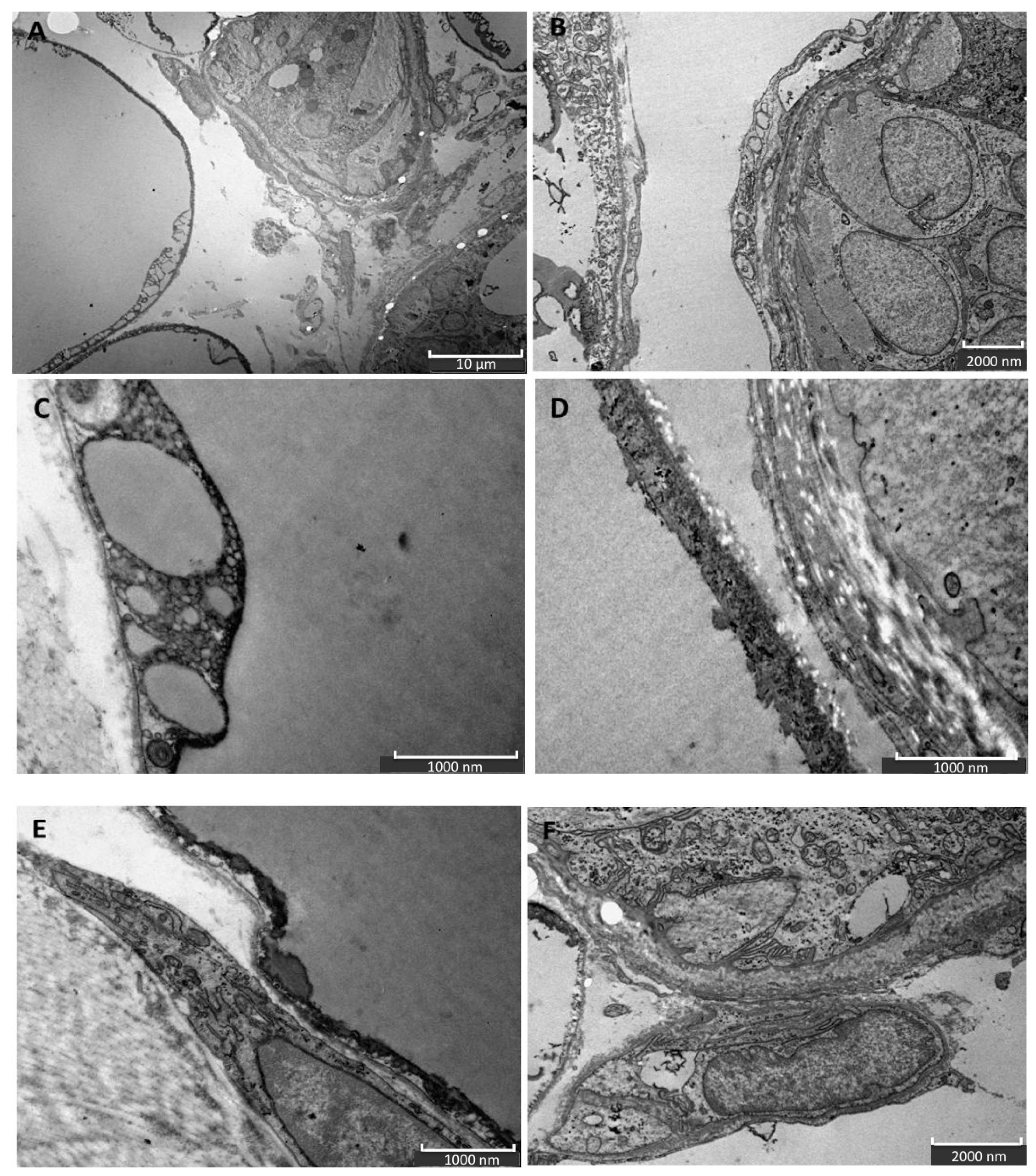
The ultrastructural analysis allowed to investigate the cellulite samples at an ultrastructural level, showing the organization of dermal lobules, composed by mature unilocular adipocytes close to the sweat glands. This represent the first findings about cellulite-affected tissue. A similar morphology was described in breast during pubertal phase, in which the sebaceous glands start to produce adipocytes, under estrogenic stimulation. In cellulite, the sweat gland has the same role.
MRI and ultrastructural results could influence the microsurgical methods proposed until now to treat the dimpling [
19,
20], or therapies based on radiofrequency [
21] and on laser employment [
22]. Most of these techniques, in fact, are based on the collagen fiber remodeling or cutting, but we are not sure that the remodeling of collagen septa is the most efficient method to treat cellulite. These techniques have been developed based on the previously theories [
6,
7] about dermis differences between male and female, which are refuted by the data showed by our results (
Figure 1).
