Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms
Abstract
:1. Introduction
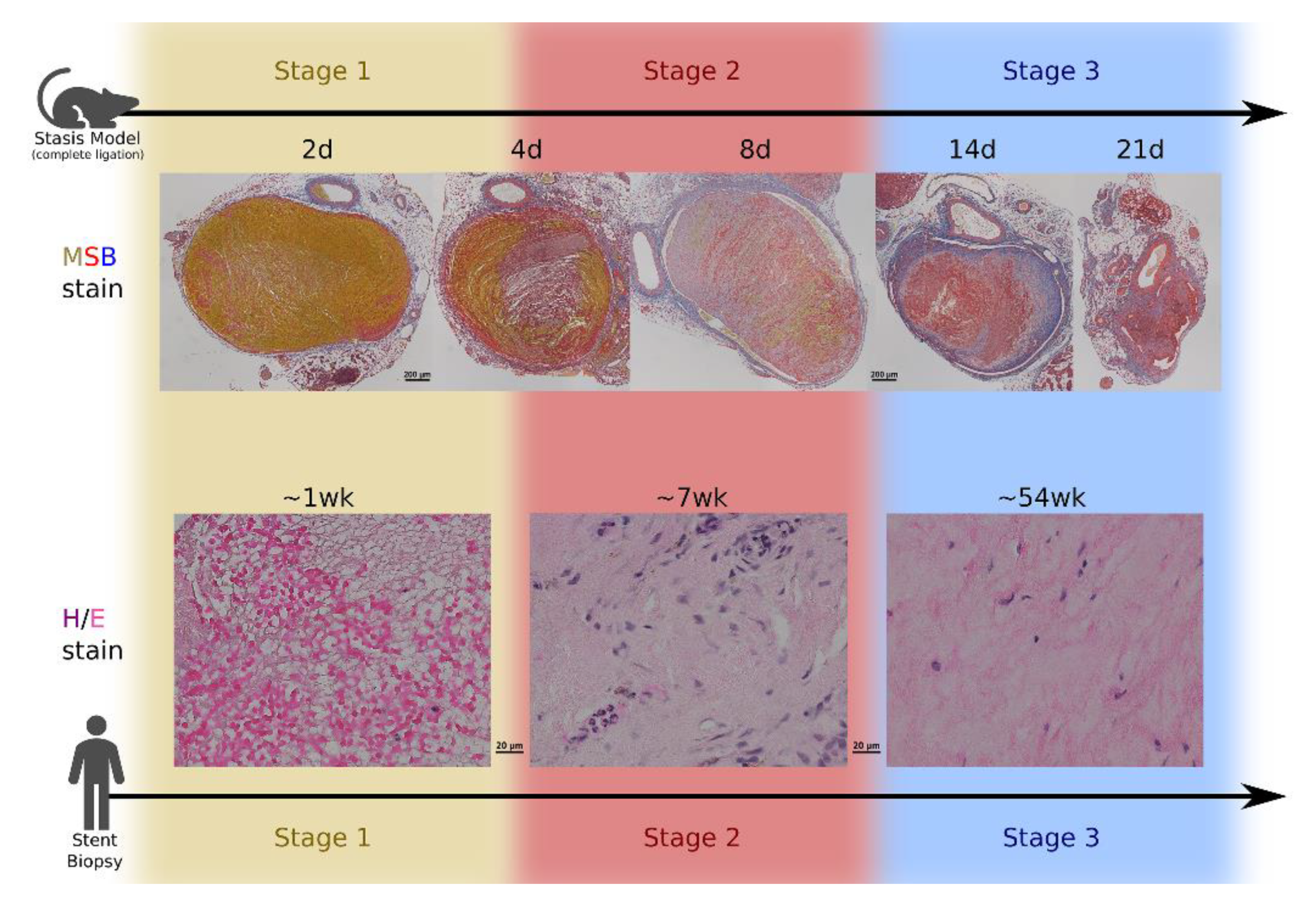
2. Overview of Venous Thrombus Development in Humans
3. Dependence on Murine Models
4. Immune Cells: Attack, then Repair
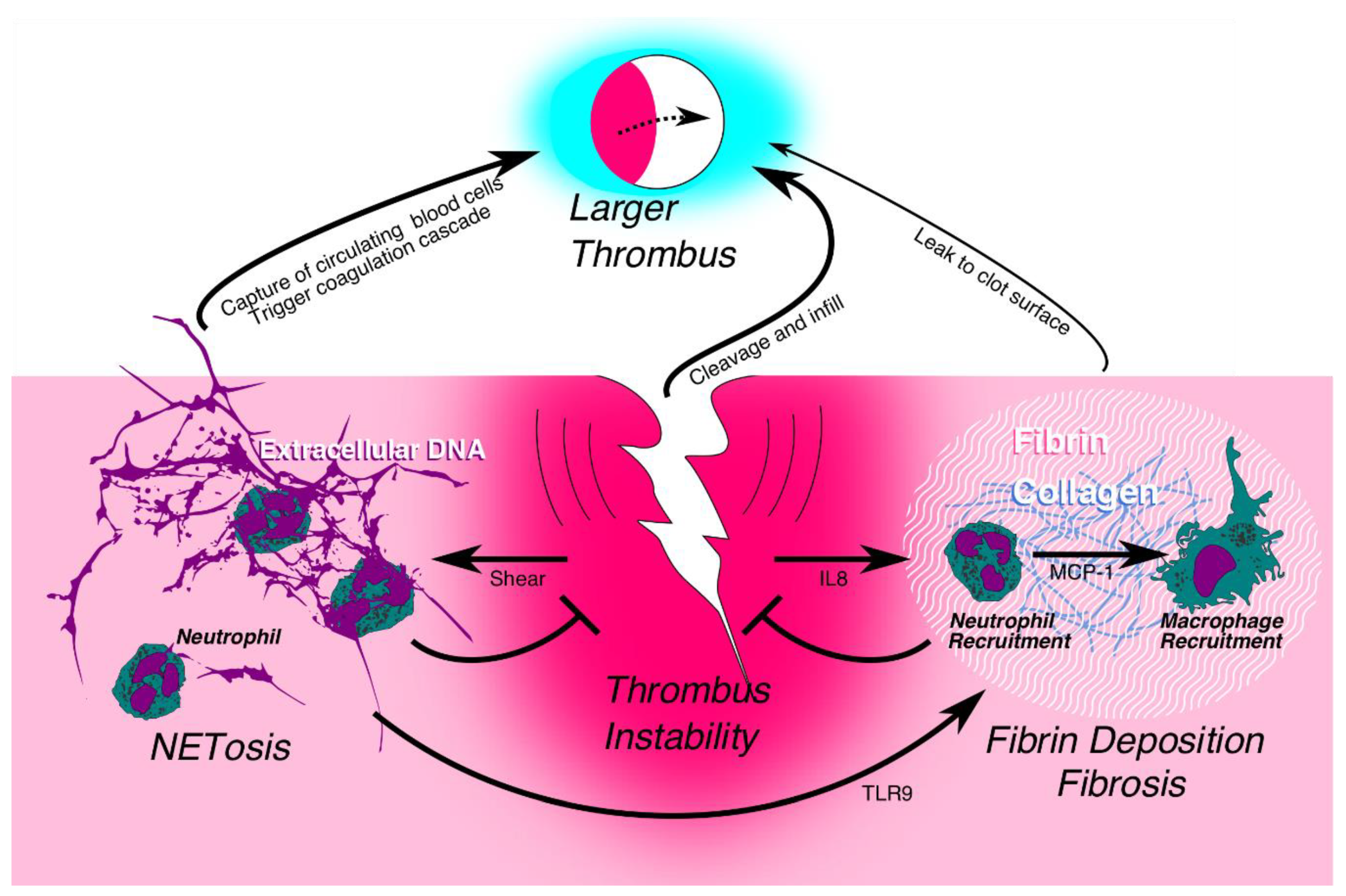
5. Neutrophils and the Potential Regulation of Early Structural Stability
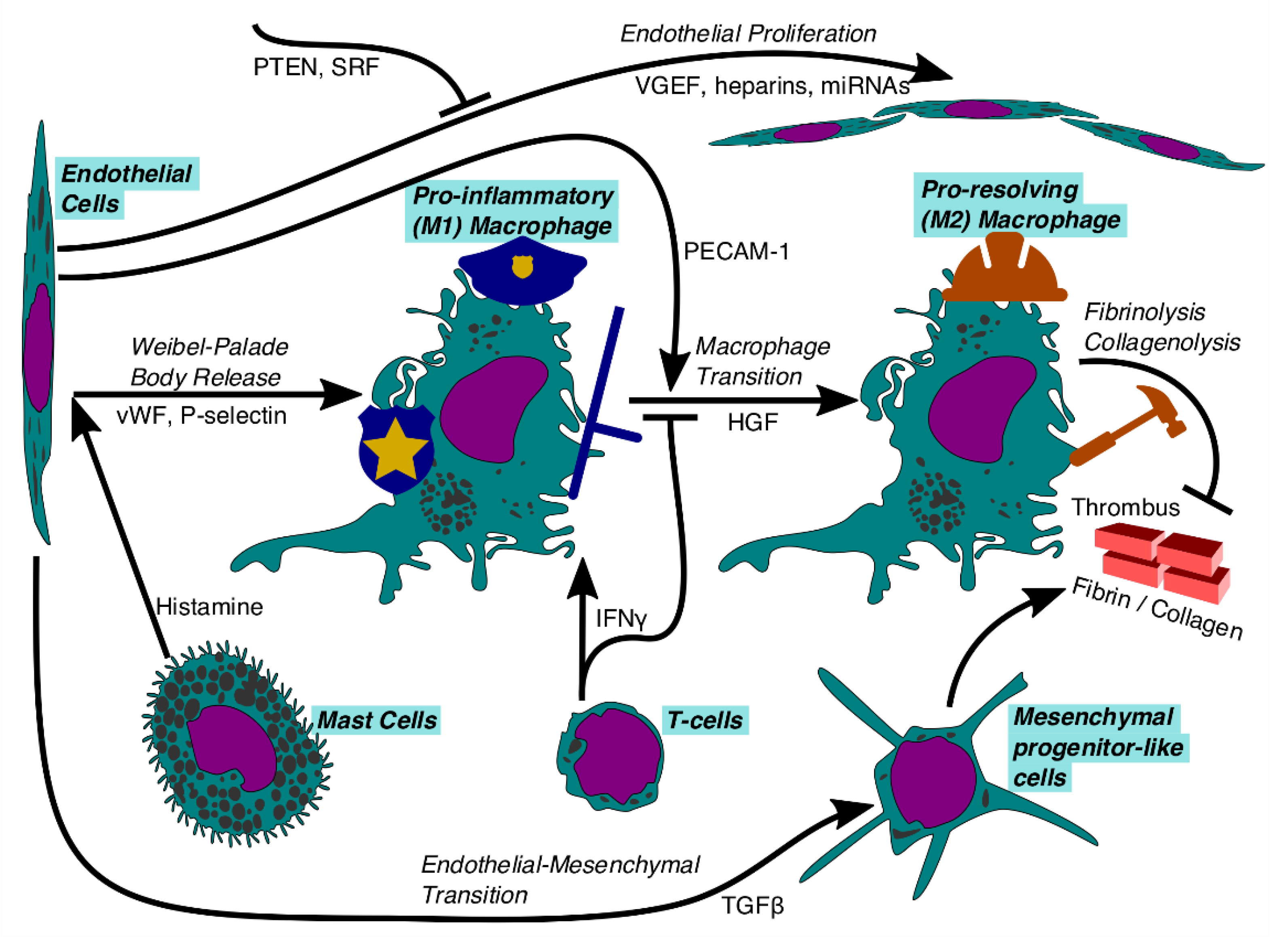
6. Monocyte-Derived Macrophages and Thrombus Resolution
7. Endothelial Cells
8. Fibrinolysis
9. Collagenolysis
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kochanek, K.D.; Murphy, S.L.; Xu, J.; Arias, E. National Vital Statistics Reports Volume 68, Number 9, 24 June 2019. Deaths: Final Data for 2017. Available online: https://www.cdc.gov/nchs/products/index.htm (accessed on 12 September 2019).
- Huang, W.; Goldberg, R.J.; Anderson, F.A.; Kiefe, C.I.; Spencer, F.A. Secular trends in occurrence of acute venous thromboembolism: The worcester VTE study (1985–2009). Am. J. Med. 2014, 127, 829–839. [Google Scholar] [CrossRef]
- Alotaibi, G.S.; Wu, C.; Senthilselvan, A.; McMurtry, M.S. Secular Trends in Incidence and Mortality of Acute Venous Thromboembolism: The AB-VTE Population-Based Study. Am. J. Med. 2016, 129, 879. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Isaksen, T.; Hansen, J.B.; Brækkan, S.K. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur. J. Epidemiol. 2017, 32, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delluc, A.; Tromeur, C.; le Ven, F.; Gouillou, M.; Paleiron, N.; Bressollette, L.; Nonent, M.; Salaun, P.-Y.; Lacut, K.; Leroyer, C.; et al. Current incidence of venous thromboembolism and comparison with 1998: A community-based study in Western France. Thromb. Haemost. 2016, 116, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Tagalakis, V.; Patenaude, V.; Kahn, S.R.; Suissa, S. Incidence of and mortality from venous thromboembolism in a real-world population: The Q-VTE study cohort. Am. J. Med. 2013, 126, 832. [Google Scholar] [CrossRef]
- Yusuf, H.R.; Tsai, J.; Atrash, H.K.; Boulet, S.; Grosse, S.D. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. Morb. Mortal. Wkly. Rep. 2012, 61, 401–404. [Google Scholar]
- Galanaud, J.P.; Monreal, M.; Kahn, S.R. Epidemiology of the post-thrombotic syndrome. Thromb. Res. 2018, 164, 100–109. [Google Scholar] [CrossRef]
- Wiener, R.S.; Schwartz, L.M.; Woloshin, S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch. Intern. Med. 2011, 171, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Arshad, N.; Bjøri, E.; Hindberg, K.; Isaksen, T.; Hansen, J.B.; Brækkan, S.K. Recurrence and mortality after first venous thromboembolism in a large population-based cohort. J. Thromb. Haemost. 2017, 15, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Søgaard, K.K.; Schmidt, M.; Pedersen, L.; Horváth-Puhó, E.; Sørensen, H.T. 30-year mortality after venous thromboembolism: A population-based cohort study. Circulation 2014, 130, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Streiff, M.B.; Agnelli, G.; Connors, J.M.; Crowther, M.; Eichinger, S.; Lopes, R.; McBane, R.D.; Moll, S.; Ansell, J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J. Thromb. Thrombolysis. 2016, 41, 32–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzolai, L.; Aboyans, V.; Ageno, W.; Agnelli, G.; Alatri, A.; Bauersachs, R.; Brekelmans, M.P.A.; Büller, H.R.; Elias, A.; Farge, D.; et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Heart J. 2018, 39, 4208–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendrum, A.C.; Fraser, D.S.; Slidders, W.; Henderson, R. Studies on the character and staining of fibrin. J. Clin. Path. 1962, 15, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irniger, W. Histologische Altersbestimmung von Thrombosen und Embolien. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1963, 336, 220–237. [Google Scholar] [CrossRef]
- Fineschi, V.; Turillazzi, E.; Neri, M.; Pomara, C.; Riezzo, I. Histological age determination of venous thrombosis: A neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci. Int. 2009, 186, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, G.; Costa, D.; Capasso, E.; Varavallo, F.; Brunitto, G.; Caserta, R.; Esposito, S.; Niola, M.; Sardu, C.; Marfella, R.; et al. The dating of thrombus organization in cases of pulmonary embolism: An autopsy study. BMC Cardiovasc. Disord. 2019, 19, 250. [Google Scholar] [CrossRef]
