Abstract
Melanization is a common phenomenon in insects, and melanin synthesis is a conserved physiological process that occurs in epidermal cells. Moreover, a comprehensive understanding of the mechanisms of melanin synthesis influencing insect pigmentation are well-suited for investigating phenotype variation. The Asian multi-colored (Harlequin) ladybird beetle, Harmonia axyridis, exhibits intraspecific polymorphism based on relative levels of melanization. However, the specific characteristics of melanin synthesis in H. axyridis remains elusive. In this study, we performed gene-silencing analysis of the pivotal inverting enzyme, tyrosine hydroxylase (TH), and DOPA decarboxylase (DDC) in the tyrosine metabolism pathway to investigate the molecular and regulatory mechanism of melanin synthesis in H. axyridis. Using RNAi of TH and DDC genes in fourth instar larvae, we demonstrated that dopamine melanin was the primary contributor to the overall body melanization of H. axyridis. Furthermore, our study provides the first conclusive evidence that dopamine serves as a melanin precursor for synthesis in the early pupal stage. According to transcription factor Pannier, which is essential for the formation of melanic color on the elytra in H. axyridis, we further demonstrated that suppression of HaPnr can significantly decrease expression levels of HaTH and HaDDC. These results in their entirety lead to the conclusion that transcription factor Pannier can regulate dopamine melanin synthesis in the dorsal elytral epidermis of H. axyridis.
1. Introduction
The Asian multi-colored (Harlequin) ladybird beetle, Harmonia axyridis (Coleoptera: Coccinellidae), has been widely used for biological control of pests in fields and greenhouse crops for a long time [1]. It also exhibits intraspecific polymorphism based on the influence of melanization with over 200 different elytral color forms [2]. This striking intraspecific variation has prompted investigation into its genetic, biochemical, and evolutionary meaning [3,4] and forms the basis of the current study. In recent years, an association between melanization of H. axyridis and different phenotypes have been investigated, including variation in fitness parameters [5], prey capacity [6,7], behavioral characteristics [8], aggregation behavior [9], assortative mating [10], fertility [11], and responses to insecticide stress [12]. In addition, the degree of melanization has a linear relationship with temperature variation in H. axyridis [13]; for instance, melanization increased linearly with lower temperatures [14] and decreased linearly with elevated temperature [15]. The abovementioned research also highlighted that different phenotypic individuals exhibited varying behavioral traits. Thus, elucidating the molecular mechanism of melanin synthesis in H. axyridis would provide crucial information on the sole ontogenies and evolutionary histories of the attendant melanization.
Melanin is the final product in the melanization process and has a prominent role in wound healing, cuticle sclerotization, innate immunity, defensive reactions, cuticular coloration, and camouflage in insects [16,17,18]. Melanin synthesis is a conserved physiological process in insects that occurs in epidermal cells [19,20], and in the melanin synthesis pathway, the precursor DOPA (3, 4-dihydroxyphenylalanine) and dopamine are synthesized from tyrosine hydroxylase (TH) and DOPA decarboxylase (DDC) enzymes, respectively [21]. Tyrosine hydroxylase and DOPA decarboxylase enzymes are abound in central nervous system and epidermal cells of insects. Dopamine is an important catecholamine neurotransmitter in invertebrates and vertebrates [18]. In insects, dopamine is the major contributor for melanin biosynthesis, while in mammals DOPA is dominated [17,18]. The biochemistry of tyrosine metabolism is abounding in insects because tyrosine is the initiatory material of melanin formation. Tyrosine hydroxylase is the first key enzyme in this pathway that can convert tyrosine to DOPA. Then, DOPA decarboxylase catalyzes DOPA to dopamine. DOPA and dopamine are crucial precursors of sclerotization and melanization in insects. There was research reported that sclerotization takes precedence over melanization in insects. Dopamine firstly uses to synthesis NBAD by NBAD synthetase (ebony) and NADA by N-acetyldopamine transferase (aaNAT) for sclerotization. The redundant NBAD can be hydrolyzed back to dopamine by NBAD hydrolase (Tan). Then, the dopamine is oxidized by phenoloxidase (laccase 2) to dopaminequinone and further converted to melanin [17]. Many aspects of this process also influence the ecology, development, genetics, and physiology of insects [19]. Due to its important role in insect physiology, it is imperative to understand the role this plays in all vital processes through a comprehensive investigation of the characteristics of melanin synthesis.
The availability of sophisticated molecular techniques has enabled the disentangling of complex melanin synthesis processes in H. axyridis [22]. As previously reported, Ando et al. [3] delimited the melanin synthesis process of H. axyridis revealing that it begins immediately after eclosion in regions complementary to the red-pigment regions. Furthermore, we have previously demonstrated that dopamine melanin is the primary contributor in the elytra in H. axyridis through silencing DDC in the third instar larvae and DDC can regulate H. axyridis fecundity [23]. Recently, a single GATA transcription factor gene, Pannier, was identified in genome studies and was revealed to promote melanin production and suppress carotenoid synthesis during elytral development in H. axyridis [2,3]. Pannier is believed to regulate a subset of enzyme genes in the melanin synthesis pathway, because this process and genes of the enzymes that catalyze various chemical reactions are relatively conservative in animals. [3]. However, the specific mechanism of how Pannier regulates pigmentation patterns and the gene regulatory network remains unclear. The objectives of this study were to determine: (1) the melanin component in overall body of H. axyridis; (2) establish the synthesis time of melanin in H. axyridis; and (3) identify the regulatory mechanism of transcription factor Pannier on melanin synthesis in H. axyridis.
2. Results
2.1. Developmental Stage Expression Patterns of HaTH
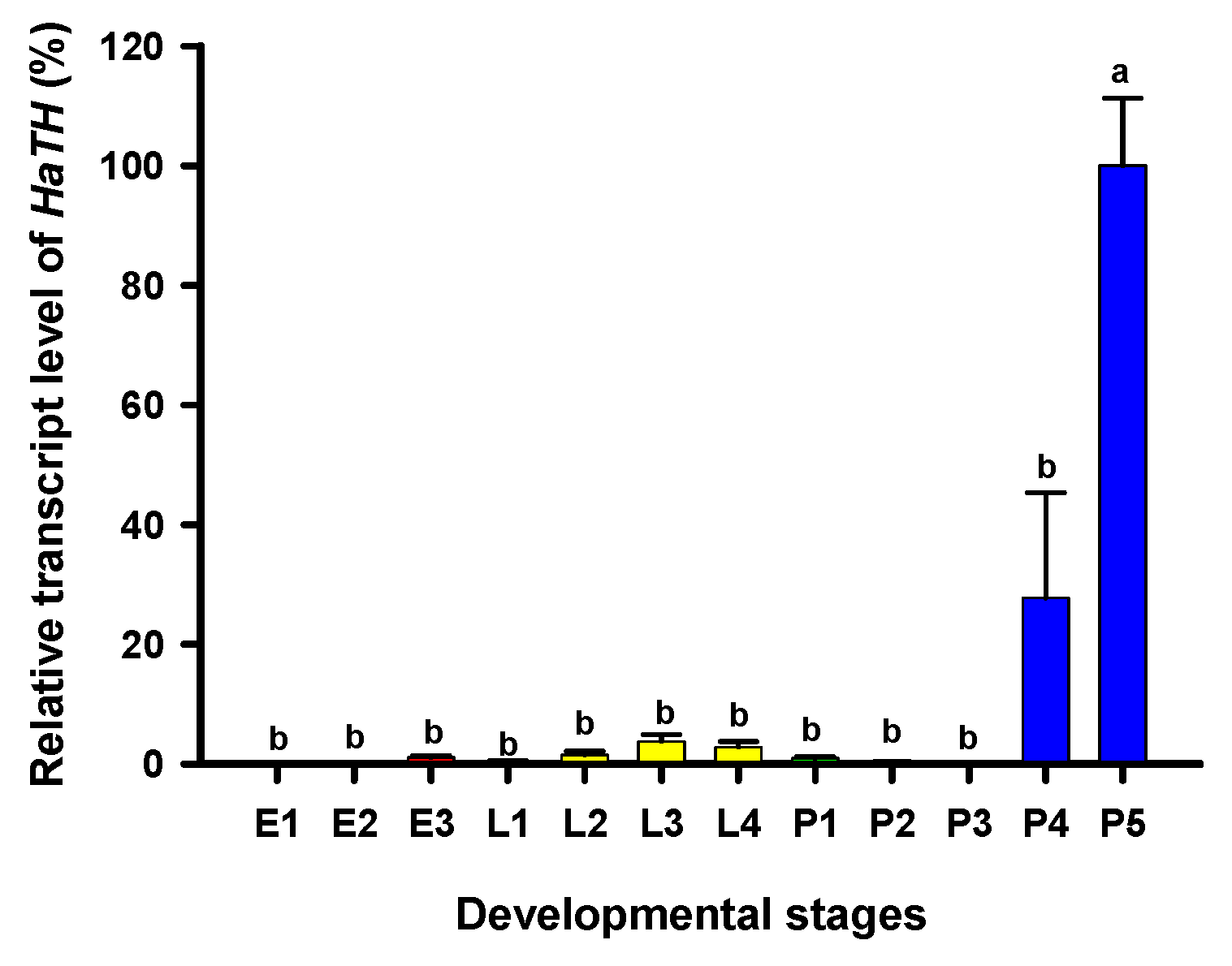
Tyrosine hydroxylase (TH) is the first key enzyme in melanin synthesis, which catalyzes tyrosine converting into DOPA. The relative transcript levels of HaTH in each developmental stage were analyzed by RT-qPCR. Transcripts of HaTH were observed at all test stages and the highest levels of HaTH transcripts were detected on the fifth day of pupal development (pharate adult) (Figure 1).
