Environmental Signals on Microglial Function during Brain Development, Neuroplasticity, and Disease
Abstract
:1. Introduction
2. Neuroinflammatory Cross-Talk in Response to Brain Lesions and Plasticity
2.1. Neuroinflammation and Brain Injury in the Adult CNS
2.2. Neuroinflammation and Microglial Function in Lesion Recovery during the Critical Period of Brain Development
2.3. Neuroinflammation and Microglial Function in Infectious Conditions
2.4. Neuroinflammation in Fetal Alcohol Spectrum Disorder (FASD)
2.5. Neuroinflammation in Congenital Hypothyroidism
3. The Cross-Talk between Diet, Microglia, and the Endocannabinoid System
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Herz, J.; Filiano, A.J.; Smith, A.; Yogev, N.; Kipnis, J. Myeloid Cells in the Central Nervous System. Immunity 2017, 46, 943–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mildner, A.; Schmidt, H.; Nitsche, M.; Merkler, D.; Hanisch, U.K.; Mack, M.; Heikenwalder, M.; Bruck, W.; Priller, J.; Prinz, M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007, 10, 1544–1553. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [Green Version]
- Wakselman, S.; Bechade, C.; Roumier, A.; Bernard, D.; Triller, A.; Bessis, A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8138–8143. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martinez-Cerdeno, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [Green Version]
- Pont-Lezica, L.; Beumer, W.; Colasse, S.; Drexhage, H.; Versnel, M.; Bessis, A. Microglia shape corpus callosum axon tract fasciculation: Functional impact of prenatal inflammation. Eur. J. Neurosci. 2014, 39, 1551–1557. [Google Scholar] [CrossRef]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551. [Google Scholar] [CrossRef]
- Marin-Teva, J.L.; Dusart, I.; Colin, C.; Gervais, A.; van Rooijen, N.; Mallat, M. Microglia promote the death of developing Purkinje cells. Neuron 2004, 41, 535–547. [Google Scholar] [CrossRef]
- Weinhard, L.; di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; Nabekura, J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Cho, M.H.; Shim, W.H.; Kim, J.K.; Jeon, E.Y.; Kim, D.H.; Yoon, S.Y. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 2017, 22, 1576–1584. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [Green Version]
- Hagemeyer, N.; Hanft, K.M.; Akriditou, M.A.; Unger, N.; Park, E.S.; Stanley, E.R.; Staszewski, O.; Dimou, L.; Prinz, M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017, 134, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Du, X.F.; Liu, C.S.; Wen, Z.L.; Du, J.L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Eyo, U.B.; Bispo, A.; Liu, J.; Sabu, S.; Wu, R.; DiBona, V.L.; Zheng, J.; Murugan, M.; Zhang, H.; Tang, Y.; et al. The GluN2A Subunit Regulates Neuronal NMDA receptor-Induced Microglia-Neuron Physical Interactions. Sci. Rep. 2018, 8, 828. [Google Scholar] [CrossRef] [Green Version]
- Pósfai, B.; Cserép, C.; Orsolits, B.; Dénes, Á. New Insights into Microglia-Neuron Interactions: A Neuron’s Perspective. Neuroscience 2019, 405, 103–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyo, U.B.; Wu, L.J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013, 2013, 456857. [Google Scholar] [CrossRef] [PubMed]
- Hoshiko, M.; Arnoux, I.; Avignone, E.; Yamamoto, N.; Audinat, E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J. Neurosci. Off. J. Soc. Neurosc. 2012, 32, 15106–15111. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef]