- McGuinness, C.L.; Humphries, J.; Waltham, M.; Burnand, K.G.; Collins, M.; Smith, A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb. Haemost. 2001, 85, 1018–1024. [Google Scholar] [CrossRef]
- Geier, B.; Barbera, L.; Muth-Werthmann, D.; Siebers, S.; Ermert, H.; Philippou, S.; Mumme, A. Ultrasound elastography for the age determination of venous thrombi. Evaluation in an animal model of venous thrombosis. Thromb. Haemost. 2005, 93, 368–374. [Google Scholar]
- Comerota, A.J.; Oostra, C.; Fayad, Z.; Gunning, W.; Henke, P.; Luke, C.; Lynn, A.; Lurie, F. A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb. Res. 2015, 135, 882–887. [Google Scholar] [CrossRef]
- Metz, A.K.; Luke, C.E.; Dowling, A.; Henke, P.K. Acute experimental venous thrombosis impairs venous relaxation but not contraction. J. Vasc. Surg. 2019. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Garry, J.; Gasparis, A.; Labropoulos, N. Vein wall remodeling in patients with acute deep vein thrombosis and chronic postthrombotic changes. J. Thromb. Haemost. 2017, 15, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Deatrick, K.B.; Elfline, M.; Baker, N.; Luke, C.E.; Blackburn, S.; Stabler, C.; Wakefield, T.W.; Henke, P.K. Postthrombotic vein wall remodeling: Preliminary observations. J. Vasc. Surg. 2011, 53, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.A.; Obi, A.T.; Myers, D.D.; Wrobleski, S.K.; Henke, P.K.; MacKman, N.; Wakefield, T.W. Critical review of mouse models of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.A.; Saha, P.; Cooley, B.; Palmer, O.R.; Grover, S.P.; Mackman, N.; Wakefield, T.W.; Henke, P.K.; Smith, A.; Lal, B.K. Choosing a mouse model of venous thrombosis: A consensus assessment of utility and application. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sevitt, S. The structure and growth of valve-pocket thrombi in femoral veins. J. Clin. Pathol. 1974, 27, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, G.; Chen, B.; Pu, Y.; Nie, P.; Li, X.; Li, Z.; Su, K. Numerical simulation of hemodynamics in portal vein with thrombosis by computational fluid dynamics. J. Mech. Med. Biol. 2014, 14. [Google Scholar] [CrossRef]
- Henke, P.K.; Varga, A.; De, S.; Deatrick, C.B.; Eliason, J.; Arenberg, D.A.; Sukheepod, P.; Thanaporn, P.; Kunkel, S.L.; Upchurch, G.R.; et al. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1130–1137. [Google Scholar] [CrossRef] [Green Version]
- Varma, M.R.; Varga, A.J.; Knipp, B.S.; Sukheepod, P.; Upchurch, G.R.; Kunkel, S.L.; Wakefield, T.W.; Henke, P.K. Neutropenia impairs venous thrombosis resolution in the rat. J. Vasc. Surg. 2003, 38, 1090–1098. [Google Scholar] [CrossRef] [Green Version]
- Albadawi, H.; Witting, A.A.; Pershad, Y.; Wallace, A.; Fleck, A.R.; Hoang, P.; Khademhosseini, A.; Oklu, R. Animal models of venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7, S197–S206. [Google Scholar] [CrossRef]
- Wernersson, R.; Schierup, M.H.; Jørgensen, F.G.; Gorodkin, J.; Panitz, F.; Stærfeldt, H.H.; Christensen, O.F.; Mailund, T.; Hornshøj, H.; Klein, A.; et al. Pigs in sequence space: A 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Perleberg, C.; Kind, A.; Schnieke, A. Genetically engineered pigs as models for human disease. DMM Dis. Model. Mech. 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fineschi, V.; Bafunno, V.; Bello, S.; de Stefano, F.; Margaglione, M.; Neri, M.; Riezzo, I.; Turillazzi, E.; Bonsignore, A.; Vecchione, G.; et al. Fatal pulmonary thromboembolism. A retrospective autopsy study: Searching for genetic thrombophilias (Factor V Leiden (G1691A) and FII (G20210A) gene variants) and dating the thrombus. Forensic Sci. Int. 2012, 214, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Shaya, S.A.; Saldanha, L.J.; Vaezzadeh, N.; Zhou, J.; Ni, R.; Gross, P.L. Comparison of the effect of dabigatran and dalteparin on thrombus stability in a murine model of venous thromboembolism. J. Thromb. Haemost. 2016, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sweetland, S.; Green, J.; Liu, B.; de González, A.B.; Canonico, M.; Reeves, G.; Beral, V. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: Prospective cohort study. BMJ 2009, 339, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, Y.; Cushman, M.; Zakai, N.; Rosamond, W.D.; Folsom, A.R. Tv viewing and incident venous thromboembolism: The atherosclerotic risk in communities study. J. Thromb. Thrombolysis. 2018, 45, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Suadicani, P.; Hannerz, H.; Bach, E.; Gyntelberg, F. Jobs encompassing prolonged sitting in cramped positions and risk of venous thromboembolism: Cohort study. JRSM Short Rep. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Pascarella, L.; Schmid-Schönbein, G.W.; Bergan, J. An animal model of venous hypertension: The role of inflammation in venous valve failure. J. Vasc. Surg. 2005, 41, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef]
- Heit, J.A.; Rooke, T.W.; Silverstein, M.D.; Mohr, D.N.; Lohse, C.M.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the incidence of venous stasis syndrome and venous ulcer: A 25-year population-based study. J. Vasc. Surg. 2001, 33, 1022–1027. [Google Scholar] [CrossRef] [Green Version]
- Maurins, U.; Hoffmann, B.H.; Lösch, C.; Jöckel, K.-H.; Rabe, E.; Pannier, F. Distribution and prevalence of reflux in the superficial and deep venous system in the general population—Results from the Bonn Vein Study, Germany. J. Vasc. Surg. 2008, 48, 680–687. [Google Scholar] [CrossRef] [Green Version]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doeing, D.C.; Borowicz, J.L.; Crockett, E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin. Pathol. 2003, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balduini, C.L.; Noris, P. Platelet count and aging. Haematologica 2014, 99, 953–955. [Google Scholar] [CrossRef] [Green Version]
- Levin, J.; Ebbe, S. Why are recently published platelet counts in normal mice so low? Blood 1994, 83, 3829–3831. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, M.; Chan, X.Y.; Tan, S.Y.; Subramaniam, S.; Fan, Y.; Loh, E.; Chang, K.T.E.; Tan, T.C.; Chen, Q. Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 2017, 130, 1995–2005. [Google Scholar] [CrossRef] [Green Version]
- Baaten, C.C.; Meacham, S.; de Witt, S.M.; Feijge, M.A.H.; Adams, D.J.; Akkerman, J.W.N.; Cosemans, J.M.E.M.; Grassi, L.; Jupe, S.; Kostadima, M.; et al. A synthesis approach of mouse studies to identify genes and proteins in arterial thrombosis and bleeding. Blood 2018, 132, E35–E46. [Google Scholar] [CrossRef] [Green Version]
- Oostra, C.; Henke, P.; Luke, C.; Acino, R.; Lurie, F.; Comerota, A.J. Human Chronic Post-Thrombotic Intraluminal Venous Obstruction Involves Neovascularization. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 110. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S. Sepsis: Early recognition and optimized treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef]
- Zinsser, H.H.; Pryde, A.W. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann. Surg. 1952, 136, 818–827. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Suzuki, K. Changes of Expression of the Protein C Pathway Components in LPSInduced Endotoxemia–Implication for Sepsis. Cardiovasc. Hematol. Disord. Targets. 2015, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.; Fialho, A.; Schenone, A.; Thota, P.; McCullough, A.; Shen, B. Association between small intestinal bacterial overgrowth and deep vein thrombosis. Gastroenterol. Rep. 2016, 4, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Branchford, B.R.; Carpenter, S.L. The role of inflammation in venous thromboembolism. Front. Pediatr. 2018, 6. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Kimball, A.S.; Obi, A.T.; Diaz, J.A.; Henke, P.K. The emerging role of NETs in venousthrombosis and immunothrombosis. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Laridan, E.; Martinod, K.; de Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; von Bruhl, M.L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef]
- Dyer, M.R.; Chen, Q.; Haldeman, S.; Yazdani, H.; Hoffman, R.; Loughran, P.; Tsung, A.; Zuckerbraun, B.S.; Simmons, R.L.; Neal, M.D. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Goel, M.S.; Diamond, S.L. Neutrophil Enhancement of Fibrin Deposition Under Flow Through Platelet-Dependent and-Independent Mechanisms. Available online: http://www.ahajournals (accessed on 12 September 2019).