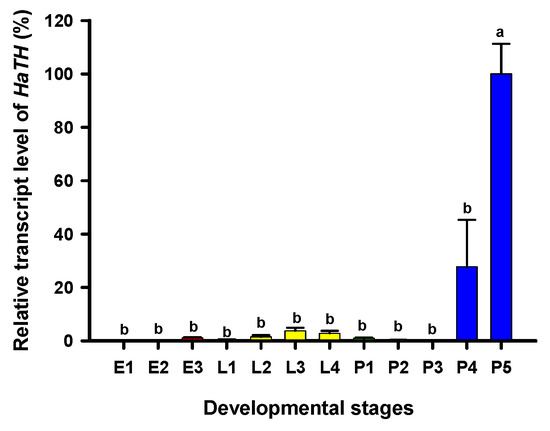
Figure 1.
Relative transcript levels of HaTH at different developmental stages of Harmonia axyridis as determined by RT-qPCR. E1, E2, and E3 represent 1-, 2-, and 3-day eggs, respectively; L1, L2, L3, and L4 represent first, second, third, and fourth instar larvae, respectively; P1, P2, P 3, P4, and P5 represent 1-, 2-, 3-, 4-, and 5-day pupae, respectively. Different letters above the standard error bars indicate significant differences based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05). H. axyridis ribosomal protein 49 (HaRp49) was used as an internal reference gene to normalize the differences among the samples. Relative expression levels for HaTH were calculated based on the highest expressions of HaTH in 5-day pupae (P5) as 100% in the developmental stage.
2.2. The Melanin Synthesis Pathway in H. axyridis
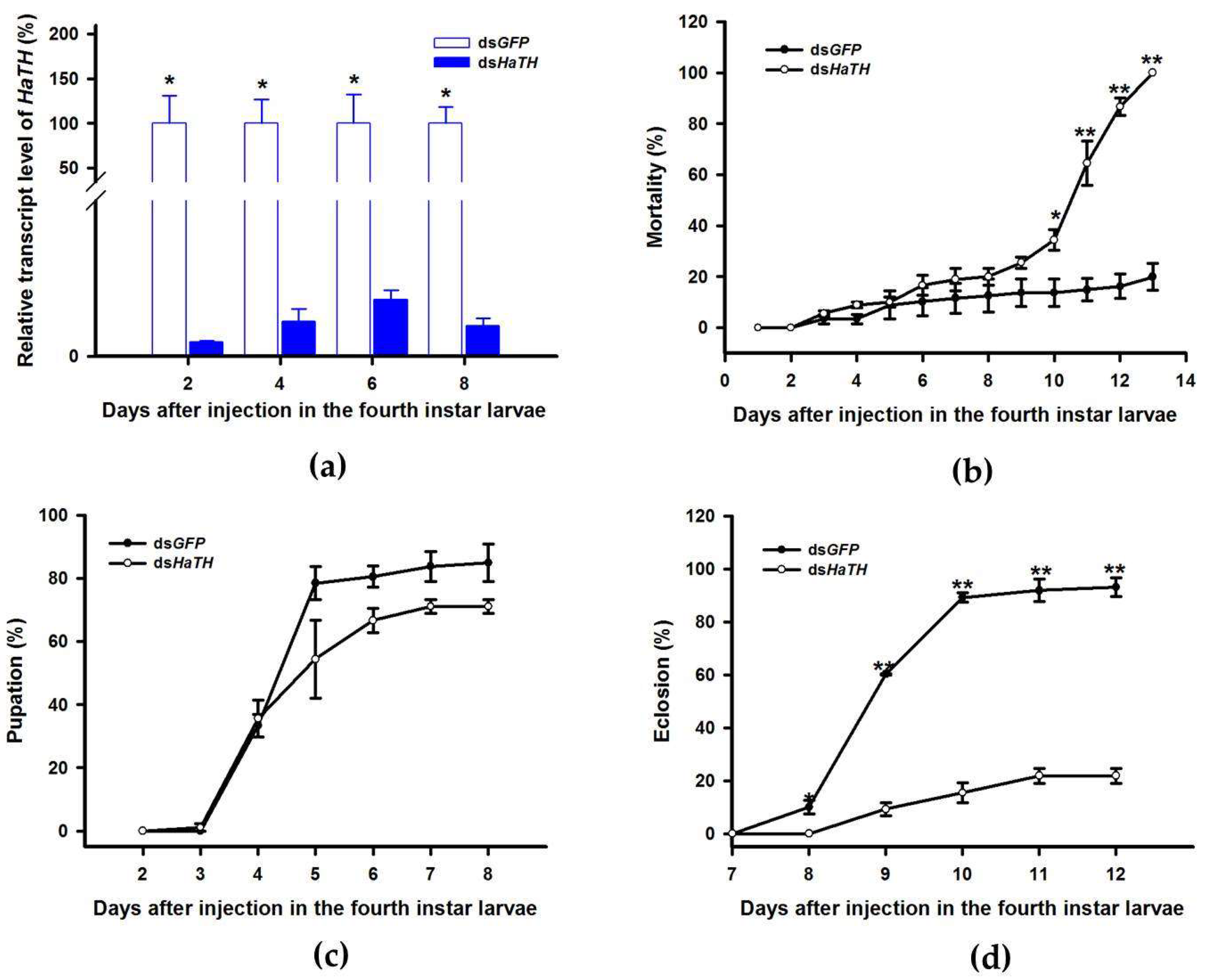
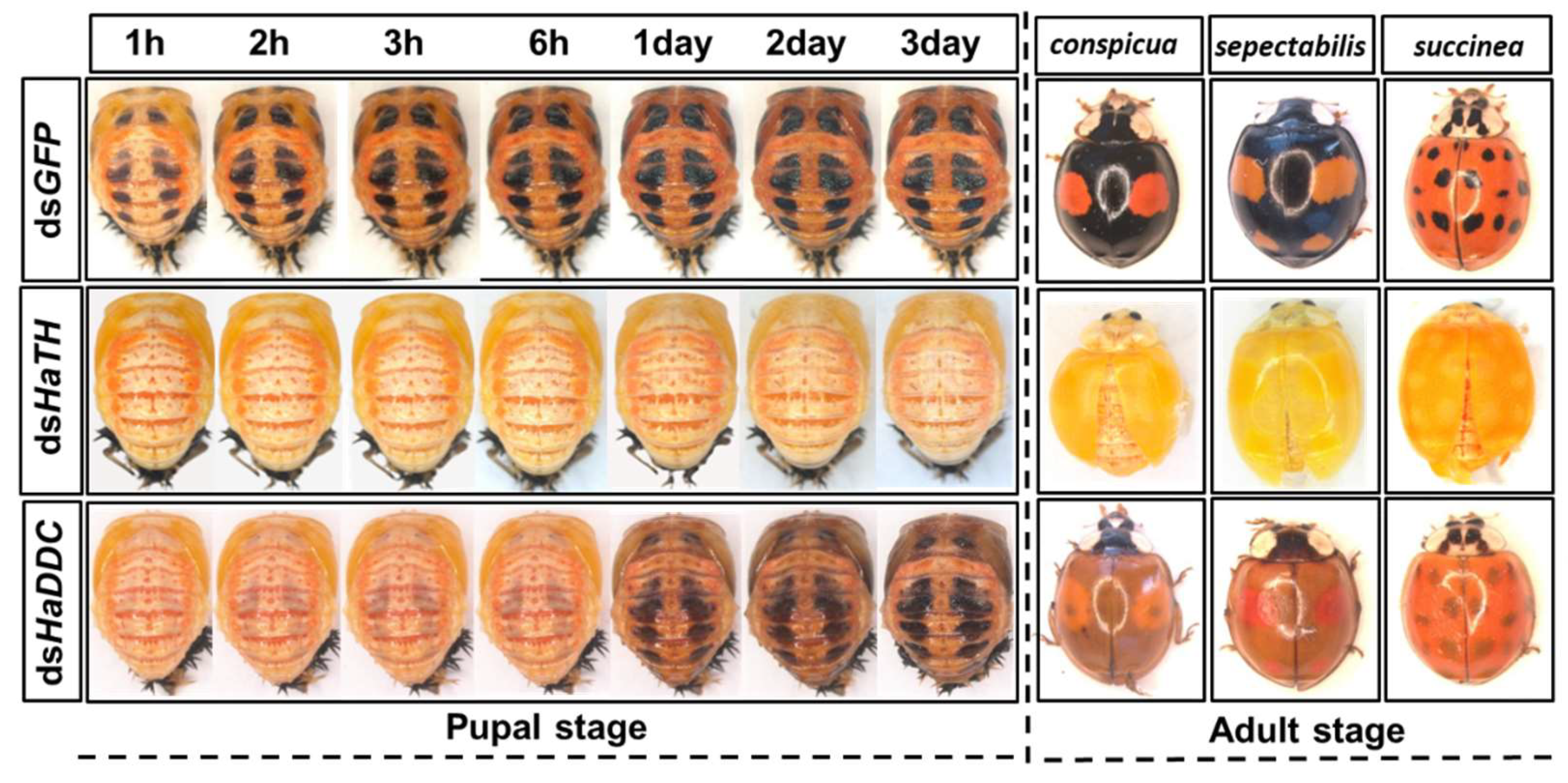
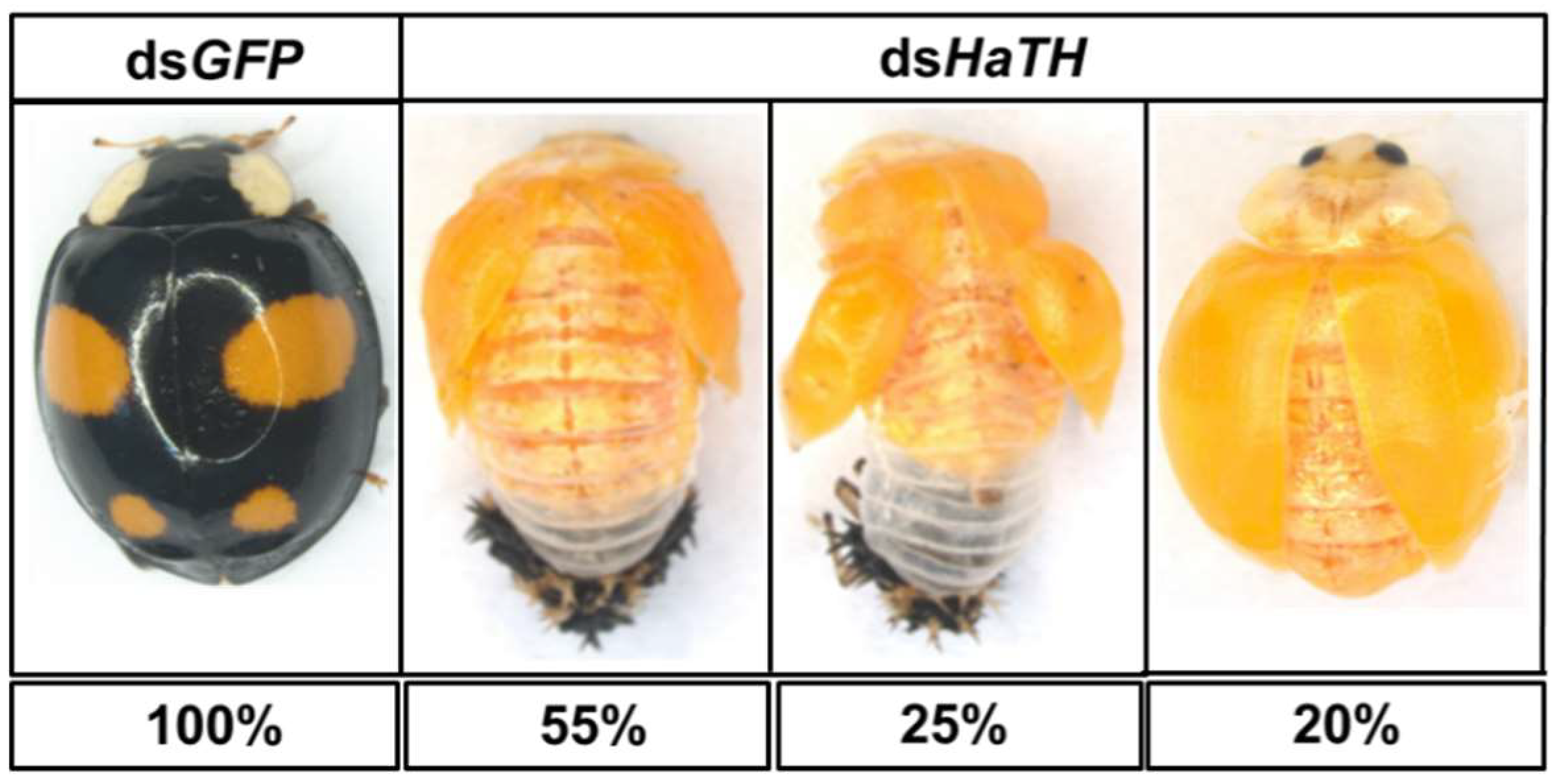
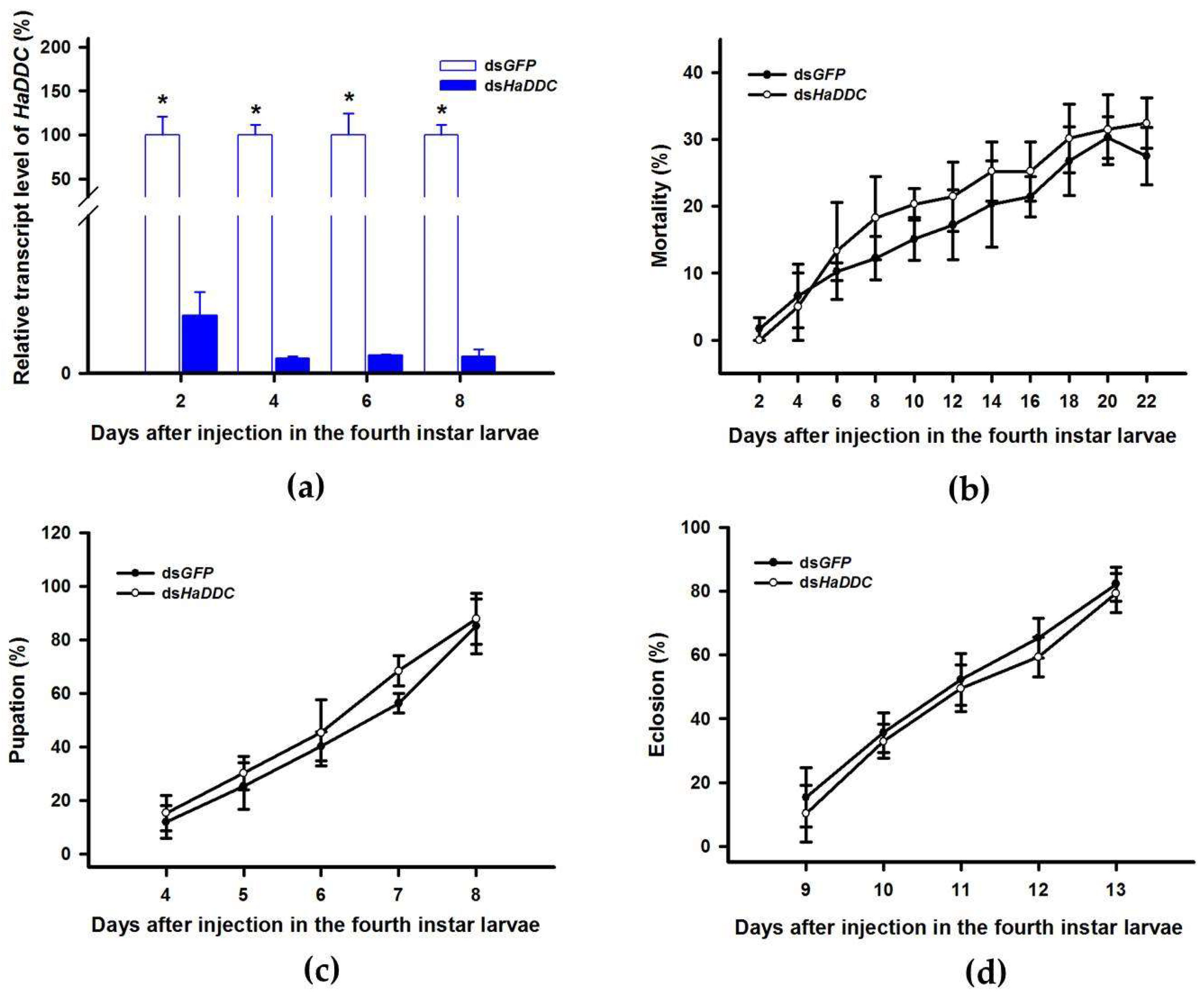
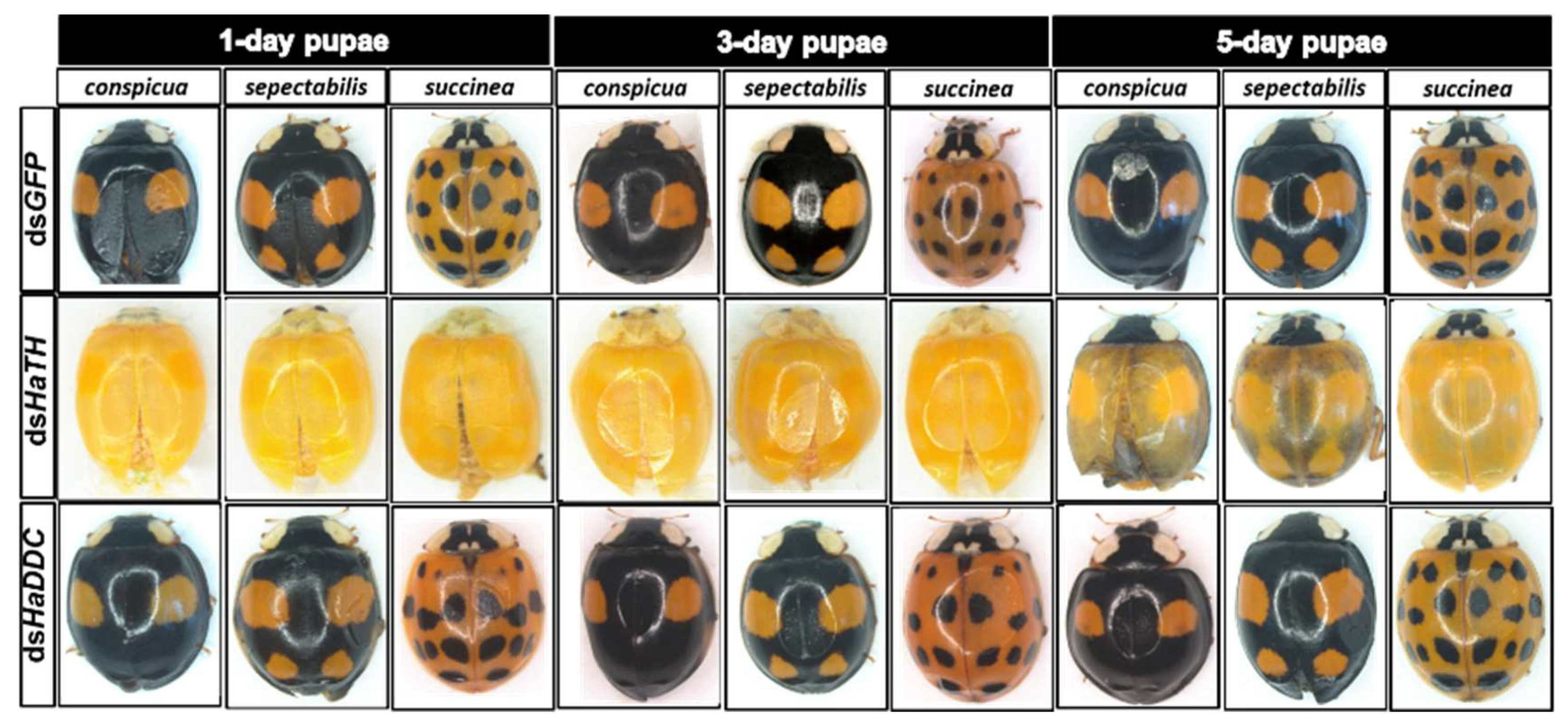
Our previous study examining melanin synthesis pathways suggested that dopamine melanin is the major melanin in the elytra through silencing HaDDC in third instar larvae of H. axyridis [23]. In this study, we continue to investigate how the melanin synthesis pathway contributes to the overall body in H. axyridis. Due to the crucial role of TH in the melanin synthesis pathway, we first silenced HaTH in fourth instar larvae. The transcript levels of HaTH were significantly suppressed on days 2, 4, 6, and 8 after injection (Figure 2a). However, we did not find significant differences of pupation rate between HaTH silenced ladybirds and its control (Figure 2c). HaTH silenced pupae showed obvious abnormal phenotypes with a loss of melanic color throughout the body compared