- Beggs, S.; Trang, T.; Salter, M.W. P2X4R + microglia drive neuropathic pain. Nat. Neurosci. 2012, 15, 1068–1073. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Doran, S.J.; Ritzel, R.M.; Glaser, E.P.; Henry, R.J.; Faden, A.I.; Loane, D.J. Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. J. Neurotrauma 2019, 36, 1040–1053. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Ajdacic-Gross, V.; Aleksandrowicz, A.; Rodgers, S.; Mutsch, M.; Tesic, A.; Muller, M.; Kawohl, W.; Rossler, W.; Seifritz, E.; Castelao, E.; et al. Infectious, atopic and inflammatory diseases, childhood adversities and familial aggregation are independently associated with the risk for mental disorders: Results from a large Swiss epidemiological study. World J. Psychiatry 2016, 6, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Yazdani, U.; Gadad, B.; Zaman, S.; Hynan, L.S.; Roatch, N.; Schutte, C.; Marti, C.N.; Hewitson, L.; German, D.C. Serum thyroid-stimulating hormone and interleukin-8 levels in boys with autism spectrum disorder. J. Neuroinflamm. 2017, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.H.; Bjorke-Monsen, A.L.; Teixeira, A.L.; Silverman, M.N. Maternal stress, nutrition and physical activity: Impact on immune function, CNS development and psychopathology. Brain Res. 2015, 1617, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, L.S.; Schwarz, J.M. Effects of Moderate Prenatal Alcohol Exposure during Early Gestation in Rats on Inflammation across the Maternal-Fetal-Immune Interface and Later-Life Immune Function in the Offspring. J. Neuroimmune Pharmacol. 2016, 11, 680–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; Spinelli, C.C.; Martucciello, S.; Nori, S.L.; Capunzo, M.; Puca, A.A.; Ciaglia, E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018, 103, 509–524. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Schwarz, J.M. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilhardt, F. Microglia: Phagocyte and glia cell. Int. J. Biochem. Cell Biol. 2005, 37, 17–21. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ferretti, M.T. Function and Dysfunction of Microglia during Brain Development: Consequences for Synapses and Neural Circuits. Front. Synaptic Neurosci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.C.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef]
- Goldman, D.; Song, X.; Kitai, R.; Casadevall, A.; Zhao, M.L.; Lee, S.C. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: Role of specific antibody and soluble capsular polysaccharide. Infect. Immun. 2001, 69, 1808–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.S.; Novak, R.; Herzog, K.H.; Bodner, S.M.; Cleveland, J.L.; Tuomanen, E.I. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 1999, 5, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Johnson, K.R.; McGavern, D.B. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J. Exp. Med. 2015, 212, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaafsma, W.; Basterra, L.B.; Jacobs, S.; Brouwer, N.; Meerlo, P.; Schaafsma, A.; Boddeke, E.; Eggen, B.J.L. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 2017, 106, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shine, H.D. Neuroimmune processes associated with Wallerian degeneration support neurotrophin-3-induced axonal sprouting in the injured spinal cord. J. Neurosci. Res. 2013, 91, 1280–1291. [Google Scholar] [CrossRef]
- Jin, X.; Yamashita, T. Microglia in central nervous system repair after injury. J. Biochem. 2016, 159, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; DeLeo, J.A. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008, 38, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.; Dawes, J.M.; Bennett, D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012, 11, 629–642. [Google Scholar] [CrossRef]