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kondo, T. Time-dependent appearance of intrathrombus neutrophils and macrophages in a stasis-induced deep vein thrombosis model and its application to thrombus age determination. Int. J. Legal Med. 2009, 123, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Henke, P.K.; Wakefield, T.W.; Kadell, A.M.; Linn, M.J.; Varma, M.R.; Sarkar, M.; Hawley, A.; Fowlkes, J.B.; Strieter, R.M. Interleukin-8 administration enhances venous thrombosis resolution in a rat model. J. Surg. Res. 2001, 99, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R.; Jaffer, M.A.; Woodburne, V.E.; Sewell, T.; Kelly, S.L.; Kirsch, R.E.; Shephard, E.G. Fibrinogen is degraded and internalized during incubation with neutrophils, and fibrinogen products localize to electron lucent vesicles. Biochem. J. 2002, 364, 403–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yao, Y.; Yao, C.; Jiang, Q. Predictive value of lymphocyte to monocyte ratio and monocyte to high-density lipoprotein ratio for acute deep vein thrombosis after total joint arthroplasty: A retrospective study. J. Orthop. Surg. Res. 2018, 13, 211. [Google Scholar] [CrossRef]
- Henke, P.; Varma, M.; Deatrick, K.; Dewyer, N.; Lynch, E.; Moore, A.; Dubay, D.; Sukheepod, P.; Pearce, C.; Upchurch, G.; et al. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb. Haemost. 2006, 95, 272–281. [Google Scholar]
- Cherpokova, D.; Jouvene, C.C.; Libreros, S.; DeRoo, E.P.; Chu, L.; de la Rosa, X.; Norris, P.C.; Wagner, D.D.; Serhan, C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019, 134, 1458–1468. [Google Scholar] [CrossRef]
- Obi, A.T.; Andraska, E.; Kanthi, Y.; Kessinger, C.W.; Elfline, M.; Luke, C.; Siahaan, T.J.; Jaffer, F.A.; Wakefield, T.W.; Henke, P.K. Endotoxaemia-augmented murine venous thrombosis is dependent on TLR-4 and ICAM-1, and potentiated by neutropenia. Thromb. Haemost. 2017, 117, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef] [Green Version]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like Receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Sundquist, K.; Zöller, B.; Svensson, P.J.; Sundquist, J.; Memon, A.A. Association between TLR9 rs5743836 polymorphism and risk of recurrent venous thromboembolism. J. Thromb. Thrombolysis. 2017, 44, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, Y.W.; Bouman, A.C.; Castoldi, E.; Wielders, S.J.; Spronk, H.M.H.; Cate, H.T.; Cate-Hoek, A.J.T.; Wolde, M.T. Toll-like receptor 9 gene expression in the post-thrombotic syndrome, residual thrombosis and recurrent deep venous thrombosis: A case-control study. Thromb. Res. 2016, 140, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Henke, P.K.; Mitsuya, M.; Luke, C.E.; Elfline, M.A.; Baldwin, J.F.; Deatrick, K.B.; Diaz, J.A.; Sood, V.; Upchurch, G.R.; Wakefield, T.W.; et al. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewyer, N.A.; El-Sayed, O.M.; Luke, C.E.; Elfline, M.; Kittan, N.; Allen, R.; Laser, A.; Oostra, C.; Comerota, A.; Hogaboam, C.; et al. Divergent effects of Tlr9 deletion in experimental late venous thrombosis resolution and vein wall injury. Thromb. Haemost. 2015, 114, 1028–1037. [Google Scholar] [PubMed] [Green Version]
- El-Sayed, O.M.; Dewyer, N.A.; Luke, C.E.; Elfline, M.; Laser, A.; Hogaboam, C.; Kunkel, S.L.; Henke, P.K. Intact Toll-like receptor 9 signaling in neutrophils modulates normal thrombogenesis in mice. J. Vasc. Surg. 2016, 64, 1450–1458. [Google Scholar] [CrossRef] [Green Version]
- Budnik, I.; Brill, A. Immune Factors in Deep Vein Thrombosis Initiation. Trends Immunol. 2018, 39, 610–623. [Google Scholar] [CrossRef]
- Saha, P.; Humphries, J.; Modarai, B.; Mattock, K.; Waltham, M.; Evans, C.E.; Ahmad, A.; Patel, A.S.; Premaratne, S.; Lyons, O.T.A.; et al. Leukocytes and the natural history of deep vein thrombosis: Current concepts and future directions. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Mechanisms of venous thrombosis and resolution. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.; McGuinness, C.L.; Smith, A.; Waltham, M.; Poston, R.; Burnand, K.G. Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J. Vasc. Surg. 1999, 30, 894–899. [Google Scholar] [CrossRef]
- Miyazaki, S.; Matsukawa, A.; Ohkawara, S.; Takagi, K.; Yoshinaga, M. Neutrophil infiltration as a crucial step for monocyte chemoattractant protein (MCP)-1 to attract monocytes in lipopolysaccharide-induced arthritis in rabbits. Inflamm. Res. 2000, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Takahashi, M. IFN-γ-Mediated Survival Enables Human Neutrophils to Produce MCP-1/CCL2 in Response to Activation by TLR Ligands. J. Immunol. 2007, 179, 1942–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezende, S.M.; Lijfering, W.M.; Rosendaal, F.R.; Cannegieter, S.C. Hematologic variables and venous thrombosis: Red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica 2014, 99, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Humphries, J.; Burnand, K.; Sawyer, B.; Bursill, C.; Channon, K.; Greaves, D.; Rollins, B.; Charo, I.F.; Smith, A. Monocyte recruitment in venous thrombus resolution. J. Vasc. Surg. 2006, 43, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Hogg, N. Human monocytes are associated with the formation of fibrin. J. Exp. Med. 1983, 157, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar]
- Porrello, A.; Leslie, P.L.; Harrison, E.B.; Gorentla, B.K.; Kattula, S.; Ghosh, S.K.; Azam, S.H.; Holtzhausen, A.; Chao, Y.L.; Hayward, M.C.; et al. Factor XIIIA-expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Pang, X.; Wang, Y.; Liu, M. M1-macrophage polarization is upregulated in deep vein thrombosis and contributes to the upregulation of adhesion molecules. Hum. Immunol. 2019, 80, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-De-arellano, M. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE. 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Ley, K. M1 and M2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1(Lps+) vs. Classically and M2(LPS-) vs. Alternatively activated macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Schönfelder, T.; Brandt, M.; Kossmann, S.; Knopp, T.; Münzel, T.; Walter, U.; Karbach, S.H.; Wenzel, P. Lack of T-bet reduces monocytic interleukin-12 formation and accelerates thrombus resolution in deep vein thrombosis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Mukaida, N.; Kondo, T. Absence of IFN-γ accelerates thrombus resolution through enhanced MMP-9 and VEGF expression in mice. J. Clin. Investig. 2011, 121, 2911–2920. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.F.; Goods, B.A.; Askenase, M.H.; Hammond, M.D.; Renfroe, S.C.; Steinschneider, A.F.; Landreneau, M.J.; Ai, Y.; Beatty, H.E.; da Costa, L.H.A.; et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J. Clin. Investig. 2018, 128, 607–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.; Burnand, K.G.; Collins, M.; Luttun, A.; Collen, D.; Boelhouwer, B.; Smith, A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: Rescue by normal bone marrow-derived cells. Circulation 2003, 107, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, N.A.; de Vries, B.M.W.; Hillebrands, J.L.; Harlaar, N.J.; Tio, R.A.; Slart, R.H.J.A.; van Dam, G.M.; Boersma, H.H.; Zeebregts, C.J.; Westra, J. Distribution of Matrix Metalloproteinases in Human Atherosclerotic Carotid Plaques and Their Production by Smooth Muscle Cells and Macrophage Subsets. Mol. Imaging Biol. 2016, 18, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Meznarich, J.; Malchodi, L.; Helterline, D.; Ramsey, S.A.; Bertko, K.; Plummer, T.; Plawman, A.; Gold, E.; Stempien-Otero, A. Urokinase Plasminogen Activator Induces Pro-Fibrotic/M2 Phenotype in Murine Cardiac Macrophages. PLoS ONE 2013, 8, e57837. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Achuthan, A.; Schultz, H.; Nansen, A.; Almholt, K.; Usher, P.; Hamilton, J.A. Urokinase Plasminogen Activator Is a Central Regulator of Macrophage Three-Dimensional Invasion, Matrix Degradation, and Adhesion. J. Immunol. 2014, 192, 3540–3547. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Yang, Y.; Cui, Y.; He, S.; Wang, L.; Liu, L.; He, Q.; Lv, T.; Han, W.; Yu, W.; et al. M2 macrophage-mediated interleukin-4 signalling induces myofibroblast phenotype during the progression of benign prostatic hyperplasia article. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Henke, P.K.; Pearce, C.G.; Moaveni, D.M.; Moore, A.J.; Lynch, E.M.; Longo, C.; Varma, M.; Dewyer, N.A.; Deatrick, K.B.; Upchurch, G.R.; et al. Targeted Deletion of CCR2 Impairs Deep Vein Thombosis Resolution in a Mouse Model. J. Immunol. 2006, 177, 3388–3397. [Google Scholar] [CrossRef] [Green Version]
- Modarai, B.; Burnand, K.G.; Humphries, J.; Waltham, M.; Smith, A. The role of neovascularisation in the resolution of venous thrombus. Thromb. Haemost. 2005, 93, 801–809. [Google Scholar] [CrossRef]
- Varma, M.R.; Moaveni, D.M.; Dewyer, N.A.; Varga, A.J.; Deatrick, K.B.; Kunkel, S.L.; Upchurch, G.R.; Wakefield, T.W.; Henke, P.K. Deep vein thrombosis resolution is not accelerated with increased neovascularization. J. Vasc. Surg. 2004, 40, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Alias, S.; Redwan, B.; Panzenböck, A.; Winter, M.P.; Schubert, U.; Voswinckel, R.; Frey, M.K.; Jakowitsch, J.; Alimohammadi, A.; Hobohm, L.; et al. Defective angiogenesis delays thrombus resolution: A potential pathogenetic mechanism underlying chronic thromboembolic pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Obi, A.T.; Elfline, M.A.; Hogikyan, E.; Luke, C.E.; Henke, S.; Coleman, D.; Henke, P.K. Alterations in macrophage phenotypes in experimental venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ikezumi, Y.; Suzuki, T.; Yamada, T.; Hasegawa, H.; Kaneko, U.; Hara, M.; Yanagihara, T.; Nikolic-Paterson, D.J.; Saitoh, A. Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr. Nephrol. 2015, 30, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Austin, J.C.; Hare, B.D.; Burdick, M.D.; Greenfield, L.J.; Wakefield, T.W. IL-10 regulates thrombus-induced vein wall inflammation and thrombosis. J. Immunol. 1998, 161, 1471–1476. [Google Scholar] [PubMed]
- Furukoji, E.; Gi, T.; Yamashita, A.; Moriguchi-Goto, S.; Kojima, M.; Sugita, C.; Sakae, T.; Sato, Y.; Hirai, T.; Asada, Y. CD163 macrophage and erythrocyte contents in aspirated deep vein thrombus are associated with the time after onset: A pilot study. Thromb. J. 2016, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Kimball, A.S.; Obi, A.T.; Luke, C.E.; Dowling, A.R.; Cai, Q.; Adili, R.; Jankowski, H.; Schaller, M.; Holinstadt, M.; Jaffer, F.A.; et al. Ly6CLo Monocyte/Macrophages are Essential for Thrombus Resolution in a Murine Model of Venous Thrombosis. Thromb. Haemost. 2019. [Google Scholar] [CrossRef]
- Rabinovich, A.; Cohen, J.M.; Cushman, M.; Wells, P.S.; Rodger, M.A.; Kovacs, M.J.; Anderson, D.R.; Tagalakis, V.; Lazo-Langner, A.; Solymoss, S.; et al. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J. Thromb. Haemost. 2015, 13, 398–408. [Google Scholar] [CrossRef]
- Du, T.; Tan, Z. Relationship between deep venous thrombosis and inflammatory cytokines in postoperative patients with malignant abdominal tumors. Braz. J. Med. Biol. Res. 2014, 47, 1003–1007. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Jin, X.; Gao, P.; Wang, M.; Yang, L. Bone marrow stromal cells transplantation promotes the resolution and recanalization of deep vein thrombosis in rabbits through regulating macrophage infiltration and angiogenesis. J. Cell. Biochem. 2019, 120, 11680–11689. [Google Scholar] [CrossRef]
- Choi, W.; Lee, J.; Lee, J.; Lee, S.H.; Kim, S. Hepatocyte growth factor regulates macrophage transition to the M2 phenotype and promotes murine skeletal muscle regeneration. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Casella, G.; Garzetti, L.; Gatta, A.T.; Finardi, A.; Maiorino, C.; Ruffini, F.; Martino, G.; Muzio, L.; Furlan, R. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J. Neuroinflammation 2016, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Ozaki, M.; Tanaka, A.; Mukaida, N.; Kondo, T. Crucial Involvement of IL-6 in Thrombus Resolution in Mice via Macrophage Recruitment and the Induction of Proteolytic Enzymes. Front. Immunol. 2020, 10, 3150. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, B.M.; Wrobleski, S.K.; Hawley, A.E.; Wakefield, T.W.; Myers, D.D.; Diaz, J.A. Interleukin-6: A potential target for post-thrombotic syndrome. Ann. Vasc. Surg. 2011, 25, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Alciato, F.; Sainaghi, P.P.; Sola, D.; Castello, L.; Avanzi, G.C. TNF-α, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J. Leukoc. Biol. 2010, 87, 869–875. [Google Scholar] [CrossRef]
- Bertin, F.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Modarai, B.; Burnand, K.G.; Sawyer, B.; Smith, A. Endothelial progenitor cells are recruited into resolving venous thrombi. Circulation 2005, 111, 2645–2653. [Google Scholar] [CrossRef] [Green Version]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [Green Version]
- Sieweke, M.H.; Allen, J.E. Beyond stem cells: Self-renewal of differentiated macrophages. Science 2013, 342. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, A.; Schaller, M.; Joshi, A.; Davis, F.M.; DenDekker, A.; Boniakowski, A.; Bermick, J.; Obi, A.; Moore, B.; Henke, P.K.; et al. Ly6CHiblood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1102–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, N.; Shahneh, F.; Brähler, M.; Krebs, F.; Jäckel, S.; Subramaniam, S.; Stanger, C.; Schönfelder, T.; Kleis-Fischer, B.; Reinhardt, C.; et al. Innate Effector-Memory T-Cell Activation Regulates Post-Thrombotic Vein Wall Inflammation and Thrombus Resolution. Circ. Res. 2016, 119, 1286–1295. [Google Scholar] [CrossRef]
- Lauvau, G.; Soudja, S.M. Mechanisms of memory T Cell activation and effective immunity. Adv. Exp. Med. Biol. 2015, 850, 73–80. [Google Scholar]
- Lu, S.; Li, D.; Xi, L.; Calderone, R. Interplay of interferon-gamma and macrophage polarization during Talaromyces marneffei infection. Microb. Pathog. 2019, 134. [Google Scholar] [CrossRef]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function: A New Vision of an Old Cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Ponomaryov, T.; Payne, H.; Fabritz, L.; Wagner, D.D.; Brill, A. Mast Cells Granular Contents Are Crucial for Deep Vein Thrombosis in Mice. Circ. Res. 2017, 121, 941–950. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J. Allergy and Venous Thromboembolism: A Casual or Causative Association. Semin. Thromb. Hemost. 2015, 42, 63–68. [Google Scholar]