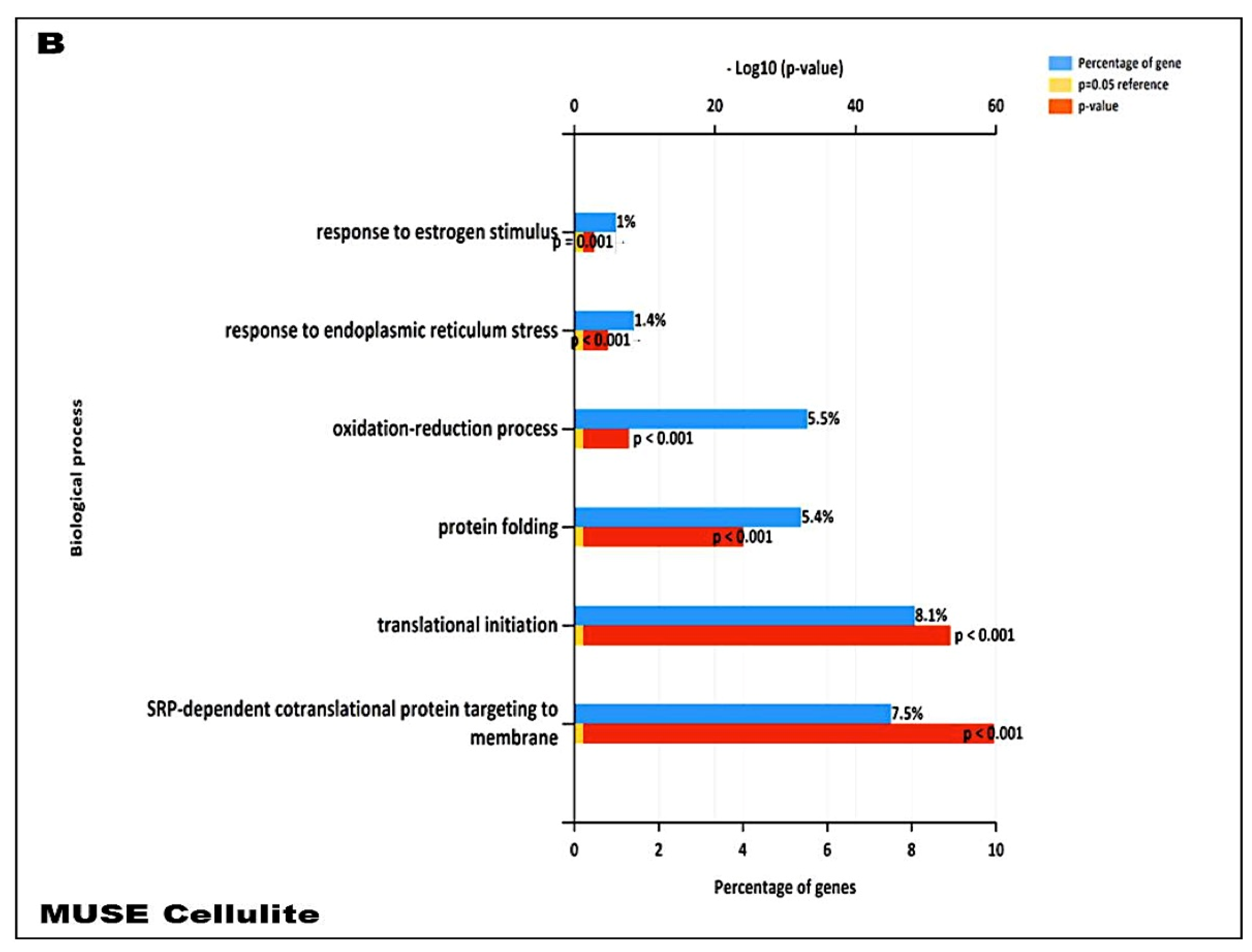
In addition, our data suggest that during cellulite development the main role is played by mesenchymal stem cells, which are rich in estrogen receptor and are recruited after stress stimuli, represented by oxidative stress, matrix remodeling, reactive oxygen species production, as demonstrated by proteomic analysis (
Figure 7). Cellulite appeared characterized by a high amount of mesenchymal stem cells, a feature related to the stimulation of mesenchymal stem cells that could be represented by the observation of numerous vesicles in the extracellular matrix of the dermis that were detected by transmission electron microscopy, in the interspace between adipocytes and sweat glands. Obviously, the electron microscopy alone is not sufficient to study the significance of these vesicles, but the proteomic analysis, using the functional annotation analysis, confirmed the presence of proteins involved in exosome secretion. Exosomes are lipid vesicles secreted by adipocytes, or by adipose mesenchymal stem cells, with the aim to release molecular mediators, in particular in stress conditions [
23], and are considered a critical messenger for cell-cell communication. The relevant presence of exosomes supports the existence of a crosstalk between adipocytes and sweat glands, which leads the cellulite progression. This complex could be defined as dermic papilla and it could be considered a peculiar microenvironment in which all the molecular pathways of cellulite were activated. Moreover, the proteomic analysis of cellulite showed the presence of numerous families of proteins related to oxidative stress and extracellularmatrix remodeling and deposition (
Figure 7). The alteration of reactive oxygen species level and a massive extracellular matrix deposition is a frequent feature in many chronic inflammatory processes, supporting the theories of Gruber and Huber [
10,
11]. Additionally, a correlation between oxidative stress and fibrotic processes are known in skin [
24], and ROS can play a crucial role in fibrotic degeneration, including growing stimulation of fibroblasts.