- De Laere, M.; Berneman, Z.N.; Cools, N. To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Mundt, S.; Mrdjen, D.; Utz, S.G.; Greter, M.; Schreiner, B.; Becher, B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 2019, 4, eaau8380. [Google Scholar] [CrossRef]
- Baruch, K.; Schwartz, M. CNS-specific T cells shape brain function via the choroid plexus. Brain Behav. Immun. 2013, 34, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.T.; Watson, N.; Kipnis, J. T cells in the central nervous system: Messengers of destruction or purveyors of protection? Immunology 2014, 141, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kunis, G.; Baruch, K.; Rosenzweig, N.; Kertser, A.; Miller, O.; Berkutzki, T.; Schwartz, M. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain A J. Neurol. 2013, 136 Pt 11, 3427–3440. [Google Scholar] [CrossRef] [Green Version]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of learning and memory by meningeal immunity: A key role for IL-4. J. Exp. Med. 2010, 207, 1067–1080. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Tang, Y.; Feng, J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011, 89, 141–146. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef]
- Shechter, R.; London, A.; Varol, C.; Raposo, C.; Cusimano, M.; Yovel, G.; Rolls, A.; Mack, M.; Pluchino, S.; Martino, G.; et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 2009, 6, e1000113. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Andjelkovic, A.V.; Song, L.; Dzenko, K.A.; Cong, H.; Pachter, J.S. Functional expression of CCR2 by human fetal astrocytes. J. Neurosci. Res. 2002, 70, 219–231. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Li, W.; Deng, W.; Ning, M.; Lo, E.H. A potential gliovascular mechanism for microglial activation: Differential phenotypic switching of microglia by endothelium versus astrocytes. J. Neuroinflamm. 2018, 15, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamoto-Combs, K.; McNeal, D.W.; Morecraft, R.J.; Combs, C.K. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J. Neurotrauma 2007, 24, 1719–1742. [Google Scholar] [CrossRef] [PubMed]
- Zanier, E.R.; Pischiutta, F.; Riganti, L.; Marchesi, F.; Turola, E.; Fumagalli, S.; Perego, C.; Parotto, E.; Vinci, P.; Veglianese, P.; et al. Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics 2014, 11, 679–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain A J. Neurol. 2013, 136 Pt 1, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. Off. J. Soc. Neurosc. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [Green Version]
- Almad, A.; Sahinkaya, F.R.; McTigue, D.M. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 2011, 8, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Puntambekar, S.S.; Saber, M.; Lamb, B.T.; Kokiko-Cochran, O.N. Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury. Brain Behav. Immun. 2018, 71, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Ji, S.; Dingman, A.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 2007, 100, 893–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalancette-Hebert, M.; Swarup, V.; Beaulieu, J.M.; Bohacek, I.; Abdelhamid, E.; Weng, Y.C.; Sato, S.; Kriz, J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. Off. J. Soc. Neurosc. 2012, 32, 10383–10395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serfaty, C.A.; Campello-Costa, P.; Linden, R. Rapid and long-term plasticity in the neonatal and adult retinotectal pathways following a retinal lesion. Brain Res. Bull. 2005, 66, 128–134. [Google Scholar] [CrossRef]