- Preston, R.J.S.; O’Sullivan, J.M.; O’Donnell, J.S. Advances in understanding the molecular mechanisms of venous thrombosis. Br. J. Haematol. 2019, 186, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, X.; Xu, Z.; Nakamura, T.; Pang, Y.; Yao, C.; Wang, F.; Chen, D.; Dai, J.; Jiang, Q. P-selectin: An unpredicted factor for deep vein thrombosis after total hip arthroplasty. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Bittar, L.F.; da Silva, L.Q.; Orsi, F.L.; Zapponi, K.C.S.; Mazetto, B.; de Paula, E.V.; Montalvão, S.A.; Annichino-Bizzacchi, J.M. Increased inflammation and endothelial markers in patients with late severe post-thrombotic syndrome. PLoS ONE 2020, 15, e0227150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.A.; Wrobleski, S.K.; Alvarado, C.M.; Hawley, A.E.; Doornbos, N.K.; Lester, P.A.; Lowe, S.E.; Gabriel, J.E.; Roelofs, K.J.; Henke, P.K.; et al. P-Selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von willebrand factor. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, T.R.; Myers, D.D.; Wrobleski, S.K.; Zajkowski, P.J.; Hawley, A.E.; Bedard, P.W.; Ballard, N.E.; Londy, F.J.; Kaila, N.; Vlasuk, G.P.; et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb. Haemost. 2008, 99, 343–351. [Google Scholar]
- Ramacciotti, E.; Myers, D.D.; Wrobleski, S.K.; Deatrick, K.B.; Londy, F.J.; Rectenwald, J.E.; Henke, P.K.; Schaub, R.G.; Wakefield, T.W. P-selectin/ PSGL-1 Inhibitors versus enoxaparin in the resolution of venous thrombosis: A meta-analysis. Thromb. Res. 2010, 125. [Google Scholar] [CrossRef] [Green Version]
- Myers, D.; Lester, P.; Adili, R.; Hawley, A.; Durham, L.; Dunivant, V.; Reynolds, G.; Sood, S.; Fogler, W.; Magnani, J.; et al. E-Selectin Inhibition: A New Way to Treat Proximal Deep Venous Thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 290. [Google Scholar] [CrossRef]
- Culmer, D.L.; Dunbar, M.L.; Hawley, A.E.; Sood, S.; Sigler, R.E.; Henke, P.K.; Wakefield, T.W.; Magnani, J.L.; Myers, D.D. E-selectin inhibition with GMI-1271 decreases venous thrombosis without profoundly affecting tail vein bleeding in a mouse model. Thromb. Haemost. 2017, 117, 1171–1181. [Google Scholar] [CrossRef]
- Kellermair, J.; Redwan, B.; Alias, S.; Jabkowski, J.; Panzenboeck, A.; Kellermair, L.; Winter, M.P.; Weltermann, A.; Lang, I.M. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood 2013, 122, 3376–3384. [Google Scholar] [CrossRef] [Green Version]
- Gaugler, M.H.; Vereycken-Holler, V.; Squiban, C.; Aigueperse, J. PECAM-1 (CD31) is required for interactions of platelets with endothelial cells after irradiation. J. Thromb. Haemost. 2004, 2, 2020–2026. [Google Scholar] [CrossRef]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Privratsky, J.R.; Newman, D.K.; Newman, P.J. PECAM-1: Conflicts of interest in inflammation. Life Sci. 2010, 87, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.D.; Li, X.Q. Endothelial progenitor cells accelerate the resolution of deep vein thrombosis. Vascul. Pharmacol. 2016, 83, 10–16. [Google Scholar] [CrossRef]
- Alessio, A.M.; Beltrame, M.P.; Nascimento, M.C.F.; Vicente, C.P.; de Godoy, J.A.P.; Silva, J.C.R.S.; Bittar, L.F.; Lorand-Metze, I.; de Paula, E.V.; Annichino-Bizzacchi, J.M. Circulating progenitor and mature endothelial cells in deep vein thrombosis. Int. J. Med. Sci. 2013, 10, 1746–1754. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Montagne, K.; Nishihara, A.; Watabe, T.; Miyazono, K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J. Biochem. 2008, 143, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L.; Orlando, C.; Maggi, C.A.; Ledda, F.; Ziche, M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am. J. Physiol. 1995, 269, H686–H695. [Google Scholar] [CrossRef]
- Moaveni, D.K.; Lynch, E.M.; Luke, C.; Sood, V.; Upchurch, G.R.; Wakefield, T.W.; Henke, P.K. Vein wall re-endothelialization after deep vein thrombosis is improved with low-molecular-weight heparin. J. Vasc. Surg. 2008, 47, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Xiao, L.; Du, X.; Hong, L.; Li, C.; Jiao, J.; Li, W.; Li, X. MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression. J. Cell. Mol. Med. 2019, 23, 8493–8504. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Hong, L.; Sun, L.; Sang, H.; Qian, A.; Li, W.; Zhuang, H.; Liang, H.; Song, D.; Li, C.; et al. miR-21 induces endothelial progenitor cells proliferation and angiogenesis via targeting FASLG and is a potential prognostic marker in deep venous thrombosis. J. Transl. Med. 2019, 17, 270. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhu, X.; Du, X.; Xu, A.; Yuan, X.; Zhan, Y.; Liu, M.; Wang, S. MiR-150 promotes angiogensis and proliferation of endothelial progenitor cells in deep venous thrombosis by targeting SRCIN1. Microvasc. Res. 2019, 123, 35–41. [Google Scholar] [CrossRef]
- Kong, L.; Hu, N.; Du, X.; Wang, W.; Chen, H.; Li, W.; Wei, S.; Zhuang, H.; Li, X.; Li, C. Upregulation of miR-483-3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhang, L.; Wu, X.; Zhao, R.; Meng, Z.; Wang, K.; Wang, B.; Wang, H.; Shi, Z.; Li, G. Homocysteine inhibits the viability and migration ability of human umbilical vein endothelial cells by downregulating the expression of vascular endothelial growth factor. Exp. Ther. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wang, N.; Zhang, T.C. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 2012, 64, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Hong, L.; Du, X.; You, T.; Sun, L.; Li, W.; Xiao, L.; Lu, H.; Wang, W.; Li, X. Reciprocal enhancement of thrombosis by endothelial-to-mesenchymal transition induced by iliac vein compression. Life Sci. 2019, 233. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Leidinger, C.; Rosinus, N.S.; Gogiraju, R.; Guth, S.; Hobohm, L.; Jurk, K.; Mayer, E.; Münzel, T.; Lankeit, M.; et al. Activated Endothelial TGFβ1 Signaling Promotes Venous Thrombus Nonresolution in Mice Via Endothelin-1. Circ. Res. 2020, 126, 162–181. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, S.; Liu, T.; Liu, Q.; Chen, Y.; Tan, D.; Ma, R.; Lu, X. MCP2 activates NF-κB signaling pathway promoting the migration and invasion of ESCC cells. Cell Biol. Int. 2018, 42, 365–372. [Google Scholar] [CrossRef]
- Lee, Y.U.; Lee, A.Y.; Humphrey, J.D.; Rausch, M.K. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology 2015, 52, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Truelove, J.; Tawakol, A.; Wojtkiewicz, G.R.; Hucker, W.J.; MacNabb, M.H.; Brownell, A.L.; Jokivarsi, K.; Kessinger, C.W.; Jaff, M.R.; et al. FDG-PET/CT enables the detection of recurrent same-site deep vein thrombosis by illuminating recently formed, neutrophil-rich thrombus. Circulation 2014, 103, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
- Cesarman-Maus, G.; Hajjar, K.A. Molecular mechanisms of fibrinolysis. Br. J. Haematol. 2005, 129, 307–321. [Google Scholar] [CrossRef]
- Adam, S.S.; Key, N.S.; Greenberg, C.S. D-dimer antigen: Current concepts and future prospects. Blood 2009, 113, 2878–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marder, V.J. Historical perspective and future direction of thrombolysis research: The re-discovery of plasmin. J. Thromb. Haemost. 2011, 9, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Summaria, L.; Hsieh, B.; Robbins, K.C. The specific mechanism of activation of human plasminogen to plasmin. J. Biol. Chem. 1967, 242, 4279–4283. [Google Scholar]
- Wang, H.; Lottenberg, R.; Boyle, M.D. Analysis of the interaction of group A streptococci with fibrinogen, streptokinase and plasminogen. Microb. Pathog. 1995, 18, 153–166. [Google Scholar] [CrossRef]
- Bokarewa, M.I.; Jin, T.; Tarkowski, A. Staphylococcus aureus: Staphylokinase. Int. J. Biochem. Cell Biol. 2006, 38, 504–509. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yasui, H.; Brzoska, T.; Mogami, H.; Urano, T. Surface-retained tPA is essential for effective fibrinolysis on vascular endothelial cells. Blood 2011, 118, 3182–3185. [Google Scholar] [CrossRef]
