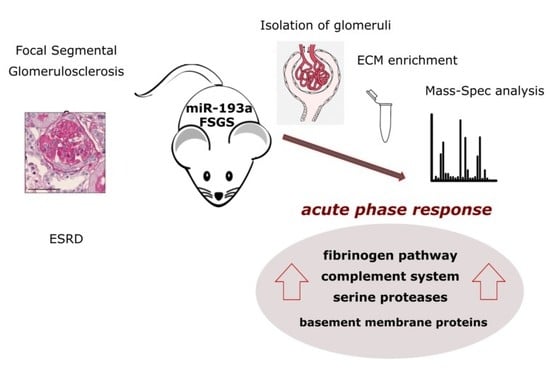
ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Transgenic Mice
4.2. Isolation of Mouse Glomeruli and ECM
4.3. Glomerular ECM Sample Preparation for Analysis by Mass Spectrometry
4.4. Hybrid Quadrupole–Orbitrap Mass Spectrometry
4.5. Data Processing for Protein Identification and Quantification
4.6. RNA Data Analysis and Human Data
4.7. Histology and Antibody Stainings
4.8. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin converting enzyme 2 |
| AGC | automatic gain control |
| APOA1 | Apolipoprotein A1 |
| BMP | bone morphogenic protein |
| CFP | properdin |
| Col1a1 | collagen 1 |
| COL12A1 | collagen type XII, alpha-1 chain |
| COL3A1 | collagen type III, alpha-1 chain |
| CRP | C-reactive protein |
| C1R | complement C1r |
| C4B | complement C4-B |
| DDA | data-dependent acquisition |
| DTT | dithiotreitol, 1,4-bis-(sulfanil)-butan-2,3-diol |
| ECM | extra-cellular matrix |
| ESRD | end-stage renal disease |
| FGA, FGB, FGG | fibrinogen α, β and γ chains |
| FSGS | focal segmental glomerulosclerosis |
| GBM | glomerular basement membrane |
| HBA1 | α-globin |
| HBB-B1 | β1-globin |
| HBB-B2 | β2-globin |
| HBSS | Hank’s Balanced Salt Solution |
| HCD | high energy collision dissociation |
| HDGFL3, HRP3 | Hepatoma-Derived Growth Factor-Related Protein 3 |
| HDL | high-density lipoprotein |
| HPLC-MS | high-performance liquid chromatography mass spectrometry |
| ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 |
| IGF | insulin-like growth factor |
| IGFBP1 | insulin growth factor binding protein 1 |
| MS | mass spectrometry |
| NCE | normalized collision energy |
| NFκB | nuclear factor κB |
| PIGR | Polymeric immunoglobulin receptor |
| PPT1 | Palmitoyl-protein thioesterase 1 |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| TFA | trifluoro acetic acid |
| TGF-β | transformin growth factor beta |
| TLR4 | Toll-like receptor 4 |
| SERPINA1 | serine proteinase A1 |
| SERPINA3 | serine proteinase A3 |
| SFTPD | pulmonary surfactant-associated protein D |
| SOST | sclerostin |
| SPP1 | osteopontin |
| TINAG | tubulointerstitial nephritis antigen |
| UMOD | Uromodulin, Tamm-Horsfall protein |
| VEGFA | vascular endothelial cell growth factor-alpha |
| WT1 | Wilms’ tumor 1 |
References
- Lennon, R.; Randles, M.J.; Humphries, M.J. The Importance of Podocyte Adhesion for a Healthy Glomerulus. Front. Endocrinol. 2014, 5, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, W. Genetic basis of adult-onset nephrotic syndrome and focal segmental glomerulosclerosis. Front. Med. 2017, 11, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Randles, M.J.; Humphries, M.J.; Lennon, R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017, 57–58, 12–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Sonnenberg, A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013, 9, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Voziyan, P.A.; Hudson, B.G.; Zent, R. Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr. Pharm. Des. 2009, 15, 1318–1333. [Google Scholar] [CrossRef]
- Randles, M.J.; Woolf, A.S.; Huang, J.L.; Byron, A.; Humphries, J.D.; Price, K.L.; Kolatsi-Joannou, M.; Collinson, S.; Denny, T.; Knight, D.; et al. Genetic Background is a Key Determinant of Glomerular Extracellular Matrix Composition and Organization. J. Am. Soc. Nephrol. 2015, 26, 3021–3034. [Google Scholar] [CrossRef]
- Fogo, A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2014, 11, 76–87. [Google Scholar] [CrossRef]