to respective controls (Figure 3). Furthermore, these pale pupae eclosed into the adult stage, but only 20% emerged into normal morphological adults (Figure 2d). This led to approximately 80% of abnormal eclosion, which was manifested with a deformed elytron with the puparium still attached in the body (Figure 4). In addition, melanin in the three subspecies (conspicua, sepectabilis, and succinea) of TH silenced ladybirds totally disappeared compared with their respective controls (Figure 3). We did not observe any movement or feeding in TH silenced ladybirds and they all died within 2 days of eclosion (Figure 2b).
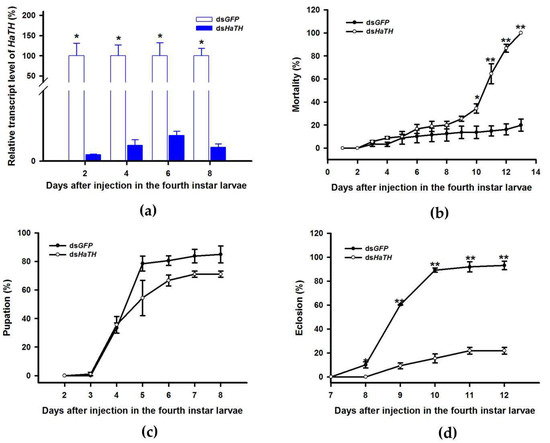
Figure 2.
Time-dependent suppression of HaTH transcript in fourth instar larvae of H. axyridis injected with dsHaTH at 300 ng/larva or dsGFP at 300 ng/larva as determined by RT-qPCR (a), time-dependent mortality (b), pupation rate (c), and eclosion rate (d) in dsHaTH and dsGFP-injected larvae. The relative transcription levels (%) are presented as the mean and SE of three replicates with three insects. There were three replicates in the determination of mortality, pupation rate, and emergence rate, each of which had at least 30 fourth instar larvae. Notability analysis was based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) within the same time point. Asterisk indicates a significant difference in the rate of eclosion and relative transcript level of HaTH between insect injected with dsHaTH and dsGFP (*: significant difference; **: extremely significant difference).
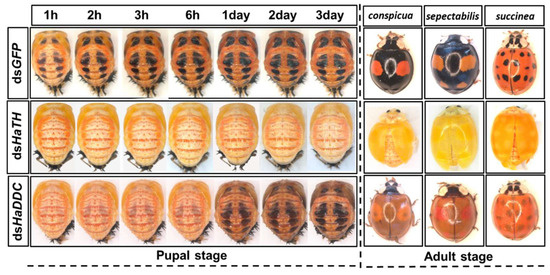
Figure 3.
The phenotypes of abnormal pupae and adults from the fourth instar larvae of H. axyridis injected with dsHaTH and dsHaDDC, respectively.
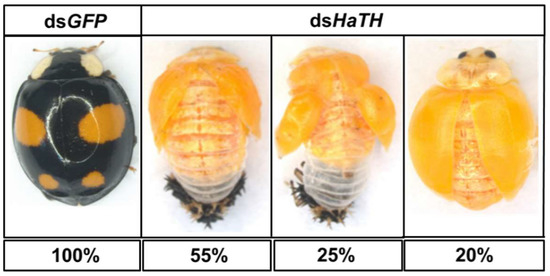
Figure 4.
The phenotypes of an abnormal adult from the fourth instar larvae of H. axyridis injected with dsHaTH.