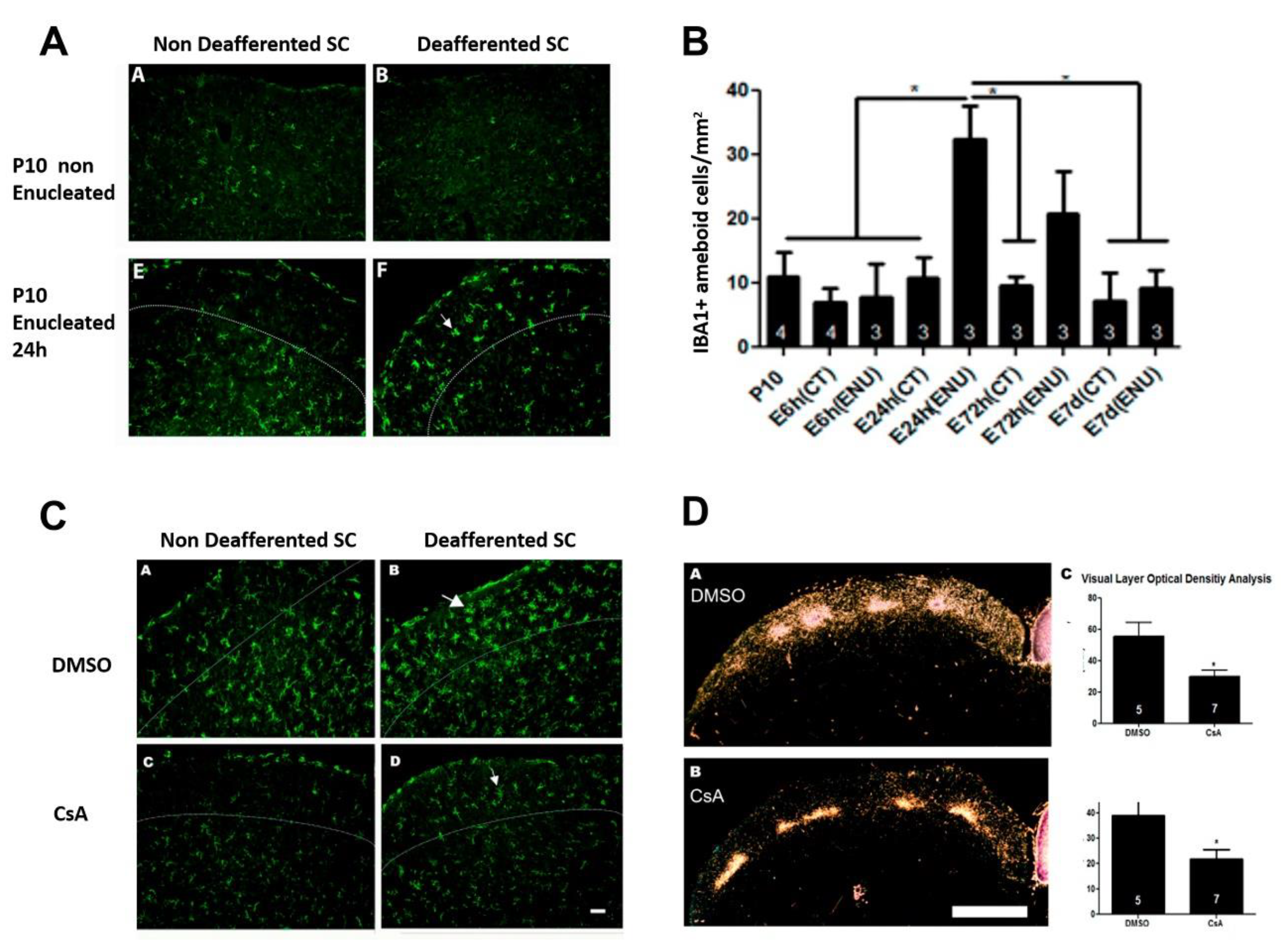
- Chagas, L.D.S.; Trindade, P.; Gomes, A.L.T.; Mendonca, H.R.; Campello-Costa, P.; Faria Melibeu, A.D.C.; Linden, R.; Serfaty, C.A. Rapid plasticity of intact axons following a lesion to the visual pathways during early brain development is triggered by microglial activation. Exp. Neurol. 2019, 311, 148–161. [Google Scholar] [CrossRef]
- Liberman, A.C.; Trias, E.; da Silva Chagas, L.; Trindade, P.; Dos Santos Pereira, M.; Refojo, D.; Hedin-Pereira, C.; Serfaty, C.A. Neuroimmune and Inflammatory Signals in Complex Disorders of the Central Nervous System. Neuroimmunomodulation 2018, 25, 246–270. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Silva, P.; Jurgilas, P.B.; Trindade, P.; Campello-Costa, P.; Perales, J.; Savino, W.; Serfaty, C.A. Matrix metalloproteinase-9 is involved in the development and plasticity of retinotectal projections in rats. Neuroimmunomodulation 2007, 14, 144–149. [Google Scholar] [CrossRef]
- Olah, M.; Patrick, E.; Villani, A.C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A transcriptomic atlas of aged human microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Fyfe, I. Neurodegenerative disease: Gene expression in aged microglia is related to neurodegenerative disease. Nat. Rev. Neurol. 2018, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Ikegaya, Y. Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 2015, 100, 1–5. [Google Scholar] [CrossRef] [PubMed]
- de Fernandez Cossio, L.; Guzman, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 2018, 48, 979–991. e8. [Google Scholar] [CrossRef] [Green Version]
- Kowal, C.; Athanassiou, A.; Chen, H.; Diamond, B. Maternal antibodies and developing blood-brain barrier. Immunol. Res. 2015, 63, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Bloise, E.; Petropoulos, S.; Iqbal, M.; Kostaki, A.; Ortiga-Carvalho, T.M.; Gibb, W.; Matthews, S.G. Acute Effects of Viral Exposure on P-Glycoprotein Function in the Mouse Fetal Blood-Brain Barrier. Cell. Physiol. Biochem. 2017, 41, 1044–1050. [Google Scholar] [CrossRef]
- Kinder, J.M.; Stelzer, I.A.; Arck, P.C.; Way, S.S. Immunological implications of pregnancy-induced microchimerism. Nat. Rev. Immunol. 2017, 17, 483–494. [Google Scholar] [CrossRef]
- Prins, J.R.; Eskandar, S.; Eggen, B.J.L.; Scherjon, S.A. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment? J. Reprod. Immunol. 2018, 126, 18–22. [Google Scholar] [CrossRef]
- Lum, F.M.; Low, D.K.; Fan, Y.; Tan, J.J.; Lee, B.; Chan, J.K.; Renia, L.; Ginhoux, F.; Ng, L.F. Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin. Infect. Dis. 2017, 64, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Brizic, I.; Susak, B.; Arapovic, M.; Huszthy, P.C.; Hirsl, L.; Kvestak, D.; Juranic Lisnic, V.; Golemac, M.; Pernjak Pugel, E.; Tomac, J.; et al. Brain-resident memory CD8(+) T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. Eur. J. Immunol. 2018, 48, 950–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmac, K.; Bantug, G.R.; Pugel, E.P.; Cekinovic, D.; Jonjic, S.; Britt, W.J. Glucocorticoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development. PLoS Pathog. 2013, 9, e1003200. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012, 71, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Favrais, G.; van de Looij, Y.; Fleiss, B.; Ramanantsoa, N.; Bonnin, P.; Stoltenburg-Didinger, G.; Lacaud, A.; Saliba, E.; Dammann, O.; Gallego, J.; et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011, 70, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgartner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. Off. J. Soc. Neurosc. 2018, 38, 3060–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bright, H.R.; Babata, K.; Allred, E.N.; Erdei, C.; Kuban, K.C.K.; Joseph, R.M.; O’Shea, T.M.; Leviton, A.; Dammann, O. Neurocognitive Outcomes at 10 Years of Age in Extremely Preterm Newborns with Late-Onset Bacteremia. J. Pediatr. 2017, 187, 43–49. e1. [Google Scholar] [CrossRef]
