High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout
Abstract
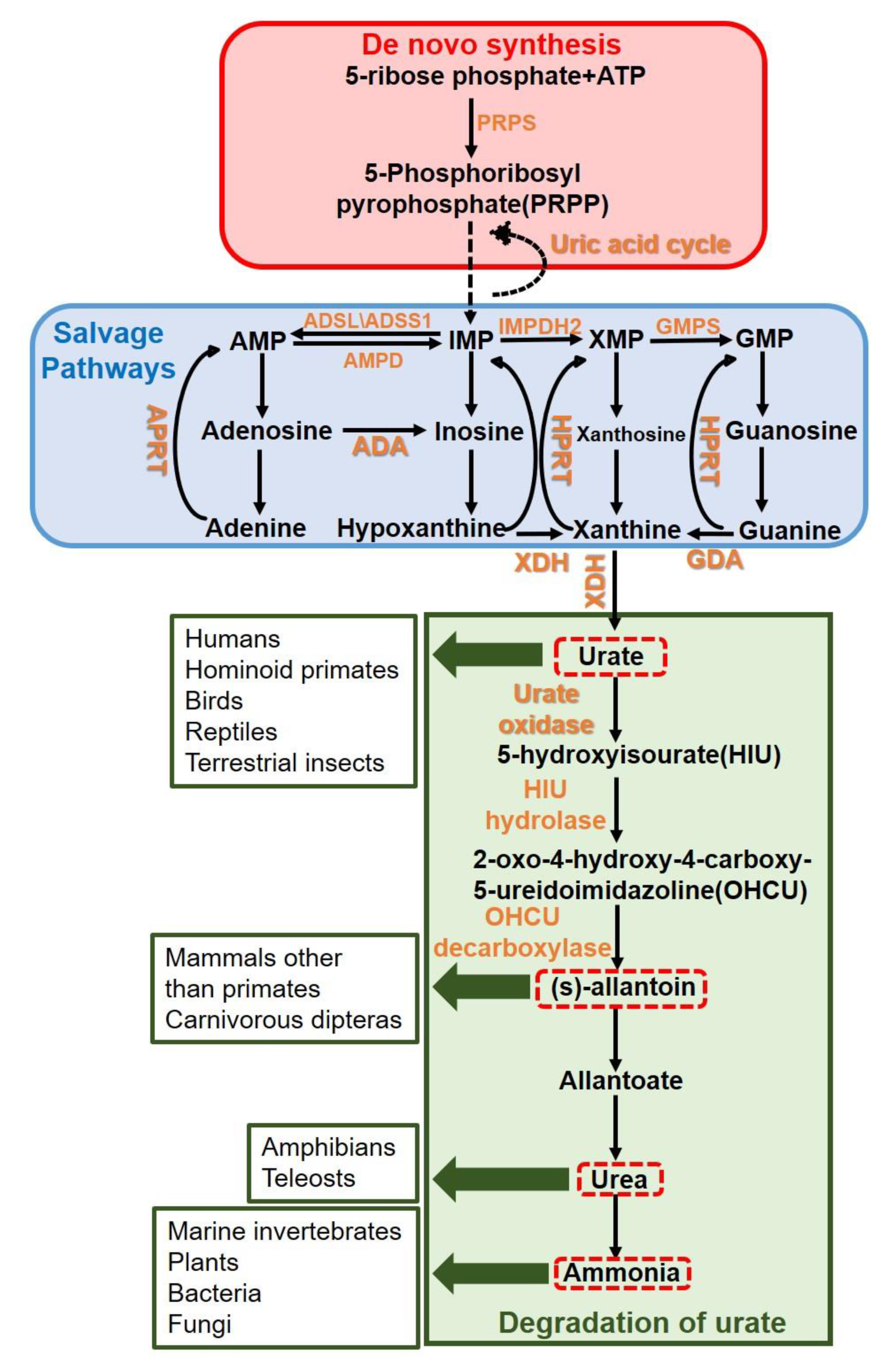
:1. Introduction