DOPA decarboxylase (DDC) is the second important inverting enzyme that was investigated in our study. When fourth instar larvae of H. axyridis were injected with dsHaDDC at 300 ng /larva, the transcript levels of HaDDC were significantly suppressed at 2, 4, 6, and 8 days after injection (Figure 5a). However, we did not find significant differences of mortality, pupation rate, and eclosion rate between the HaDDC silenced group and their respective controls (Figure 5b–d). When HaDDC was silenced in fourth instar larvae, all injected ladybirds successfully pupated but melanin was not synthesized until 6h after pupation, later than their respective controls (Figure 3). Furthermore, although these pupae emerged into adults, melanin in the three subspecies (conspicua, sepectabilis, and succinea) of H. axyridis partially disappeared. Although the head and pronotum were normal, the elytral melanin was significantly decreased compared to respective controls (Figure 3).
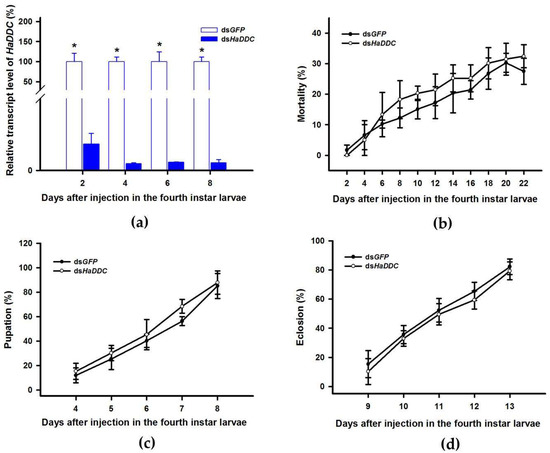
Figure 5.
Time-dependent suppression of HaDDC transcript in fourth instar larvae of H. axyridis injected with dsHaDDC at 300 ng/larva or dsGFP at 300 ng/larva as determined by RT-qPCR (a), time-dependent mortality (b), pupation rate (c), and eclosion rate (d) in dsHaTH and dsGFP-injected larvae. The relative transcription levels (%) are presented as the mean and SE of three replicates with three insects. There were three replicates in the determination of mortality, pupation rate, and emergence rate, each of which had at least 30 fourth instar larvae. Notability analysis was based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) within the same time point. “*” indicates a significant difference in relative transcript level of HaDDC between insect injected with dsHaDDC and dsGFP.