- Killewich, L.A.; Macko, R.F.; Cox, K.; Franklin, D.R.; Benjamin, M.E.; Lilly, M.P.; Flinn, W.R. Regression of proximal deep venous thrombosis is associated with fibrinolytic enhancement. J. Vasc. Surg. 1997, 26, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Lai, R.; Li, J.; Luo, M.; Wang, Y.; Sheng, W. The -7351C/T Polymorphism in the TPA Gene and Ischemic Stroke Risk: A Meta-Analysis. PLoS ONE 2013, 8, e53558. [Google Scholar] [CrossRef] [Green Version]
- Urano, T.; Castellino, F.J.; Suzuki, Y. Regulation of plasminogen activation on cell surfaces and fibrin. J. Thromb. Haemost. 2018, 16, 1487–1497. [Google Scholar] [CrossRef]
- Baldwin, J.F.; Sood, V.; Elfline, M.A.; Luke, C.E.; Dewyer, N.A.; Diaz, J.A.; Myers, D.D.; Wakefield, T.; Henke, P.K. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. J. Vasc. Surg. 2012, 56, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Gossage, J.A.; Humphries, J.; Modarai, B.; Burnand, K.G.; Smith, A. Adenoviral urokinase-type plasminogen activator (uPA) gene transfer enhances venous thrombus resolution. J. Vasc. Surg. 2006, 44, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alias, S.; Lang, I.M. Coagulation and the vessel wall in pulmonary embolism. Pulm. Circ. 2013, 3, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Humphries, J.; Gossage, J.A.; Modarai, B.; Burnand, K.G.; Sisson, T.H.; Murdoch, C.; Smith, A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J. Vasc. Surg. 2009, 50, 1127–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paland, N.; Aharoni, S.; Fuhrman, B. Urokinase-type plasminogen activator (uPA) modulates monocyte-to-macrophage differentiation and prevents Ox-LDL-induced macrophage apoptosis. Atherosclerosis 2013, 231, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L.; Houng, A.K.; Singh, S.; Wang, D. α2-Antiplasmin: New Insights and Opportunities for Ischemic Stroke. Semin. Thromb. Hemost. 2017, 43, 191–199. [Google Scholar] [PubMed] [Green Version]
- Weitz, J.I.; Leslie, B.; Hirsh, J.; Klement, P. α2-Antiplasmin supplementation inhibits tissue plasminogen activator- induced fibrinogenolysis and bleeding with little effect on thrombolysis. J. Clin. Investig. 1993, 91, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, S.L.; Mathew, P. α2-antiplasmin and its deficiency: Fibrinolysis out of balance. Haemophilia 2008, 14, 1250–1254. [Google Scholar] [CrossRef]
- Matsuno, H.; Okada, K.; Ueshima, S.; Matsuo, O.; Kozawa, O. α 2-Antiplasmin plays a significant role in acute pulmonary embolism. J. Thromb. Haemost. 2003, 1, 1734–1739. [Google Scholar] [CrossRef]
- Bajzar, L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2511–2518. [Google Scholar] [CrossRef] [Green Version]
- Plug, T.; Meijers, J.C.M. Structure-function relationships in thrombin-activatable fibrinolysis inhibitor. J. Thromb. Haemost. 2016, 14, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Colucci, M.; Semeraro, N. Thrombin activatable fibrinolysis inhibitor: At the nexus of fibrinolysis and inflammation. Thromb. Res. 2012, 129, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Morser, J.; Gabazza, E.C.; Myles, T.; Leung, L.L.K. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J. Thromb. Haemost. 2010, 8, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, T.; Suzuki, Y.; Sano, H.; Suzuki, S.; Tomczyk, M.; Tanaka, H.; Urano, T. Imaging analyses of coagulation-dependent initiation of fibrinolysis on activated platelets and its modification by thrombin-activatable fibrinolysis inhibitor. Thromb. Haemost. 2017, 117, 682–690. [Google Scholar] [CrossRef]
- Burley, K.; Whyte, C.S.; Westbury, S.K.; Walker, M.; Stirrups, K.E.; Turro, E.; Chapman, O.G.; Reilly-Stitt, C.; Mutch, N.J.; Mumford, A.D. Altered fibrinolysis in autosomal dominant thrombomodulin-associated coagulopathy. Blood 2016, 128, 1879–1883. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of factor XIII is exclusively expressed through α2-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef] [Green Version]
- Sherman, P.M.; Lawrence, D.A.; Verhamme, I.M.; Paielli, D.; Shore, J.D.; Ginsburg, D. Identification of tissue-type plasminogen activator-specific plasminogen activator inhibitor-1 mutants. Evidence that second sites of interaction contribute to target specificity. J. Biol. Chem. 1995, 270, 9301–9306. [Google Scholar] [CrossRef] [Green Version]
- Klarin, D.; Busenkell, E.; Judy, R.; Lynch, J.; Levin, M.; Haessler, J.; Aragam, K.; Chaffin, M.; Haas, M.; Lindström, S.; et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat. Genet. 2019, 51, 1574–1579. [Google Scholar] [CrossRef]
- Klarin, D.; Emdin, C.A.; Natarajan, P.; Conrad, M.F.; Kathiresan, S. Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ. Cardiovasc. Genet. 2017, 10. [Google Scholar] [CrossRef]
- Obi, A.T.; Diaz, J.A.; Ballard-Lipka, N.L.; Roelofs, K.J.; Farris, D.M.; Lawrence, D.A.; Wakefield, T.W.; Henke, P.K. Plasminogen activator-1 overexpression decreases experimental postthrombotic vein wall fibrosis by a non-vitronectin-dependent mechanism. J. Thromb. Haemost. 2014, 12, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Role of selectins and fibrinolysis in VTE. Thromb. Res. 2009, 123. [Google Scholar] [CrossRef]
- Baxi, S.; Crandall, D.L.; Meier, T.R.; Wrobleski, S.; Hawley, A.; Farris, D.; Elokdah, H.; Sigler, R.; Schaub, R.G.; Wakefield, T.; et al. Dose-dependent thrombus resolution due to oral plasminogen activator inhibitor (PAI)-1 inhibition with tiplaxtinin in a rat stenosis model of venous thrombosis. Thromb. Haemost. 2008, 99, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Mikus, P.; Urano, T.; Liljeström, P.; Ny, T. Plasminogen-activator inhibitor type 2 (PAI-2) is a spontaneously polymerising SERPIN: Biochemical characterisation of the recombinant intracellular and extracellular forms. Eur. J. Biochem. 1993, 218, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Siefert, S.A.; Chabasse, C.; Mukhopadhyay, S.; Hoofnagle, M.H.; Strickland, D.K.; Sarkar, R.; Antalis, T.M. Enhanced venous thrombus resolution in plasminogen activator inhibitor type-2 deficient mice. J. Thromb. Haemost. 2014, 12, 1706–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, K.M.; Pearson, J.M.; Yang, A.Y.; Westrick, R.J.; Baker, M.S.; Ginsburg, D. The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. Proc. Natl. Acad. Sci. USA 1999, 96, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Medcalf, R.L. Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 499, pp. 105–134. [Google Scholar]
- Lee, J.A.; Yerbury, J.J.; Farrawell, N.; Shearer, R.F.; Constantinescu, P.; Hatters, D.M.; Schroder, W.A.; Suhrbier, A.; Wilson, M.R.; Saunders, D.N.; et al. SerpinB2 (PAI-2) Modulates Proteostasis via Binding Misfolded Proteins and Promotion of Cytoprotective Inclusion Formation. PLoS ONE 2015, 10, e0130136. [Google Scholar] [CrossRef] [Green Version]