The proteomic analysis of cellulite and of MUSE cells, isolated from cellulite specimens, showed the presence of estrogens receptors on the multilineage differentiating stress enduring cells membranes, demonstrating the pivotal role of these cells in the cellulite development. According to the previous theories about cellulite progression, our results recognize the importance of estrogenic stimulation [
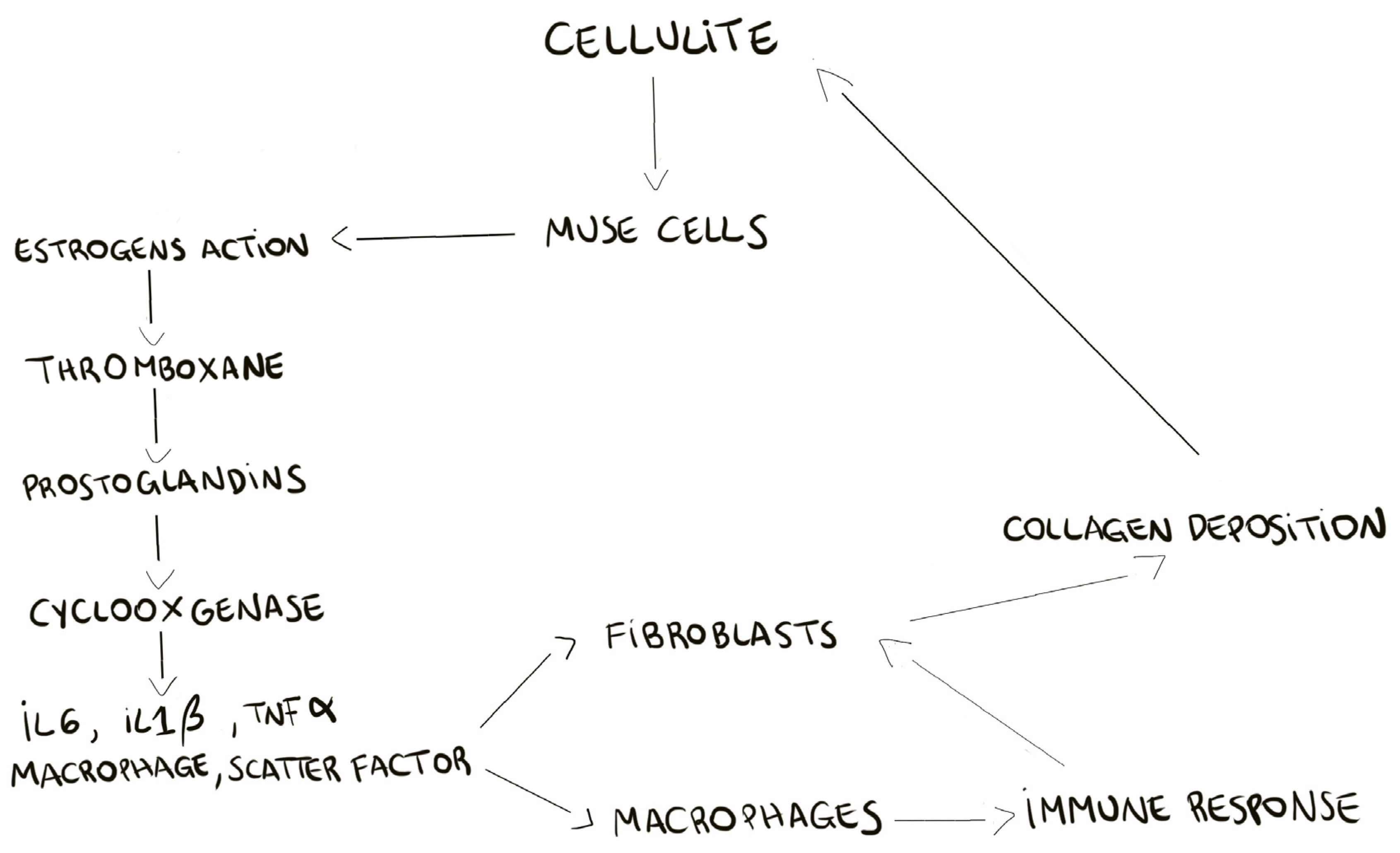
2]. Our hypothesis on MUSE role, is that these cells, after estrogenic stimuli, could activate all the bio chemical cascades of prostaglandins production, cyclooxygenases expression, and of stimulation of matrix metalloproteinases and elastases. A schematic summary is reported in
Figure 8, in which we try to clarify the development of cellulite. The recruitment of MUSE, bringing the estrogens receptor, induces the deep remodeling of the dermis and the activation of pro-inflammatory pathways.