2.3. The Critical Period of Melanin Synthesis in H. axyridis
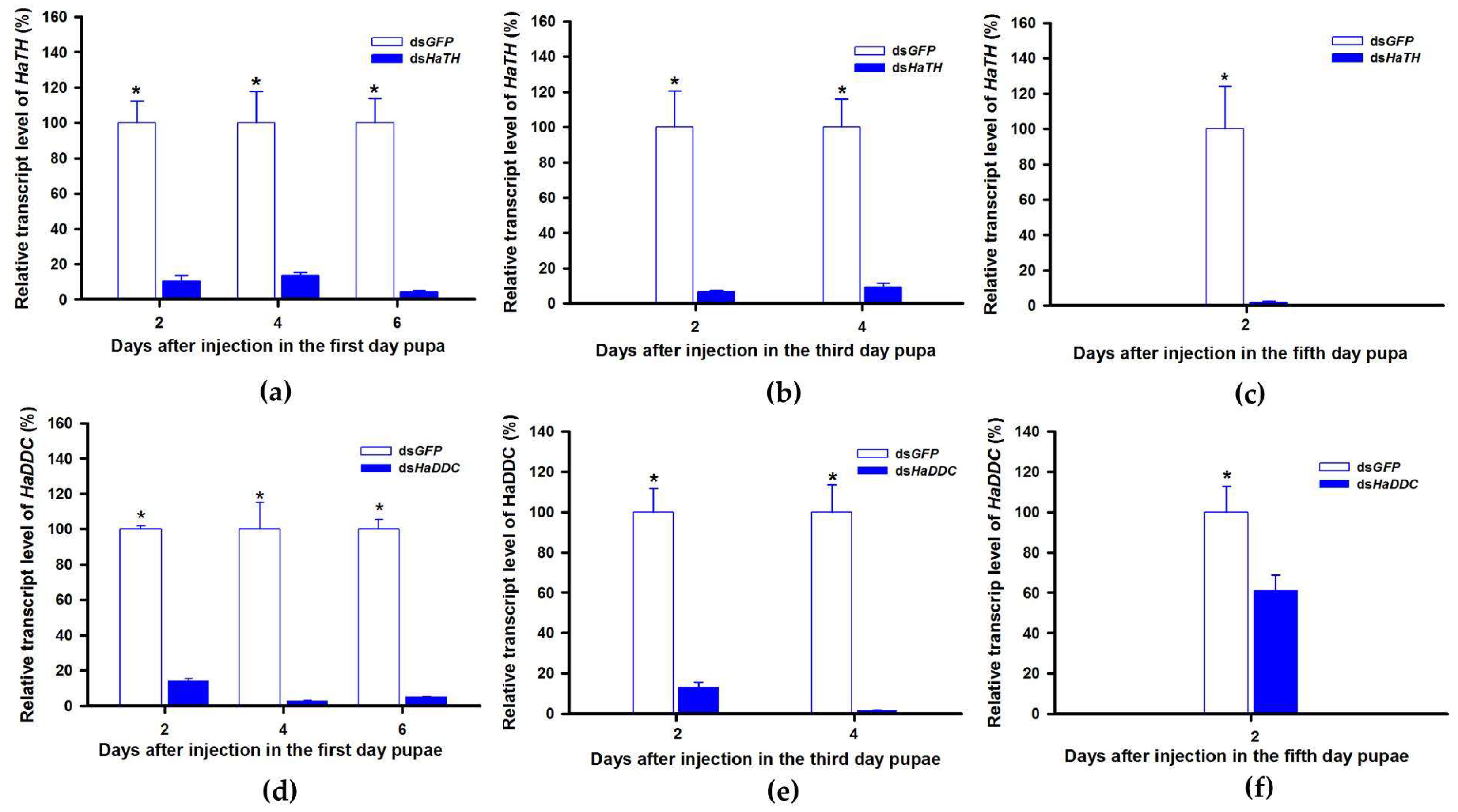
In holometabolous insects, the pupal stage is considered the key material accumulation stage of melanization. To further delimit the critical period of melanin synthesis in H. axyridis, TH and DDC were silenced in 1-, 3-, and 5-day pupae. When the pupae (1-, 3-, and 5-day) were injected with dsHaTH (or dsHaDDC) at 300 ng /pupa, the transcript levels of HaTH (or HaDDC) were significantly suppressed on days 2, 4, and 6 compared to respective controls (Figure 6a–f). For the three subspecies of H. axyridis, 1- and 3-day pupal RNAi targeting TH resulted in complete disappearance of melanin in the overall body. However, when TH was silenced in 5-day pupae, the head and pronotum showed normal melanization with the control, but melanin in the elytra was significantly decreased (Figure 7). We obtained unexpected results with all three subspecies of H. axyridis showing normal melanization in the overall body even though transcript levels of HaDDC were significantly decreased after dsHaDDC injection (Figure 7).
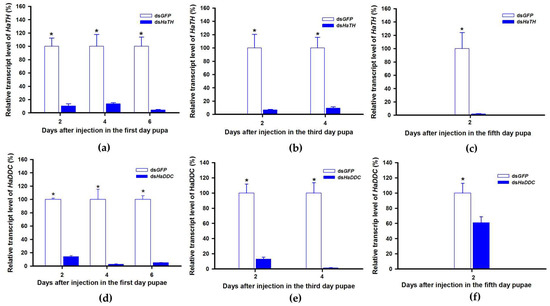
Figure 6.
Time-dependent suppression of HaTH (a–c) and HaDDC (d–f) transcript in the 1-, 3-, and 5-day pupae of H. axyridis injected with dsHaTH and dsHaDDC at 300 ng/pupa or dsGFP at 300 ng/pupa as determined by RT-qPCR, respectively. The relative transcription levels (%) are presented as the mean and SE of three replicates with three insects. Notability analysis was based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) within the same time point. “*” indicates a significant difference in relative transcript level of HaTH and HaDDC between insect injected with dsHaTH (or dsHaDDC) and dsGFP.
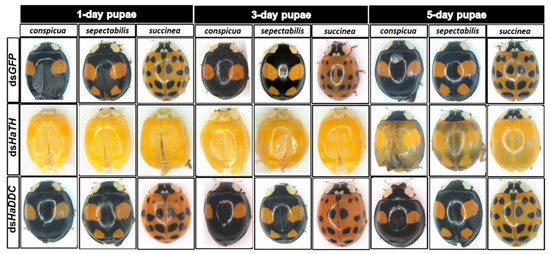
Figure 7.
The phenotypes of adult from the pupae of H. axyridis injected with dsHaTH and dsHaDDC, respectively.
2.4. The Regulatory Mechanism of Transcription Factor Pannier on Melanin Synthesis in H. axyridis
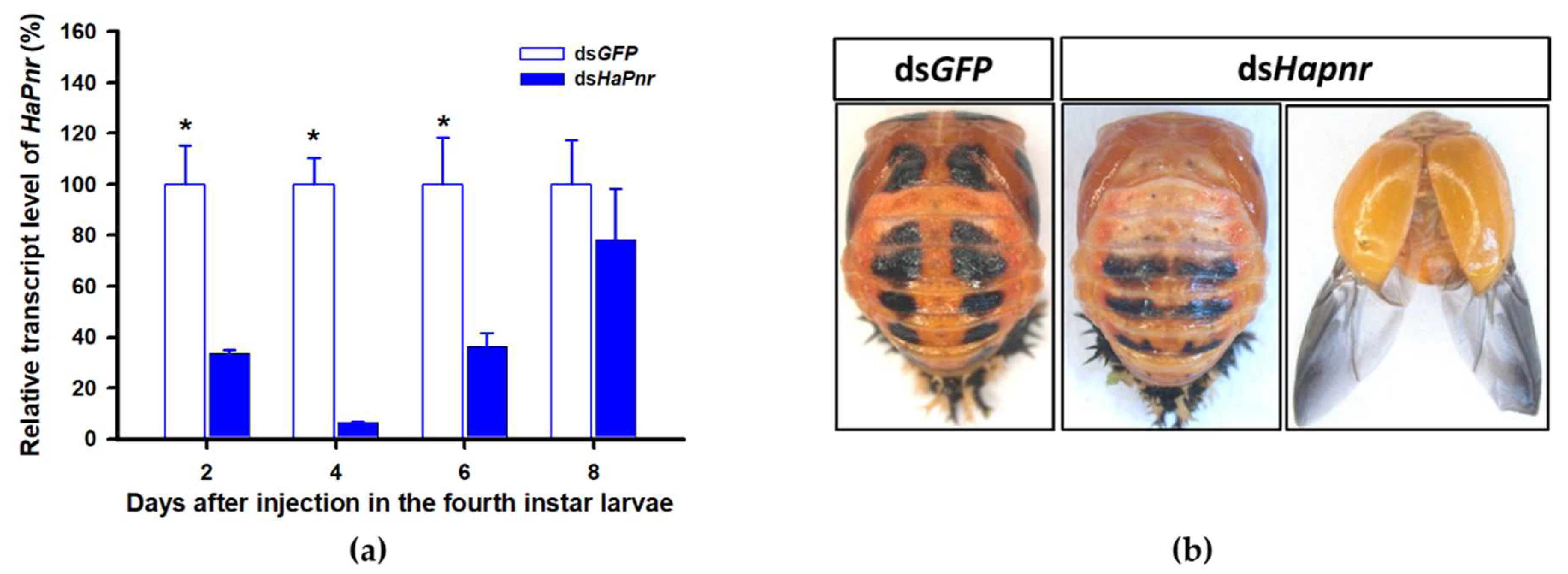
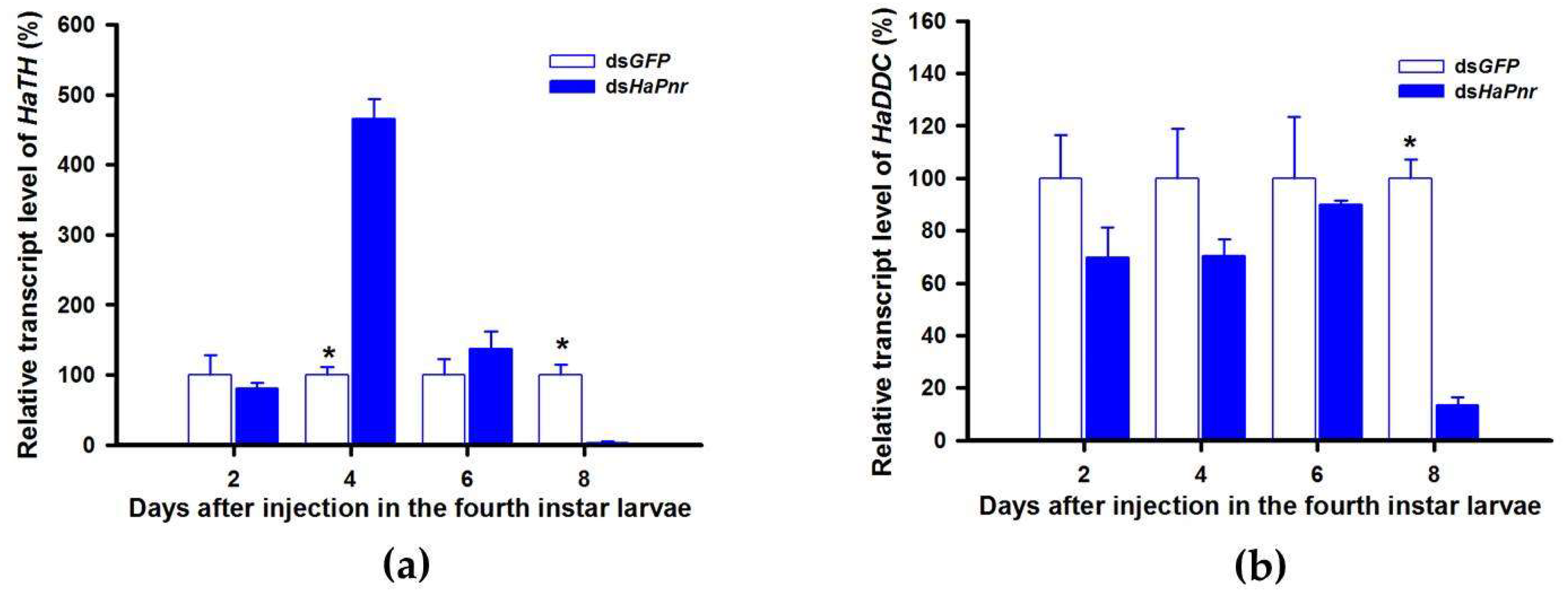
In a recent study, the transcript factor gene Pannier was identified as regulating melanin synthesis during the ontogeny process in H. axyridis [2,3]. To investigate the regulatory mechanism of Pannier on melanin synthesis, we first examined the function of Pannier on development in H. axyridis using RNAi. When fourth instar larvae of H. axyridis were injected with dsHaPnr at 300 ng /larva, transcript levels of HaPnr were significantly suppressed on days 2, 4, 6, and 8 after injection (Figure 8a). The fourth instar larvae of HaPnr that were suppressed successfully pupated, but the pupae showed an abnormal phenotype and melanin was absent from half of the body (Figure 8b). Furthermore, these pupae successfully eclosed into adults, but melanin totally disappeared from the overall body compared to controls (Figure 8b).
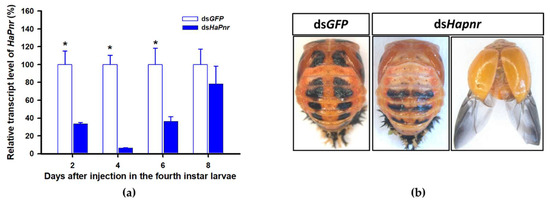
Figure 8.
Time-dependent suppression of HaPnr transcript in the fourth instar larvae of H. axyridis injected with dsHaPnr at 300 ng/larva or dsGFP at 300 ng/larva as determined by RT-qPCR (a) and the phenotype of abnormal pupae and adult from fourth instar larvae injected with dsHaPnr (b). The relative transcription levels (%) are presented as the mean and SE of three replicates with three insects. Notability analysis was based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) within the same time point. ”*” indicates a significant difference in relative transcript level of HaPnr between insect injected with dsHaPnr and dsGFP.