- Schroder, W.A.; Hirata, T.D.; Le, T.T.; Gardner, J.; Boyle, G.M.; Ellis, J.; Nakayama, E.; Pathirana, D.; Nakaya, H.I.; Suhrbier, A. SerpinB2 inhibits migration and promotes a resolution phase signature in large peritoneal macrophages. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Schroder, W.A.; Le, T.T.; Major, L.; Street, S.; Gardner, J.; Lambley, E.; Markey, K.; MacDonald, K.P.; Fish, R.J.; Thomas, R.; et al. A Physiological Function of Inflammation-Associated SerpinB2 Is Regulation of Adaptive Immunity. J. Immunol. 2010, 184, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.A.; Ballard-Lipka, N.E.; Farris, D.M.; Hawley, A.E.; Wrobleski, S.K.; Myers, D.D.; Henke, P.K.; Lawrence, D.A.; Wakefield, T.W. Impaired fibrinolytic system in ApoE gene-deleted mice with hyperlipidemia augments deep vein thrombosis. J. Vasc. Surg. 2012, 55, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.A.; Zhang, X.; Wrobleski, S.K.; Hawley, A.E.; Lawrence, D.A.; Wakefield, T.W.; Myers, D.D.; Diaz, J.A. Rosuvastatin reduced deep vein thrombosis in ApoE gene deleted mice with hyperlipidemia through non-lipid lowering effects. Thromb. Res. 2013, 131, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Norberto, E.M.S.; Gastambide, M.V.; Taylor, J.H.; García-Saiz, I.; Vaquero, C. Effects of rosuvastatin as an adjuvant treatment for deep vein thrombosis. Vasa Eur. J. Vasc. Med. 2016, 45, 133–140. [Google Scholar] [CrossRef] [Green Version]
- DeRoo, S.; Deatrick, K.B.; Henke, P.K. The vessel wall: A forgotten player in post thrombotic syndrome. Thromb. Haemost. 2010, 104, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.F.; Lourenço, D.M.; Orikaza, C.M.; Bajerl, J.A.H.; Noguti, M.A.E.; Morelli, V.M. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6 -174GC, IL-8 -251AT and MCP-1 -2518AG in the risk of venous thromboembolism: A case-control study. Thromb. Res. 2011, 128, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Henke, P.K.; Varma, M.R.; Moaveni, D.K.; Dewyer, N.A.; Moore, A.J.; Lynch, E.M.; Longo, C.; Deatrick, C.B.; Kunkel, S.L.; Upchurch, G.R.; et al. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb. Haemost. 2007, 98, 1045–1055. [Google Scholar] [PubMed]
- Deatrick, K.B.; Eliason, J.L.; Lynch, E.M.; Moore, A.J.; Dewyer, N.A.; Varma, M.R.; Pearce, C.G.; Upchurch, G.R.; Wakefield, T.W.; Henke, P.K. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J. Vasc. Surg. 2005, 42, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, G.B. Interstitial collagen catabolism. J. Biol. Chem. 2013, 288, 8785–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkedal-Hansen, H.; Taylor, R.E.; Bhown, A.S.; Katz, J.; Lin, H.Y.; Wells, B.R. Cleavage of bovine skin type III collagen by proteolytic enzymes. Relative resistance of the fibrillar form. J. Biol. Chem. 1985, 260, 16411–16417. [Google Scholar]
- Patterson, M.L.; Atkinson, S.J.; Knäuper, V.; Murphy, G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001, 503, 158–162. [Google Scholar] [CrossRef]
- Bigg, H.F.; Rowan, A.D.; Barker, M.D.; Cawston, T.E. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007, 274, 1246–1255. [Google Scholar] [CrossRef]
- Mosevoll, K.A.; Lindås, R.; Tvedt, T.H.A.; Bruserud, O.; Reikvam, H. Altered plasma levels of cytokines, soluble adhesion molecules and matrix metalloproteases in venous thrombosis. Thromb. Res. 2015, 136, 30–39. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kondo, T. Immunohistochemical detection of MMP-2 and MMP-9 in a stasis-induced deep vein thrombosis model and its application to thrombus age estimation. Int. J. Legal Med. 2010, 124, 439–444. [Google Scholar] [CrossRef]
- Deatrick, K.B.; Luke, C.E.; Elfline, M.A.; Sood, V.; Baldwin, J.; Upchurch, G.R.; Jaffer, F.A.; Wakefield, T.W.; Henke, P.K. The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post-thrombotic vein wall remodeling. J. Vasc. Surg. 2013, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.P.; McGilvray, K.C.; Puttlitz, C.M.; Mukhopadhyay, S.; Chabasse, C.; Sarkar, R. Matrix Metalloproteinase 9 (MMP-9) Regulates Vein Wall Biomechanics in Murine Thrombus Resolution. PLoS ONE 2015, 10, e0139145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, J.; Sun, Y. Transcriptional activation by p53 of the human type IV collagenase (gelatinase A or matrix metalloproteinase 2) promoter. Mol. Cell. Biol. 1997, 17, 6330–6338. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kim, C.S.; Hoffman, T.A.; Naqvi, A.; Dericco, J.; Jung, S.B.; Lin, Z.; Jain, M.K.; Irani, K. P53 Impairs endothelial function by transcriptionally repressing kruppel-Like factor 2. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochenek, M.L.; Bauer, T.; Gogiraju, R.; Nadir, Y.; Mann, A.; Schönfelder, T.; Hünig, L.; Brenner, B.; Münzel, T.; Wenzel, P.; et al. The endothelial tumor suppressor p53 is essential for venous thrombus formation in aged mice. Blood Adv. 2018, 2, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Antalis, T.M.; Nguyen, K.P.; Hoofnagle, M.H.; Sarkar, R. Myeloid p53 regulates macrophage polarization and venous thrombus resolution by inflammatory vascular remodeling in mice. Blood 2017, 129, 3245–3255. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Eriksson, P.; Hansson, G.K.; Herzfeld, I.; Klein, M.; Hansson, L.-O.; Valen, G. Expression of matrix metalloproteinase 9 and its regulators in the unstable coronary atherosclerotic plaque. Int. J. Mol. Med. 2005, 15, 57–65. [Google Scholar] [CrossRef]
- Liu, R.; Chen, B.; Chen, J.; Lan, J.U.N. Leptin upregulates smooth muscle cell expression of mmp-9 to promote plaque destabilization by activating ap-1 via the leptin receptor/mapk/erk signaling pathways. Exp. Ther. Med. 2018, 16, 5327–5333. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.-X. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef] [Green Version]
- Madsen, D.H.; Jürgensen, H.J.; Siersbæk, M.S.; Kuczek, D.E.; Cloud, L.G.; Liu, S.; Behrendt, N.; Grøntved, L.; Weigert, R.; Bugge, T.H. Tumor-Associated Macrophages Derived from Circulating Inflammatory Monocytes Degrade Collagen through Cellular Uptake. Cell Rep. 2017, 21, 3662–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabkin, S.W. Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 147, pp. 239–265. [Google Scholar]
| Cell Type | Role in VTE Resolution |
|---|---|
| Neutrophils (PMN) | Infiltrate the early developing thrombus, also maintain thrombus stability via NETosis and early fibrosis. |
| Monocyte-derived Macrophages (Mo/MΦ) | Pro-inflammatory macrophages are activated to police the thrombus by stabilizing it via fibrosis, searching for pathogens, and releasing inflammatory markers. |
| Pro-resolving (M2) macrophages phagocytize erythrocytes, conduct both fibrinolysis and collagenolysis, and promote neovascularization. | |
| Mast Cells | Trigger the activation of pro-inflammatory Mo/MΦ cells by sending inflammatory signals such as histamine to endothelial cells, causing Weibel-Palade body release. |
| T-Cells | Secrete IFNγ among other immune factors that trigger pro-inflammatory Mo/MΦ cell recruitment and prevent pro-resolving phenotype. |
| Endothelial Cells | Produce surface markers and other proteins that recruit immune cells to the site of the developing thrombus and allow them to adhere. Also proliferate to coat the developing thrombus in endothelium or transdifferentiate into mesenchymal-like cells. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicklas, J.M.; Gordon, A.E.; Henke, P.K. Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. Int. J. Mol. Sci. 2020, 21, 2080. https://doi.org/10.3390/ijms21062080
Nicklas JM, Gordon AE, Henke PK. Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. International Journal of Molecular Sciences. 2020; 21(6):2080. https://doi.org/10.3390/ijms21062080
Chicago/Turabian StyleNicklas, J. Matthew, Aviva E. Gordon, and Peter K. Henke. 2020. "Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms" International Journal of Molecular Sciences 21, no. 6: 2080. https://doi.org/10.3390/ijms21062080
APA StyleNicklas, J. M., Gordon, A. E., & Henke, P. K. (2020). Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. International Journal of Molecular Sciences, 21(6), 2080. https://doi.org/10.3390/ijms21062080