Proteomic analysis confirmed numerous aspects that were described in the previous literature about cellulite, such as the presence of proteins involved in tissue remodeling, fibroblast activation, extracellular matrix, and collagen fibers organization. Additional innovative information, provided by the analysis of proteome, is the presence of families of proteins involved in immune system activation in adipose tissue affected by cellulite, which was not observed in the proteome of MUSE cells. Surely, this aspect must be studied with higher attention, in order to comprehend how the immune system could influence cellulite development and progression.
All the collected data provided important findings about cellulite affected tissue, which had never been studied with a multidisciplinary approach, allowed the analysis of phenotypical and molecular aspects. In this study, cellulite was described starting from the skin appearance to the molecular level with the description of pathways involved in this pathological progression.
In previous published studies, in which the cellulite origin were hypothesized, only a single aspect was evaluated: either the vascular aspect, or the organization of collagen fibers, or the formation of adipose tissue papillae. In this paper, the knowledge on cellulite origin was expanded upon, and for the first time, all the aspect of this pathology were analyzed together.
Limitations of the Study
This Study has Some Limitations to Point Out
The dehydration protocol was necessary to obtain slices for morphological and ultrastructural analysis. The dehydration of the specimen may disturb the variation of cellulite in the architecture.
We did not include any images of cellulite-free area in this work. The comparison between cellulite areas and cellulite-free areas was not the goal of this work.
The sample population was small. Larger series are needed to validate these new findings.
4. Materials and Methods
4.1. Adipose Tissue Harvesting
Cellulite specimens were collected from 10 women (23–45 years old, with a BMI ranging between 24 and 27) that were subjected to surgical treatment, based on the mini invasive protocol previously published by Amore et al. [
19]. During the mini invasive surgery, it was possible to harvest punches of 0.5 mm in length and of 0.2 mm of diameter in the site of insertion of the bistoury. Punches were immersed in a 0.9% glucose sterile solution with 0.1% of human insulin and kept at 4 °C.
Others 10 biopsies of gluteo femoral area were surgically collected from cadavers (5 male, 5 female, 25–45 years old, with a BMI ranging between 24 and 27), provided by ICLO Teaching and Research Center, in Verona. Biopsies were employed for the study of cellulite affected adipose tissue phenotype using Magnetic Resonance Imaging, Transmission electron microscopy and Scanning electron microscopy.
4.2. Magnetic Resonance Imaging of Cellulite
The specimens of cellulite harvested from cadavers were observed using a spectrometer Bruker Biospin (Bruker Biospin MRI GmBH, Ettlingen, Germany) operating at 7 T, with a strength of 040 G/m and equipped with 3.5 cm i.d. transmitter/receiver birdcage coil. The samples were positioned inside the coil and the region of interest (ROI) was selected in order to collect the whole image of the sample with all the details of epidermidis. The acquisitions were performed using T1-weighted sequences at high resolution with the following parameters: TE = 25 ms, TR = 1168.2 ms, field of view = 3.0 × 3.0 cm, number of averages = 1, flip angle 180 degrees, slice thickness 1.0 mm and matrix size 256 × 256 pixels.
On the slices of MRI, an area corresponding to the space occupied by collagen was manually tracked. The software of the spectrometer provided the millimeters squared, representing collagen fraction of the field of view. Ten field of views were analyzed, in order to determine the mean of the surface occupied by collagen fibers. The analysis of MRI images has been done by two independent expert radiologists, blinded for the gender of the samples.