TH and DDC worked as important inverting enzymes, playing essential roles in the dopamine melanin synthesis process. To investigate the regulatory mechanism of Pannier on dopamine melanin synthesis in H. axyridis, we examined expression level of TH and DDC after Pannier was suppressed using RNAi. As mentioned above, results demonstrated that transcript levels of HaPnr were significantly suppressed on days 2, 4, and 6 after fourth instar larvae were injected with dsHaPnr (Figure 8a). These HaPnr suppressed samples were then used to detect expression levels of TH and DDC, and we revealed that expression levels of both TH and DDC were significantly decreased on day 8 after dsHaPnr injection (Figure 9a,b).
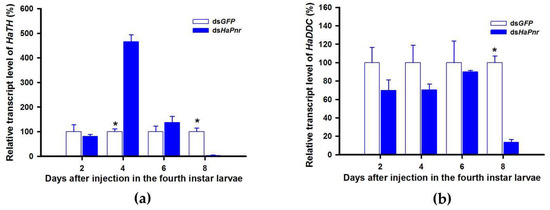
Figure 9.
Relative transcript levels of HaTH (a) and HaDDC (b) following dsHaPnr injection in the fourth instar of H. axyridis. The results are presented as the mean and standard errors of three replicates; each was performed with a RNA sample prepared from three insects. Asterisk above the standard error bars indicate significant differences based on SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) within the same time point. “*” indicates a significant difference in relative transcript level of HaTH and HaDDC between insect injected with dsHaPnr and dsGFP.
3. Discussion
The mechanism of melanin biosynthesis is conserved and has been well characterized in many insects [24,25]. The Asian multi-colored ladybird beetle, H. axyridis, exhibits intraspecific polymorphism based on melanization and is considered as an ideal model insect to investigate population diversity [1]. As previously reported, we have documented that dopamine melanin plays a significant role in elytral melanization of H. axyridis [23]. Recently, two independent studies reported that a GATA transcription factor gene, Pannier, regulates the highly diverse elytral color patterns in H. axyridis [2,3]. Based on these results, we performed gene-silencing analysis of the tyrosine metabolism pathway to investigate the molecular and regulatory mechanism of melanin synthesis in H. axyridis.
3.1. TH Has a Pleiotropic Role in the Development of H. axyridis
The first step in common melanin synthesis pathways is the hydroxylation of tyrosine to produce DOPA, and tyrosine hydroxylation (TH) catalyzes this reaction [17]. Firstly, developmental expression profiles of HaTH were analyzed by RT-qPCR and we revealed that transcripts of HaTH were dramatically increased in 5-day pupae (Figure 1). Several studies have demonstrated that TH is required in cuticle melanization, such as in Drosophila melanogaster (Diptera: Drosophilidae) [26], Tribolium castaneum (Coleoptera: Tenebrionidae) [27], Papilio xuthus (Lepidoptera: Papilionidae) [28], and Bombyx mori (Lepidoptera: Bombycidae) [20]. We obtained similar results with above studies, revealing that HaTH is also involved in melanin synthesis in H. axyridis. Suppression of the expression level of HaTH in fourth instar larvae and pupae (1-, 3-, and 5-day) resulted in the complete loss of melanic color in H. axyridis (Figure 3, Figure 7). In addition, our findings suggested that TH plays a predominant role in ecdysis and adult survival in H. axyridis because when HaTH expression was suppressed, abnormal eclosion occurred and this was lethal in the adult stage (Figure 2, Figure 4). Our results were consistent with previous reports that knockdown of tyrosine hydrolase enzymes drastically affects fundamental physiological processes such as embryogenesis, reproduction, ecdysis, and nymph survival in Rhodnius prolixus (Hemiptera: Reduviidae) [29] and T. castaneum [27]. The comprehensive analysis of this subject, coupled with our results and relative reports, confirms that TH is a pleiotropic gene in the development of insects.