- Jefferson, J.A.; Shankland, S.J. The Pathogenesis of Focal Segmental Glomerulosclerosis. Adv. Chronic Kidney Dis. 2014, 21, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Malaga-Dieguez, L.; Bouhassira, D.; Gipson, D.; Trachtman, H. Novel Therapies for FSGS: Preclinical and Clinical Studies. Adv. Chronic Kidney Dis. 2015, 22, e1–e6. [Google Scholar] [CrossRef] [Green Version]
- Lennon, R.; Byron, A.; Humphries, J.D.; Randles, M.J.; Carisey, A.; Murphy, S.; Knight, D.; Brenchley, P.E.; Zent, R.; Humphries, M.J. Global Analysis Reveals the Complexity of the Human Glomerular Extracellular Matrix. J. Am. Soc. Nephrol. 2014, 25, 939–951. [Google Scholar] [CrossRef] [Green Version]
- Byron, A.; Randles, M.J.; Humphries, J.D.; Mironov, A.; Hamidi, H.; Harris, S.; Mathieson, P.W.; Saleem, M.A.; Satchell, S.C.; Zent, R.; et al. Glomerular Cell Cross-Talk Influences Composition and Assembly of Extracellular Matrix. J. Am. Soc. Nephrol. 2014, 25, 953–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobeika, L.; Barati, M.T.; Caster, D.J.; McLeish, K.R.; Merchant, M.L. Characterization of glomerular extracellular matrix by proteomic analysis of laser-captured microdissected glomeruli. Kidney Int. 2017, 91, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebeshuber, C.A.; Kornauth, C.; Dong, L.; Sierig, R.; Seibler, J.; Reiss, M.; Tauber, S.; Bilban, M.; Wang, S.; Kain, R.; et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat. Med. 2013, 19, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteom. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-H.; Li, K.-J.; Yu, C.-L.; Tsai, C.-Y. Tamm-Horsfall Protein is a Potent Immunomodulatory Molecule and a Disease Biomarker in the Urinary System. Molecules 2018, 23, 200. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, M.E.; Wang, W.; Caberoy, N.B.; Chen, X.; Guo, F.; Alvarado, G.; Shen, C.; Wang, F.; Wang, H.; Chen, R.; et al. Hepatoma-derived growth factor-related protein-3 is a novel angiogenic factor. PLoS ONE 2015, 10, e0127904. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.R.; Czech, K.A.; Arend, L.J.; Witte, D.P.; Devarajan, P.; Potter, S.S. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp. Nephrol. 2007, 107, e30–e40. [Google Scholar] [CrossRef]
- Strassheim, D.; Renner, B.; Panzer, S.; Fuquay, R.; Kulik, L.; Ljubanović, D.; Holers, V.M.; Thurman, J.M. IgM contributes to glomerular injury in FSGS. J. Am. Soc. Nephrol. 2013, 24, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Thurman, J.M.; Wong, M.; Renner, B.; Frazer-Abel, A.; Giclas, P.C.; Joy, M.S.; Jalal, D.; Radeva, M.K.; Gassman, J.; Gipson, D.S.; et al. Complement Activation in Patients with Focal Segmental Glomerulosclerosis. PLoS ONE 2015, 10, e0136558. [Google Scholar] [CrossRef] [Green Version]
- Heybeli, C.; Oktan, M.A.; Yıldız, S.; Ünlü, M.; Celik, A.; Sarıoglu, S. Mesangial C4d deposition is independently associated with poor renal survival in patients with primary focal segmental glomerulosclerosis. Clin. Exp. Nephrol. 2019, 23, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, X.; Zhang, Y.; Ma, X.; Li, B.; Zhang, S.; Du, P.; Zhang, X.; Yi, F. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell. Physiol. Biochem. 2009, 24, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Rittirsch, D.; Flierl, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction between the coagulation and complement system. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar] [PubMed] [Green Version]