4.3. Ultrastructural Analysis of Cellulite Affected Adipose Tissue
4.3.1. Transmission Electron Microscopy
The samples of cellulite harvested during surgical treatment of cellulite, in living people, were fixed with glutaraldehyde 2% and were post-fixed in 1% osmium tetraoxide (OsO4) aqueous solution for 2 h, dehydrated in graded concentrations of acetone and immersed in an Epon–Araldite mixture (Electron Microscopy Sciences, Fort Washington, PA, USA). The semi-thin sections (1 mm thick) were examined by light microscopy and stained with toluidine blue. The ultra-thin sections of 70 nm were obtained using a microtome and placed on Cu/Rh grids with Ultracut E (Reichert, Wien, Austria), stained with lead citrate, and observed using an FEI Morgagni 268 D electron microscope (FEI Company, Eindhoven, Netherlands).
4.3.2. Scanning Electron Microscopy
The specimens of cellulite (10 living female) were fixed with glutaraldehyde 2 % in 0.1 M PB, post-fixed in 1 % osmium tetraoxide (OsO4) in the same buffer for 1 h, dehydrated in concentrations of acetone, critical point dried (CPD 030, Balzers, Vaduz, Liechtenstein), fixed to stubs with colloidal silver, sputtered with gold by an MED 010 coater (Balzers, Brugherio, Italy), and examined with an FEI XL30 scanning electron microscope (FEI Company, Eindhoven, Netherlands).
The analysis of SEM/TEM images has been done by two independent experts morphologist, blinded for the gender of the samples.
4.4. Purification of Stromal Vascular Fraction from Cellulite
The purification of stromal vascular fraction was performed by digesting fat samples collected from cellulite specimens of 10 living female with collagenase type I 0.2 % in a buffered solution containing 2% of fetal bovine albumin, at 37 °C for 45 min. After digestion samples were transferred