3.2. Melanin in H. axyridis Is Synthetized from Dopamine
In all the insect species studied to date, melanin is primarily synthesized from dopamine, which differs from mammals where melanin is synthetized from DOPA [17,18]. In our previous study, through silencing DDC in third instar larvae, we demonstrated that dopamine melanin was the primary melanin in elytra during ontogenesis of H. axyridis [23]. To comprehensively investigate melanin synthesis in H. axyridis, TH and DDC were selected to be analyzed in fourth instar larvae. Our results showed that suppressed HaTH expression in fourth instar larvae resulted in complete disappearance of melanin in the overall body of pupae and three adult subspecies (Figure 3). These findings suggest that the melanin synthesis based on tyrosine metabolism plays a particularly significant role in the melanization of H. axyridis. Furthermore, when HaDDC was suppressed in fourth instar larvae, melanin was almost entirely absent in pupae and three adult subspecies, with the exception of their head and pronotum (Figure 3). Our results corroborate previous reports on T. castaneum [24], Oncopeltus fasciatus (Hemiptera: Lygaeidae) [30], Periplaneta americana (Blattodea: Blattidae) [31], and R. prolixus [29] that suppressed DDC expression resulting in loss of melanic color in most parts of the body. As described in the conserved melanin synthesis pathway, DDC converts DOPA to dopamine, hence, the enzyme is induced with suppressing and resulted in the irreversible loss of dopamine. Consequently, our results further confirm that dopamine melanin was the primary contributor to melanin synthesis in H. axyridis.
3.3. The Crucial Synthesis time of Dopamine Melanin Precursor in H. axyridis
H. axyridis is a holometabolous insect, having an identical appearance during the larval and pupal stages. The pupa is an important developmental stage in holometabolous insects because it is involved in the dissolving of larval tissue and adult organ reproduction. Ando et al. [3] reported that during elytral development process, the elytral primordia growth occurs in fourth instar larvae and detaches from the pupal exoskeletion and differentiation into adult elytra in the early pupal stage (24h after pupation) [22]. As demonstrated in our results, when TH and DDC were silenced in fourth instar larvae of H. axyridis, an obvious loss in melanic color of adults followed (Figure 3). To confirm if the same scenario is occurring in the pupal stage, further research is required to investigate the characteristic of melanin synthesis through the silencing of TH and DDC in 1-, 3-, and 5-day pupae, respectively. We found silencing TH expression in pupal stages resulted in complete loss of melanic color in the adult stage but silencing DDC resulted in normal adults compared to the control (Figure 7). These unexpected results were consistent with previous reports in T. castaneum that 1- to 2-day old pupae treated with dsTcDDC developed normally and molted successfully into the adult stage.Furthermore, injection of dsTcDDC in 1- to 2-day pupae did not result in any decrease in levels of dopamine [24]. We can therefore speculate the reasons for eclosion of normal adults, even though the remaining HaDDC transcript level was only 1.71% of the control (Figure 6d–f). Firstly, the RNAi technique has limited ability in mRNA expression regulation resulting in target gene residues. In addition, the highest levels of HaDDC transcripts were detected in 1-day pupae of H. axyridis [23]. From these two possible reasons, we can conclude that trace amounts of HaDDC transcript may remain after dsHaDDC injection in 1-day pupae and these residual HaDDC could complete the conversion of DOPA to dopamine. Other studies have demonstrated that melanin pigments are synthesized from non-pigmentary precursors and then expressed in the corresponding region [20]. In the melanin synthesis process, DOPA and dopamine serve as a precursor stored in epidermal cells, ultimately resulting in the melanic body coloration in insects [21]. Thus, we have reasons to believe that surplus HaDDC could complete the conservation of DOPA to dopamine in 1-day pupae (early pupae stage). Subsequently, dopamine served as a precursor stored in dorsal thin epidermis cells and then oxidases to quinone when melanization commences under the effect of phenol oxidase (Laccase 2).
3.4. The Regulation Mechanism of Pannier on Melanin Synthesis in H. axyridis
A review of melanin in H. axyridis concluded that the transcript factor gene Pannier can regulate melanin synthesis during the ontogeny process [22]. The function of Pannier promotes melanin synthesis in the expressed region and suppresses carotenoids in the ventral epidermal cell [2,3]. In the current study, we confirmed that dopamine melanin is the primary contributor to overall body melanization in H. axyridis. It is therefore important to further investigate the regulatory mechanism of Pannier on dopamine melanin synthesis in H. axyridis. Firstly, we investigated the function of Pannier on melanin synthesis in H. axyridis and documented that silencing HaPnr in fourth instar larvae resulted in partial loss of melanic color in pupae and complete loss of melanic color in adults (Figure 8b). Our results were consistent with previous research stating that Pannier is necessary to produce melanic color in H. axyridis [2,3]. Subsequently, expression levels of TH and DDC were detected after HaPnr was suppressed in fourth instar larvae. Our results showed that the expression levels of both TH and DDC were significantly decreased on day 8 after dsHaPnr injection (Figure 9a,b). These results indicated that suppressing the transcript levels of Pannier can significantly decrease expression levels of TH and DDC. These results also support our conclusion that Pannier could regulate the pivotal enzymes TH and DDC in the dopamine melanin synthesis pathway. Importantly, the regulatory mechanism of transcription factors on physiological pathways in further complex studies are therefore necessary to characterize whether other genes participate in the regulatory process.
4. Materials and Methods
4.1. Insects
H. axyridis were collected from cotton fields (GPS location: 39°95′ N, 116°28′ E) at experimental fields of the Beijing Academy of Agriculture and Forestry Sciences (BAAFS), Beijing, China. Insects were reared under standardized conditions following those described by Chen et al. [23]. Three subspecies (conspicua, sepectabolis, and succinea) were common at the field site.
4.2. Total RNA Isolation and cDNA Synthesis
Total RNA was isolated from the insects by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and treated with “PrimeScriptTM RT reagent Kit with gDNA Eraser” (Takara, Dalian, China) to remove genomic DNA and synthesize the first-strand cDNA in a 20 μL reaction system according to the manufacturer’s instructions. The cDNA from untreated samples was used for the synthesis of double-stranded RNA (dsRNA) (dsHaTH, dsHaDDC, dsHaPnr) with specific primers.
4.3. Developmental Stage Expression profiles of HaTH
Developmental-stage-dependent expression profiles of HaTH were analyzed in all stage of H. axyridis at 12 time points, including embryos (1-, 2-, and 3-day eggs), larvae (1, 2, 3, and 4 instar larvae) and pupae (1-,2-, 3-,4-, and 5-day). There are three replicates and for each, 30 eggs, 10 first instar larvae, 3 second instar larvae, and fourth instar larvae and pupae were amalgamated as a biological sample. The total RNA was isolated from the insects by using TRIzol reagent and treated with “PrimeScriptTM RT reagent Kit with gDNA Eraser” (Takara, Dalian, China) to remove genomic DNA and synthesize the first-strand cDNA. Reverse transcription quantitative PCR (RT-qPCR) was performed to analyze the relative transcript levels of HaTH using “SYBR Green with the Applied Biosystems® Real-time PCR Instrument” (ABI Laboratories, Hercules, California, USA). The transcript levels of HaTH were expressed as normalized transcript abundance using the ribosomal protein S49, Harp49 (Accession number: AB552923) as an internal reference gene. The relative HaTH transcript levels were calculated according to the 2−△△Ct method [32].
4.4. Synthesis of Double-stranded RNA (dsRNA)
Specific primers for dsHaTH, dsHaDDC, and dsHaPnr were designed using E-RNAi (http://www.dkfz.de/signaling/e-rnai3/idseq.php) (Table 1). The green fluorescent protein gene (GFP) was used as a control for the RNAi experiments, which was amplified from plasmid (pMD18-T simple Vector) using T7 promoter primers. The first chain of cDNA was amplified by Premix TaqTM (TaKaRa, Dalian, China) with specific primers. The PCR products were purified by QIAquick® PCR Purification Kit (QIAGEN, Hilden, Gemany) and used as templates of double-stranded RNAs (dsRNAs), which were synthesized using “MEGAscript® RNAi Kit” (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions.

Table 1.
Primers used to synthesize dsRNA and analyze transcript levels.
4.5. RNAi Experiments
Fourth instar larvae and pupae from the first, third, and fifth day were injected in the abdomen using Nanoject II injector (Drummond Scientific, Broomall, PA, USA) with 300 ng/individual of dsHaTH. The groups of control insects were treated with same dose. Similarly, dsHaDDC was injected into fourth instar larvae and pupae from the first, third, and fifth day. However, dsHaPnr was only injected in fourth instar larvae to explore the regulatory mechanism of HaPnr on melanin synthesis. The insects injected were fed on Aphis craccivora Koch (Hemiptera: Aphididae) under standard conditions for visual monitoring of phenotypes and other analyses. The RNAi experiment was performed with three biological replicates (each with at least 40 larvae) for each control and treatment.
4.6. Analysis of Expression Profiles by Reverse Transcription Quantitative PCR
To analyze the transcription of HaTH after injection, total RNA of dsHaTH and dsGFP were isolated from whole insects after the second, fourth, sixth, and eighth day. Each time point was analyzed with three biological samples and each sample was run with three technical replications. Total RNA was isolated from the insects using TRIzol reagent. Similar experimental procedures were undertaken on the insects injected in dsHaDDC. Harp49 was used as the reference gene for these two experiments and the synthesis of cDNA was undertaken as previously described. RT-qPCR was performed with three biological replicates, each with three technical replicates. The insects injected with dsHapnr were sampled to test relative transcript levels of Hapnr, HaDDC and HaTH.
4.7. Image Processing
The phenotype of all insects in this experiment were observed and recorded by Zeiss Microscope SteREO Discovery V20 (Carl Zeiss, Oberkochen, Germany) with identical magnification, exposure time and intensity of light.