- Auger, J.L.; Haasken, S.; Binstadt, B.A. Autoantibody-mediated arthritis in the absence of C3 and activating Fcγ receptors: C5 is activated by the coagulation cascade. Arthritis Res. Ther. 2012, 14, R269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, S.; Jurk, K.; Hobohm, L.; Jäckel, S.; Saffarzadeh, M.; Schwierczek, K.; Wenzel, P.; Langer, F.; Reinhardt, C.; Ruf, W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood 2017, 129, 2291–2302. [Google Scholar] [CrossRef] [Green Version]
- Rubel, C.; Fernández, G.C.; Dran, G.; Bompadre, M.B.; Isturiz, M.A.; Palermo, M.S. Fibrinogen promotes neutrophil activation and delays apoptosis. J. Immunol. 2001, 166, 2002–2010. [Google Scholar] [CrossRef] [Green Version]
- Banas, M.C.; Banas, B.; Hudkins, K.L.; Wietecha, T.A.; Iyoda, M.; Bock, E.; Hauser, P.; Pippin, J.W.; Shankland, S.J.; Smith, K.D.; et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J. Am. Soc. Nephrol. 2008, 19, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Sörensen, I.; Susnik, N.; Inhester, T.; Degen, J.L.; Melk, A.; Haller, H.; Schmitt, R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011, 80, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zheng, C.; Xu, X.; Zhao, Y.; Lu, Y.; Liu, Z. Fibrinogen links podocyte injury with Toll-like receptor 4 and is associated with disease activity in FSGS patients. Nephrology 2018, 23, 418–429. [Google Scholar] [CrossRef]
- Róka, B.; Tod, P.; Kaucsár, T.; Vizovišek, M.; Vidmar, R.; Turk, B.; Fonović, M.; Szénási, G.; Hamar, P. The Acute Phase Response Is a Prominent Renal Proteome Change in Sepsis in Mice. Int. J. Mol. Sci. 2019, 21, 200. [Google Scholar] [CrossRef] [Green Version]
- Schneeman, T.A.; Bruno, M.E.C.; Schjerven, H.; Johansen, F.-E.; Chady, L.; Kaetzel, C.S. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: Linking innate and adaptive immune responses. J. Immunol. 2005, 175, 376–384. [Google Scholar] [CrossRef]
- Narita, I.; Kondo, D.; Goto, S.; Saito, N.; Watanabe, Y.; Yamazaki, H.; Sakatsume, M.; Saito, A.; Gejyo, F. Association of gene polymorphism of polymeric immunoglobulin receptor and IgA nephropathy. Intern. Med. 2001, 40, 867–872. [Google Scholar] [CrossRef] [Green Version]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Fornoni, A.; Merscher, S.; Kopp, J.B. Lipid biology of the podocyte--new perspectives offer new opportunities. Nat. Rev. Nephrol. 2014, 10, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Hellin, J.; Cantarell, C.; Jimeno, L.; Sanchez-Fructuoso, A.; Puig-Gay, N.; Guirado, L.; Vilariño, N.; Gonzalez-Roncero, F.M.; Mazuecos, A.; Lauzurica, R.; et al. A form of apolipoprotein a-I is found specifically in relapses of focal segmental glomerulosclerosis following transplantation. Am. J. Transpl. 2013, 13, 493–500. [Google Scholar] [CrossRef]
- Puig-Gay, N.; Jacobs-Cacha, C.; Sellarès, J.; Guirado, L.; González Roncero, F.; Jiménez, C.; Zárraga, S.; Paul, J.; Lauzurica, R.; Alonso, Á.; et al. Apolipoprotein A-Ib as a biomarker of focal segmental glomerulosclerosis recurrence after kidney transplantation: Diagnostic performance and assessment of its prognostic value—A multi-centre cohort study. Transpl. Int. 2019, 32, 313–322. [Google Scholar] [CrossRef]