in plastic tubes and centrifuged at 400× g (3000 rpm) for 7 min. The obtained pellets were suspended in 1 mL of NH4CL 160 mM in order to lyse the erythrocytes and then centrifuged at 400× g for 10 min. The pellets were suspended in 1 mL of growth medium Dulbecco modified eagle medium (DMEM) added with 10 % of fetal bovine serum (FBS) and 1% of a mixture of penicillin and streptomycin. The number of cells, of each sample, was determined using a Burker chamber, and then cell suspensions were transferred in a flask of 25 cm2 with the addition of 5 mL of growth medium. Flasks were incubated at 37 °C and 5% of CO2 for 72 h.
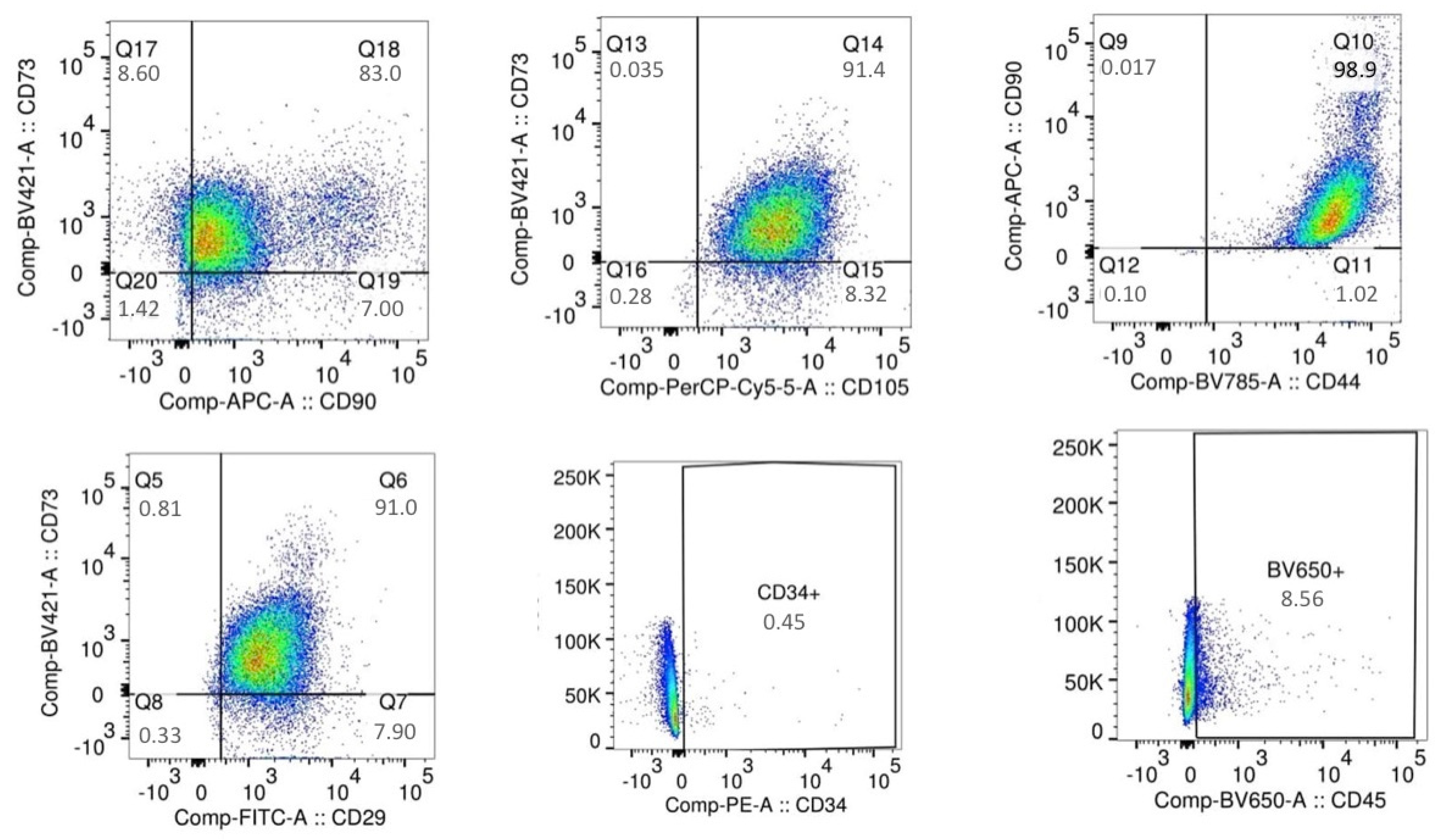
4.5. Isolation of Mesenchymal Stem Cells and of Multilineage Differentiating Stress Enduring Cells (MUSE) from Cellulite
At the end of the 72 h of initial incubation, with the same medium and in the same conditions described before, cells of each sample were detached using trypsin-EDTA 0.25%, introduced into a plastic tube, and then centrifuged at 400×
g for 5 min and plated in 75 cm
2 flasks at 37 °C and 5% of CO
2, to allow the cells growth. When the cells reached the number of 3 × 10
6, they were collected and prepared for immune phenotyping and cell sorting, following the procedures described by Conti et al. [
25], in order to identify and isolate the mesenchymal stem cells.
For the immune sorting of the mesenchymal stem cells, the following antibodies were employed: APC-conjugated CD90 (dilution 1:5), PerCP-Cyt5.5-conjugated CD105 (dilution 1:20), BV421-conjugated CD73 (dilution 1:20), BV785-conjugated CD44 (dilution 1:20), PE-conjugated CD34 (dilution1:5), FITC-conjugated CD29 (dilution 1:20), and BV650-conjugated CD45 (dilution 1:20). All these antibodies were purchased form BD Bioscences, Becton Dickinson Italy S.p.A., Milan.