- Kavas, N.; Arisoy, A.E.; Bayhan, A.; Kara, B.; Gunlemez, A.; Turker, G.; Oruc, M.; Gokalp, A.S. Neonatal sepsis and simple minor neurological dysfunction. Pediatr. Int. 2017, 59, 564–569. [Google Scholar] [CrossRef]
- Spann, M.N.; Sourander, A.; Surcel, H.M.; Hinkka-Yli-Salomaki, S.; Brown, A.S. Prenatal toxoplasmosis antibody and childhood autism. Autism. Res. 2017, 10, 769–777. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2001, 25, 159–167. [Google Scholar]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef] [Green Version]
- Chappell, T.D.; Margret, C.P.; Li, C.X.; Waters, R.S. Long-term effects of prenatal alcohol exposure on the size of the whisker representation in juvenile and adult rat barrel cortex. Alcohol 2007, 41, 239–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, A.E.; Krahe, T.E.; Coppola, D.M.; Ramoa, A.S. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J. Neurosci. Off. J. Soc. Neurosc. 2003, 23, 10002–10012. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.J.; Zhao, K.B.; Zhao, H.J.; Yu, D.M.; Niu, Y.L.; Zhang, J.S.; Deng, J.B. Prenatal alcohol exposure induces long-term changes in dendritic spines and synapses in the mouse visual cortex. Alcohol Alcohol. 2010, 45, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.A.; Akers, K.G.; Rice, J.P.; Johnson, T.E.; Candelaria-Cook, F.T.; Maes, L.I.; Rosenberg, M.; Valenzuela, C.F.; Savage, D.D. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav. Brain Res. 2010, 207, 290–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfonso-Loeches, S.; Pascual-Lucas, M.; Blanco, A.M.; Sanchez-Vera, I.; Guerri, C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. Off. J. Soc. Neurosc. 2010, 30, 8285–8295. [Google Scholar] [CrossRef]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef]
- Leclercq, S.; De Saeger, C.; Delzenne, N.; de Timary, P.; Starkel, P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol. Psychiatry 2014, 76, 725–733. [Google Scholar] [CrossRef]
- Pradier, B.; Erxlebe, E.; Markert, A.; Racz, I. Microglial IL-1beta progressively increases with duration of alcohol consumption. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 455–461. [Google Scholar] [CrossRef]
- Ahlers, K.E.; Karacay, B.; Fuller, L.; Bonthius, D.J.; Dailey, M.E. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia 2015, 63, 1694–1713. [Google Scholar] [CrossRef]
- Boschen, K.E.; Ruggiero, M.J.; Klintsova, A.Y. Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 2016, 324, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop, V.J.; Kuijpens, J.L.; van Baar, A.L.; Verkerk, G.; van Son, M.M.; de Vijlder, J.J.; Vulsma, T.; Wiersinga, W.M.; Drexhage, H.A.; Vader, H.L. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol. 1999, 50, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbel, P.; Navarro, D.; Auso, E.; Varea, E.; Rodriguez, A.E.; Ballesta, J.J.; Salinas, M.; Flores, E.; Faura, C.C.; de Escobar, G.M. Role of late maternal thyroid hormones in cerebral cortex development: An experimental model for human prematurity. Cereb. Cortex 2010, 20, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.J.; Brouwers, E.P.; Vader, H.L.; Vulsma, T.; van Baar, A.L.; de Vijlder, J.J. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin. Endocrinol. 2003, 59, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef]