4.8. Statistical Analysis
The analysis of transcript levels of HaTH, HaDDC and HaPnr in RT-qPCR were expressed as a percentage of the level in controls by dividing the relative expression value (REV) in dsRNA-injected insects by REV in dsGFP-injected insects and multiplying by 100. Percent data from developmental stage analysis and data from the RNAi experiments were arcsine square root transformed prior to analysis via SPSS (v. 22, IBM Corp. Armonk, NY, USA) followed by One-Way ANOVA (p < 0.05) to separate means.
5. Conclusions
In conclusion, our study provides an important new insight into melanization of H. axyridis suggesting that dopamine serves as a melanin precursor and could synthesize in the early pupal stage. Furthermore, we also suggest that the transcription factor Pannier could regulate the expression of TH and DDC in dopamine melanin synthesis pathways to promote melanin synthesis in H. axyridis.
Author Contributions
The individual contributions of this research articles are listed here. Conceptualization, S.W. and D.X.; methodology, X.C., M.W. and R.T.; software, R.T.; validation, F.Z. and L.Z.; formal analysis, R.T. and M.W.; investigation, X.C.; resources, D.X.; data curation, X.C. and R.T.; writing—original draft preparation, D.X. and X.C.; writing—review and editing, J.D.H.; visualization, X.C., D.X. and R.T.; supervision, F.Z.; project administration, D.X.; and funding acquisition, D.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31801794 and the Youth Scientific Research Fund of Beijing Academy of Agricultural and Forestry Sciences, grant number QNJJ201806.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Koch, R.L. The multicolored asian lady beetle, Harmonia axyridis: A review if its biology, uses in biological control, and non-target impacts. J. Insect Sci. 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Yamaguchi, J.; Foucaud, J.; Loiseau, A.; Ausset, A.; Facon, B.; Gschloessl, B.; Lagnel, J.; Loire, E.; Parrinello, H.; et al. The genomic basis of color pattern polymorphism in the harlequin ladybird. Curr. Biol. 2018, 28, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Anto, T.; Matsuda, T.; Goto, K.; Hara, K.; Ito, A.; Hirata, J.; Yatomi, J.; Kajitani, R.; Okuno, M.; Yamaguchi, K.; et al. Repeated inversions within a pannier intron drive diversification of intraspecific colour patterns of ladybird beetles. Nat. Commun. 2018, 9, 3843. [Google Scholar] [CrossRef] [PubMed]
- Bezzerides, A.L.; mCgraw, K.J.; Parker, R.S.; Husseini, J. Elytra color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 2007, 61, 1401–1408. [Google Scholar] [CrossRef]
- Soares, A.O.; Coderre, D.; Schanderl, H. Fitness of two ohenotypes of Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 2001, 98, 287–293. [Google Scholar] [CrossRef]
- Soares, A.O.; Coderre, D.; Schanderl, H. Influence of prey quality on the fitness of two phenotypes of Harmonia axyridis adults. Entomol. Exp. Appl. 2005, 114, 227–232. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, D.; Du, X.; Zhang, F.; Zang, L.; Wang, S. Impact of polymorphism and abiotic conditions on prey consumption by Harmonia axyridis. Entomol. Gen. 2019, 39, 251–258. [Google Scholar] [CrossRef]
- Seo, M.J.; Kim, G.H.; Youn, Y.N. Differences in biological and behavioural characteristics of Harmonia axyridis (Coleoptera: Coccinellidae) according to colour patterns of elytra. J. Appl. Entomol. 2008, 132, 239–247. [Google Scholar] [CrossRef]
- Wang, S.; Michaud, J.P.; Tan, X.L.; Zhang, F.; Guo, X.J. The aggregation behavior of Harmonia axyridis in its native range in northeast China. BioControl 2011, 56, 193–206. [Google Scholar] [CrossRef]
- Wang, S.; Michaud., J.P.; Zhang, R.Z.; Zhang, F. Seasonal cycles of assortative mating and reproductive hebaviour in polymorphic populations of Harmonia axyridis in China. Ecol. Entomol. 2009, 34, 483–494. [Google Scholar] [CrossRef]
- Wang, S.; Michaud, J.P.; Tan, X.; Murray, L.; Zhang, F. Melanism in a chinese population of Harmonia axyridis (Coleoptera: Coccinellidae): A criterion for male investment with pleiotropic effects on behavior and fertility. J. Insect. Behav. 2013, 26, 679–689. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, X.; Wang, W.; Zhang, F.; Desneux, N.; Wang, S. Modification of flight and l ocomotion performances, respiratory metabolism, and trnascriptome expression in the lady beetle Harmonia axyridis through sublethal pesticide exposure. Front. Physiol. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Michie, L.J.; Mallard, F.; Majerus, M.E.N.; Jiggins, F.M. Melanic through nature or nurture: Genetic polymorphism and pehnotypic plasticity in Harmonia axyridis. J. Evol. Biol. 2010, 23, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Michie, L.J.; Masson, A.; Ware, R.L.; Jiggins, F.M. Seasonal phenotypic plasticity: Wild ladybird are darker at cold temperature. Evol. Ecol. 2011, 25, 1259–1268. [Google Scholar] [CrossRef]
- Knapp, M.; Nedved, O. Gender and timing during ontogeny matter: Effects of a temporary high temperature on survival, body size and colouration in Harmonia axyridis. PLoS ONE 2013, 8, e74984. [Google Scholar] [CrossRef] [PubMed]
- Parchem, R.J.; Perry, M.W.; Patel, N.H. Pattern on the insect wing. Curr. Opin. Genet. Dev. 2007, 17, 300–308. [Google Scholar] [CrossRef]
- Barek, H.; Sugumaran, M.; Ito, S.; Wakamatsu, K. Insect cuticular melanins are distinctly different form those of mammalian epidermal melanins. Pigment Cell Melanoma Res. 2017, 31, 384–392. [Google Scholar] [CrossRef]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects ans higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Beldade, P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin. Cell Dev. Biol. 2009, 20, 71. [Google Scholar] [CrossRef]
- Ito, K.; Yoshikawa, M.; Fujii, T.; Tabunoki, H.; Yooyama, T. Melanin pigmentation gives rise to balck spots on the wings of the silkworm Bombyx mori. J. Insect Physiol. 2016, 91–92, 100–106. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Coates, C.J. Re-evaluation of insect melanogenesis research: Views from the dark side. Pigment Cell Melanoma Res. 2017, 30, 386–401. [Google Scholar] [CrossRef]
- Ando, T.; Niimi, T. Development and evolution of color patterns in ladybird beetles: A case study in Harmonia axyridis. Dev. Growth Differ. 2019, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, D.; Du, X.; Guo, X.; Zhang, F.; Desneux, N.; Zang, L.; Wang, S. The role of the dopamine melanin pathway in the ontogeny of elytral melanization in Harmonia axyridis. Front. Physiol. 2019, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Lomakin, J.; Beeman, R.W.; Muthukrishnan, S.; Gehrke, S.H.; Kanost, M.R.; Kramer, K.J. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J. Biol. Chem. 2009, 284, 16584–16594. [Google Scholar] [CrossRef]
- Fuahashi, R.; Sato, J.; Meng, Y.; Okamoto, S.; Daimon, T.; Yamamoto, K.; Suetsugu, Y.; Narukawa, J.; Takahashi, H.; Banno, Y.; et al. yellow and ebony are the responsible genes for the larval color mutants of the silkworm Bombyx mori. Genetics 2008, 180, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- True, J.R.; Edwards, K.A.; Yamamoto, D.; Carroll, S.B. Drosophila wing melanin pattern form by vein-dependent elaboration of enzymatic prepatterns. Curr. Biol. 1999, 9, 1382–1391. [Google Scholar] [CrossRef]
- Gorman, M.J.; Arakane, Y. Tyrosine hydroxylase is required for cuticle sclerotization and pigmentation in Tribilium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 267–273. [Google Scholar] [CrossRef]
- Futahashi, R.; Fujiwara, H. Melanin-synthesis enzymes coregulate stage-specific larval cuticular marking in the swallowtail butterfly, Papilio xuthus. Dev. Genes Evol. 2005, 215, 519–529. [Google Scholar] [CrossRef]
- Sterkel, M.; Ons, S.; Oliveira, P.L. DOPA decarboxylase is essential for cuticle tanning in Rhodnius prolixus (Hemiptera: Reduviidae), affecting ecdysis, survival and reproduction. Insect Biochem. Mol. Biol. 2019, 108, 24–31. [Google Scholar] [CrossRef]
- Liu, J.; Lemonds, T.R.; Popadic, A. The genetic control of aposematic black pigmentation in hemimetabolous insects: Insights from Oncopeltus fasciatus. Evol. Dev. 2014, 16, 270–277. [Google Scholar] [CrossRef]
- Lemonds, T.R.; Liu, J.; Popadic, A. The contributuion of the melanin pathway to overall body pigmentation during ontogenesis of Periplaneta americana. Insect Sci. 2016, 23, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Liang, X.; Gao, X.; Yao, J.; Zhu, K.Y. The lethal giant larvae gene in Tribolium castaneum: Molecular properties and roles in larval and pupal development as revealed by RNA interference. Intern. J. Mol. Sci. 2014, 15, 6880–6896. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).