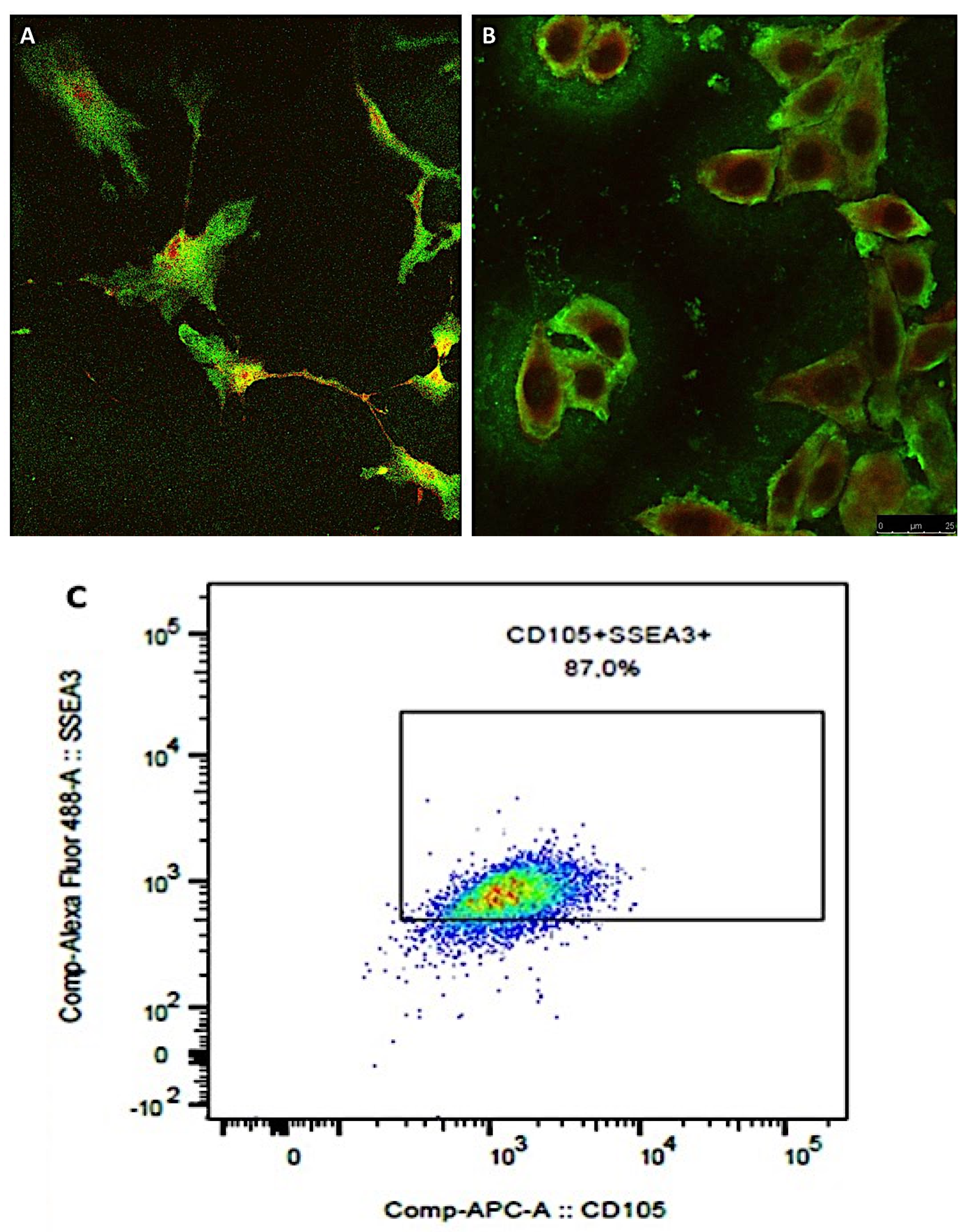
At the end of the first phase of isolation, the sorted cells were positive for CD90, CD73, CD105, CD44, and CD29 and negative for CD45 and CD34, being identified as mesenchymal stem cells that were plated on 75 cm2 flasks and expanded. When the cells reached the number of 2 × 106, they were detached with tripsin-EDTA 0.25% and collected for the immune sorting for the simultaneous expression of SEEA3 and CD105. The CD105 was used at the same dilution described before, while the Alexa Fluor-488-conjugated SEEA3 antibody was purchased from Aurogene S.R.L (Italy, Rome) and was used at the dilution of 1:200 in volume by distilled water. Immune sorting was performed using a FACS canto II (BD, Becton Dickinson Italy S.p.A Milan).
4.6. Confocal Microscopy of MUSE Cells
Confocal microscopy was used to confirm the results of immune sorting where 104 of MUSE cells, isolated from the population of mesenchymal stem cells, were seeded on a glass with a diameter of 24 mm and inserted in six well-plates, and the growth medium previously described was added. The plates were incubated at 37 °C and 5% of CO2 for 24 h. At the end of the incubation, the time cells were fixed with 4% buffered formalin for 1 h at 4 °C in dark, and then washed three times with sterile PBS. Successively, the cells were incubated with a PBS solution containing SEEA3 and CD105 antibodies in dark at 4 °C for 30 min. Antibodies were used at the same dilutions described before. At the end of the incubation with the antibodies, MUSE cells were washed with PBS and then added with 50 µL of mounting medium containing DAPI on each slice. The slices were imaged with a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) with a 40× objective. Images were prepared using Las X software.