- Freedman, B.I.; Kopp, J.B.; Langefeld, C.D.; Genovese, G.; Friedman, D.J.; Nelson, G.W.; Winkler, C.A.; Bowden, D.W.; Pollak, M.R. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J. Am. Soc. Nephrol. 2010, 21, 1422–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Shui, H.-A.; Ka, S.-M.; Yang, S.-M.; Lin, Y.-F.; Lo, Y.-F.; Chen, A. Osteopontin as an injury marker expressing in epithelial hyperplasia lesions helpful in prognosis of focal segmental glomerulosclerosis. Transl. Res. 2007, 150, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Taranta-Janusz, K.; Kuroczycka-Saniutycz, E.; Zoch-Zwierz, W. Urinary OPN excretion in children with glomerular proteinuria. Adv. Med. Sci. 2011, 56, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Al Shawaf, E.; Abu-Farha, M.; Devarajan, S.; Alsairafi, Z.; Al-Khairi, I.; Cherian, P.; Ali, H.; Mathur, A.; Al-Mulla, F.; Al Attar, A.; et al. ANGPTL4: A Predictive Marker for Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 4943191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayanthooran, S.; Magana-Arachchi, D.N.; Gunerathne, L.; Abeysekera, T. Potential diagnostic biomarkers for chronic kidney disease of unknown etiology (CKDu) in Sri Lanka: A pilot study. BMC Nephrol. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Stolz, D.B.; Kiss, L.P.; Monga, S.P.; Holzman, L.B.; Liu, Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 2009, 20, 1997–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S. Mechanisms and consequences of TGF-ß overexpression by podocytes in progressive podocyte disease. Cell Tissue Res. 2012, 347, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisternas, P.; Vio, C.P.; Inestrosa, N.C. Role of Wnt signaling in tissue fibrosis, lessons from skeletal muscle and kidney. Curr. Mol. Med. 2014, 14, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.M.; Pullen, N.; Johnson, T.S. An inhibitor of thrombin activated fibrinolysis inhibitor (TAFI) can reduce extracellular matrix accumulation in an in vitro model of glucose induced ECM expansion. Matrix Biol. 2013, 32, 277–287. [Google Scholar] [CrossRef]
- Takemoto, M.; Asker, N.; Gerhardt, H.; Lundkvist, A.; Johansson, B.R.; Saito, Y.; Betsholtz, C. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 2002, 161, 799–805. [Google Scholar] [CrossRef] [Green Version]



| ID | Symbol | Gene Name | FC |
|---|---|---|---|
| P11680 | CFP | Properidin | * |
| F8WJ05 | ITIH1 | Inter-alpha-trypsin inhibitor 1 | * |
| O70570 | PIGR | Polymeric Immunoglobulin Receptor | * |
| P50404 | SFTPD | Pulmonary surfactant-associated protein D | * |
| Q566I6 | C1R | Complement C1r | 27.8 |
| A8DUV3 | HBA1 | Hemoglobin subunit alpha 1 | 18.9 |
| Q3UER8 | FGG | Fibrinogen gamma chain | 15.4 |
| Q3TGR2 | FGB | Fibrinogen beta chain | 10.0 |
| Q91X17 | UMOD | Uromodulin | 8.8 |
| Q54AH9 | HBB-B2 | Hemoglobin subunit beta-2 | 8.7 |
| Q542I3 | CRP | C-reactive protein | 7.6 |
| A8DUK0 | HBB-B1 | Hemoglobin subunit beta-1 | 7.4 |
| P01029 | C4B | Complement C4-B | 6.2 |
| P07759 | SERPINA3 | Serine protease inhibitor A3K | 6.1 |
| A0A2P9DUN6 | SERPINA1 | Alpha-1-antitrypsin 1 | 5.6 |
| Q00623 | APOA1 | Apolipoprotein A-I | 5.3 |
| P11087 | COL1A1 | Collagen alpha-1(I) chain | 4.9 |
| Q8VBX5 | PPT1 | Palmitoyl-protein thioesterase 1 | 4.8 |
| Q9JMG7-2 | HDGFL3 | Hepatoma-derived growth factor-related protein 3 | −5.2 |
| O55186 | CD59A | CD59A glycoprotein | −5.7 |
| Gene Name | Symbol | FC in Mouse 1 | FC in Human 2 | Description |
|---|---|---|---|---|
| Insulin Like Growth Factor Binding Protein 1 | IGFBP1 | 100.6 | 9.1 | IGF signaling stimulator |
| Angiotensin I Converting Enzyme 2 | ACE2 | 95.7 | 5.9 | vasoconstrictor, hypertension |
| Tubulointerstitial Nephritis Antigen | TINAG | 65.4 | 4.3 | glycoprotein |
| Defensin Beta 1 | DEFB1 | 52.0 | 11.6 | microbicidal, cytotoxic |
| Uromodulin | UMOD | 44.2 | 51.3 | calcium crystallization inhibitor, immune response |
| Phospholipase A1 Member A | PLA1A | 42.2 | 5.0 | phospholipase, signal transduction |