- Bouvet, J.; Usson, Y.; Legrand, J. Morphometric analysis of the cerebellar Purkinje cell in the developing normal and hypothyroid chick. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 1987, 5, 345–355. [Google Scholar] [CrossRef]
- Lima, F.R.; Gervais, A.; Colin, C.; Izembart, M.; Neto, V.M.; Mallat, M. Regulation of microglial development: A novel role for thyroid hormone. J. Neurosci. Off. J. Soc. Neurosc. 2001, 21, 2028–2038. [Google Scholar] [CrossRef]
- Mori, Y.; Tomonaga, D.; Kalashnikova, A.; Furuya, F.; Akimoto, N.; Ifuku, M.; Okuno, Y.; Beppu, K.; Fujita, K.; Katafuchi, T.; et al. Effects of 3,3′,5-triiodothyronine on microglial functions. Glia 2015, 63, 906–920. [Google Scholar] [CrossRef]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am.J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef] [Green Version]
- Brites, D.; Vaz, A.R. Microglia centered pathogenesis in ALS: Insights in cell interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.R.; Dasilva, D.A.; Cluette-Brown, J.E.; Dimonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749. e1–2. [Google Scholar] [CrossRef] [Green Version]
- Harvey, L.D.; Yin, Y.; Attarwala, I.Y.; Begum, G.; Deng, J.; Yan, H.Q.; Dixon, C.E.; Sun, D. Administration of DHA Reduces Endoplasmic Reticulum Stress-Associated Inflammation and Alters Microglial or Macrophage Activation in Traumatic Brain Injury. ASN Neuro 2015, 7, 1759091415618969. [Google Scholar] [CrossRef] [Green Version]
- de Velasco, P.C.; Mendonca, H.R.; Borba, J.M.; da Andrade Costa, B.L.; Guedes, R.C.; Navarro, D.M.; Santos, G.K.; Faria-Melibeu Ada, C.; Campello Costa, P.; Serfaty, C.A. Nutritional restriction of omega-3 fatty acids alters topographical fine tuning and leads to a delay in the critical period in the rodent visual system. Exp. Neurol. 2012, 234, 220–229. [Google Scholar] [CrossRef] [Green Version]
- De Velasco, P.C.; Sandre, P.C.; Tavares Do Carmo, M.G.; Faria-Melibeu, A.C.; Campello-Costa, P.; Ferraz, A.C.; Andrade Da Costa, B.L.S.; Serfaty, C.A. A critical period for omega-3 nutritional supplementation in the development of the rodent visual system. Brain Res. 2015, 1615, 106–115. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Nadjar, A.; Joffre, C.; Costes, L.; Aubert, A.; Gregoire, S.; Bretillon, L.; Laye, S. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE 2012, 7, e36861. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 35–45. [Google Scholar] [CrossRef]
- Pu, L.; Igbavboa, U.; Wood, W.G.; Roths, J.B.; Kier, A.B.; Spener, F.; Schroeder, F. Expression of fatty acid binding proteins is altered in aged mouse brain. Mol. Cell. Biochem. 1999, 198, 69–78. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochimica et Biophysica Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Cassano, T.; Calcagnini, S.; Pace, L.; De Marco, F.; Romano, A.; Gaetani, S. Cannabinoid Receptor 2 Signaling in Neurodegenerative Disorders: From Pathogenesis to a Promising Therapeutic Target. Front. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyall, S.C. Interplay Between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, D.M.; Akbar, M.; Huang, B.X. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessaro, F.H.; Ayala, T.S.; Martins, J.O. Lipid mediators are critical in resolving inflammation: A review of the emerging roles of eicosanoids in diabetes mellitus. BioMed Res. Int. 2015, 2015, 568408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopperton, K.E.; Trepanier, M.O.; James, N.C.E.; Chouinard-Watkins, R.; Bazinet, R.P. Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2018, 69, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Hernangomez, M.; Carrillo-Salinas, F.J.; Mecha, M.; Correa, F.; Mestre, L.; Loria, F.; Feliu, A.; Docagne, F.; Guaza, C. Brain innate immunity in the regulation of neuroinflammation: Therapeutic strategies by modulating CD200-CD200R interaction involve the cannabinoid system. Curr. Pharm. Des. 2014, 20, 4707–4722. [Google Scholar] [CrossRef] [Green Version]
- Alvheim, A.R.; Malde, M.K.; Osei-Hyiaman, D.; Lin, Y.H.; Pawlosky, R.J.; Madsen, L.; Kristiansen, K.; Froyland, L.; Hibbeln, J.R. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity 2012, 20, 1984–1994. [Google Scholar] [CrossRef] [Green Version]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, N. Cannabinoid signaling in glial cells. Glia 2004, 48, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. Off. J. Soc. Neurosc. 2003, 23, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Stella, N. Endocannabinoid signaling in microglial cells. Neuropharmacology 2009, 56 (Suppl. 1), 244–253. [Google Scholar] [CrossRef] [Green Version]
- Bilkei-Gorzo, A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 3326–3341. [Google Scholar] [CrossRef]
- Scheller, A.; Kirchhoff, F. Endocannabinoids and Heterogeneity of Glial Cells in Brain Function. Front. Integr. Neurosci. 2016, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Stella, N. CB2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliu, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef]
- Tikhonenko, M.; Lydic, T.A.; Opreanu, M.; Li Calzi, S.; Bozack, S.; McSorley, K.M.; Sochacki, A.L.; Faber, M.S.; Hazra, S.; Duclos, S.; et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS ONE 2013, 8, e55177. [Google Scholar] [CrossRef] [Green Version]
- Madore, C.; Nadjar, A.; Delpech, J.C.; Sere, A.; Aubert, A.; Portal, C.; Joffre, C.; Laye, S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav. Immun. 2014, 41, 22–31. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagas, L.d.S.; Sandre, P.C.; Ribeiro e Ribeiro, N.C.A.; Marcondes, H.; Oliveira Silva, P.; Savino, W.; Serfaty, C.A. Environmental Signals on Microglial Function during Brain Development, Neuroplasticity, and Disease. Int. J. Mol. Sci. 2020, 21, 2111. https://doi.org/10.3390/ijms21062111
Chagas LdS, Sandre PC, Ribeiro e Ribeiro NCA, Marcondes H, Oliveira Silva P, Savino W, Serfaty CA. Environmental Signals on Microglial Function during Brain Development, Neuroplasticity, and Disease. International Journal of Molecular Sciences. 2020; 21(6):2111. https://doi.org/10.3390/ijms21062111
Chicago/Turabian StyleChagas, Luana da Silva, Poliana Capucho Sandre, Natalia Cristina Aparecida Ribeiro e Ribeiro, Henrique Marcondes, Priscilla Oliveira Silva, Wilson Savino, and Claudio A. Serfaty. 2020. "Environmental Signals on Microglial Function during Brain Development, Neuroplasticity, and Disease" International Journal of Molecular Sciences 21, no. 6: 2111. https://doi.org/10.3390/ijms21062111
APA StyleChagas, L. d. S., Sandre, P. C., Ribeiro e Ribeiro, N. C. A., Marcondes, H., Oliveira Silva, P., Savino, W., & Serfaty, C. A. (2020). Environmental Signals on Microglial Function during Brain Development, Neuroplasticity, and Disease. International Journal of Molecular Sciences, 21(6), 2111. https://doi.org/10.3390/ijms21062111