4.7. Purification of Proteome from Abdomen and Cellulite Affected Adipose Tissue and from Isolated Cells
Adipose tissue sections (10 µm-thick) were cut from the cellulite specimens of 10 living female (20 slices) using a cryostat and placed at −80 °C until use. The samples were placed in 1 mL cold PBS1X added with 1X Complete Mini EDTA-free protease inhibitors (Roche Italia, Monza, Italy) and 1% sodium dodecyl sulphate (SDS) (Biorad, Milan, Italy). The tissue sections were then subjected to 5–6 cycles (each cycle for 30 sec) of sonication. The whole procedure was carried out in cooled conditions. The sample tubes were kept in ice for 30 min and then centrifuged at 14,000× g at 4 °C for 15 min. The cell lysate was extracted in the aqueous layer and separated. A fourfold volume of ice-cold acetone was added to the cell lysate, vortexed and incubated at −20 °C overnight. After centrifugation at 14,000× g at 4 °C for 10 min, the supernatant was discarded and the pellet was washed with 500 µL of ice-cold acetone-methanol (8:1). For the MUSE cells, the protein extraction was performed in 1 mL of PBS1X, added with protease inhibitors and 1% SDS, through five steps of sonication on ice, each lasting 15 sec. The proteins were subjected to ice-cold acetone precipitation overnight and then recovered after centrifugation at 14,000× g at 4 °C for 15 min. The protein pellets were dried and solubilized in 1 mL of 100 mM NH4HCO3. The protein concentrations were determined by mean of the Bradford reagent (Sigma-Aldrich, Milan, Italy), using bovine serum albumin as standard. Before shotgun proteomic analysis, the proteins were subjected to in-solution digestion as previously described.
4.8. Proteomic Analysis
Protein identification by LC-MS/MS analyses were performed on the 10 living female biopsies using a micro-LC Eksigent Technologies (Dublin, USA) system interfaced with a 5600 + TripleTOF system (AB Sciex, Concord, Canada). The stationary phase was a Halo C18 column (0.5 × 100 mm, 2.7 µm; Eksigent Technologies Dublin, USA). The mobile phase was a mixture of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B), eluting at a flow-rate of 15.0 µL min with an increasing concentration of solvent B from 2% to 40% in 30 min. Injection volume was 4.0 μL and oven temperature was set to 40 °C. For identification purposes the samples were subjected to data-dependent acquisition (DDA): mass spectrometer analysis was performed using a mass range of 100–1500 Da (TOF scan with an accumulation time of 0.25 s), followed by an MS/MS product ion scan from 200 to 1250 Da (accumulation time of 5.0 ms) with the abundance threshold set at 30 cps (35 candidate ions can be monitored during every cycle). The ion source parameters in electrospray positive mode were set as follows: curtain gas (N2) at 25 psig, nebulizer gas GAS1 at 25 psig, and GAS2 at 20 psig, ion spray voltage floating (ISVF) at 5000 V, source temperature at 450 °C and declustering potential at 25 V [
26]. The DDA files were searched using Protein Pilot software v. 4.2 (SCIEX, Concord, Canada) and Mascot v. 2.4 (Matrix Science Inc., Boston, USA). Trypsin as digestion enzyme was specified for both software. For Mascot, we used two missed cleavages, set the instrument to ESI-QUAD-TOF and specified the following modifications for the assay: carbamidomethyl cysteine as fixed modification and oxidized methionine as variable modification. An assay tolerance of 50 ppm was specified for peptide mass tolerance, and 0.1 Da for MS/MS tolerance. The peptide charges to be detected were set to 2+, 3+, and 4+, and the assay was set on monoisotopic mass. The UniProt Swiss-Prot reviewed database containing human proteins (version 2015.07.07, containing 42,131 sequence entries) was used and a target-decoy database search was performed. False Discovery Rate was fixed at 1%.
4.9. Bio-Informatics Analysis
The functional annotation analysis of the identified proteins was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID, v6.8,
http://david.abcc.ncifcrf.gov/) [
27] (
Table S1). The functional annotation chart report was used to identify Gene Ontology (GO) biological processes, molecular function, and cellular component. Gene enrichment analysis was performed by setting the threshold of an EASE Score, a modified Fisher Exact
p-value, to 0.1, and a
p-value correction smaller than 0.05 (
Table S2).
The identified proteins present in the adipose tissues and in the MUSE cells were analyzed using STRING software (
http://string-db.org) [
28]. STRING analysis was performed by setting Homo Sapiens as the species under investigation and a high confidence level, namely 0.7; we retrieved interactions based exclusively on experimental and database knowledge, while excluding all other prediction methods implemented in STRING (such as text mining and gene fusion). Reactome pathways were reported by setting FDR < 1% (
Table S3).
4.10. Human and Animal Rights or Ethical Approval
Written informed consent was obtained from each patient prior to the study. Our institutional ethics committee (Comitato di Approvazione per la Ricerca sull’Uomo—C.A.R.U., University of Verona, Italy) approved the study design (n. 2019-UNVRCLE-0175710).