| Complement Factor I | CFI | 37.9 | 6.9 | complement cascade regulator |
| Serpin Family A Member 1f | SERPINA1 | 28.3 | 6.3 | coagulation cascade |
| Osteopontin | SPP1 | 26.2 | 122.8 | cell signaling modulator (inflammation, TGF-β, Wnt) |
| Kininogen 1 | KNG1 | 26.1 | 26.3 | coagulation cascade |
| EPH Receptor B2 | EPHB2 | 20.8 | 3.2 | ephrin signaling |
| Lumican | LUM | 17.6 | 45.5 | proteoglycan |
| Secreted Frizzled Related Protein 1 | SFRP1 | 17.3 | 16.6 | Wnt signaling modulator |
| Reelin | RELN | 16.1 | 6.1 | cell positioning/migration |
| WAP Four-Disulfide Core Domain 2 | WFDC2 | 15.2 | 3.5 | protease inhibitor |
| Complement Factor B | CFB | 12.8 | 7.0 | complement cascade regulator |
| Secretogranin V | SCG5 | 9.5 | 9.3 | chaperone |
| Fibulin 1 | FBLN1 | 8.4 | 3.1 | glycoprotein |
| Mucin 1 | MUC1 | 8.0 | 3.8 | signal transduction |
| Complement C7 | C7 | 7.5 | 5.3 | complement cascade regulator |
| Semaphorin 4D | SEMA4D | 6.9 | 5.0 | glycoprotein, cell signaling |
| C-X-C Motif Chemokine Ligand 14 | CXCL14 | 6.9 | 29.2 | cytokine, immune response |
| Tissue Factor Pathway Inhibitor 2 | TFPI2 | 6.9 | 3.5 | coagulation cascade |
| Laminin Subunit Gamma 2 | LAMC2 | 6.4 | 3.3 | structural glycoprotein |
| Stanniocalcin 1 | STC1 | 6.0 | 4.4 | calcium/phosphate balance regulator |
| Glypican 4 | GPC4 | 5.0 | 4.4 | Glycoprotein |
| Clusterin | CLU | 4.9 | 9.0 | Chaperone |
| Serine Protease 23 | PRSS23 | 4.8 | 25.7 | serine protease |
| Insulin Like Growth Factor Binding Protein 3 | IGFBP3 | 4.4 | 10.5 | IGF signaling stimulator |
| Collagen Triple Helix Repeat Containing 1 | CTHRC1 | 3.9 | 19.6 | vascular remodeling, Wnt signaling |
| Asporin | ASPN | 3.9 | 5.4 | calcium-binding, TGF-β inhibitor |
| N-Acetylgalactosaminyltransferase 12 | GALNT12 | 3.9 | 16.3 | protein glycosylation |
| Sushi, Nidogen And EGF Like Domains 1 | SNED1 | 0.27 | 0.33 | insulin-responsive |
| Phospholipase A2 Receptor 1 | PLA2R1 | 0.25 | 0.17 | phospholipase signaling |
| Vascular Endothelial Growth Factor A | VEGFA | 0.16 | 0.19 | proliferation, migration, survival |
| Collagen Type IV Alpha 3 Chain | COL4A3 | 0.15 | 0.20 | structural glycoprotein |
| Sclerostin | SOST | 0.02 | 0.14 | cell signaling (Wnt, TGF-β) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukosza, E.N.; Kornauth, C.; Hummel, K.; Schachner, H.; Huttary, N.; Krieger, S.; Nöbauer, K.; Oszwald, A.; Razzazi Fazeli, E.; Kratochwill, K.; et al. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. Int. J. Mol. Sci. 2020, 21, 2095. https://doi.org/10.3390/ijms21062095
Bukosza EN, Kornauth C, Hummel K, Schachner H, Huttary N, Krieger S, Nöbauer K, Oszwald A, Razzazi Fazeli E, Kratochwill K, et al. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. International Journal of Molecular Sciences. 2020; 21(6):2095. https://doi.org/10.3390/ijms21062095
Chicago/Turabian StyleBukosza, Eva Nora, Christoph Kornauth, Karin Hummel, Helga Schachner, Nicole Huttary, Sigurd Krieger, Katharina Nöbauer, André Oszwald, Ebrahim Razzazi Fazeli, Klaus Kratochwill, and et al. 2020. "ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS" International Journal of Molecular Sciences 21, no. 6: 2095. https://doi.org/10.3390/ijms21062095
APA StyleBukosza, E. N., Kornauth, C., Hummel, K., Schachner, H., Huttary, N., Krieger, S., Nöbauer, K., Oszwald, A., Razzazi Fazeli, E., Kratochwill, K., Aufricht, C., Szénási, G., Hamar, P., & Gebeshuber, C. A. (2020). ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. International Journal of Molecular Sciences, 21(6), 2095. https://doi.org/10.3390/ijms21062095