2. Results
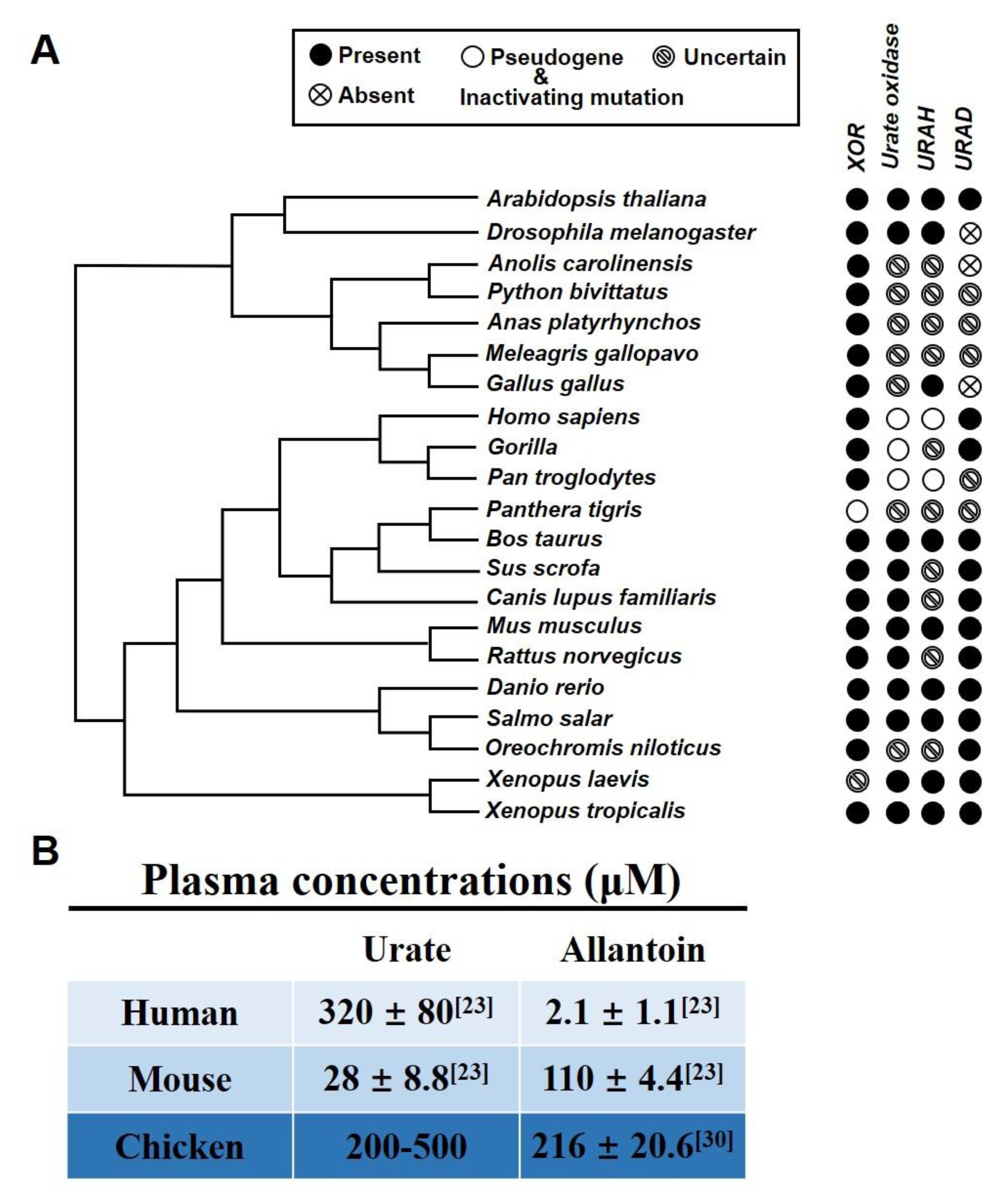
2.1. Chicken as a Suitable Hyperuricemia Model among Various Organisms
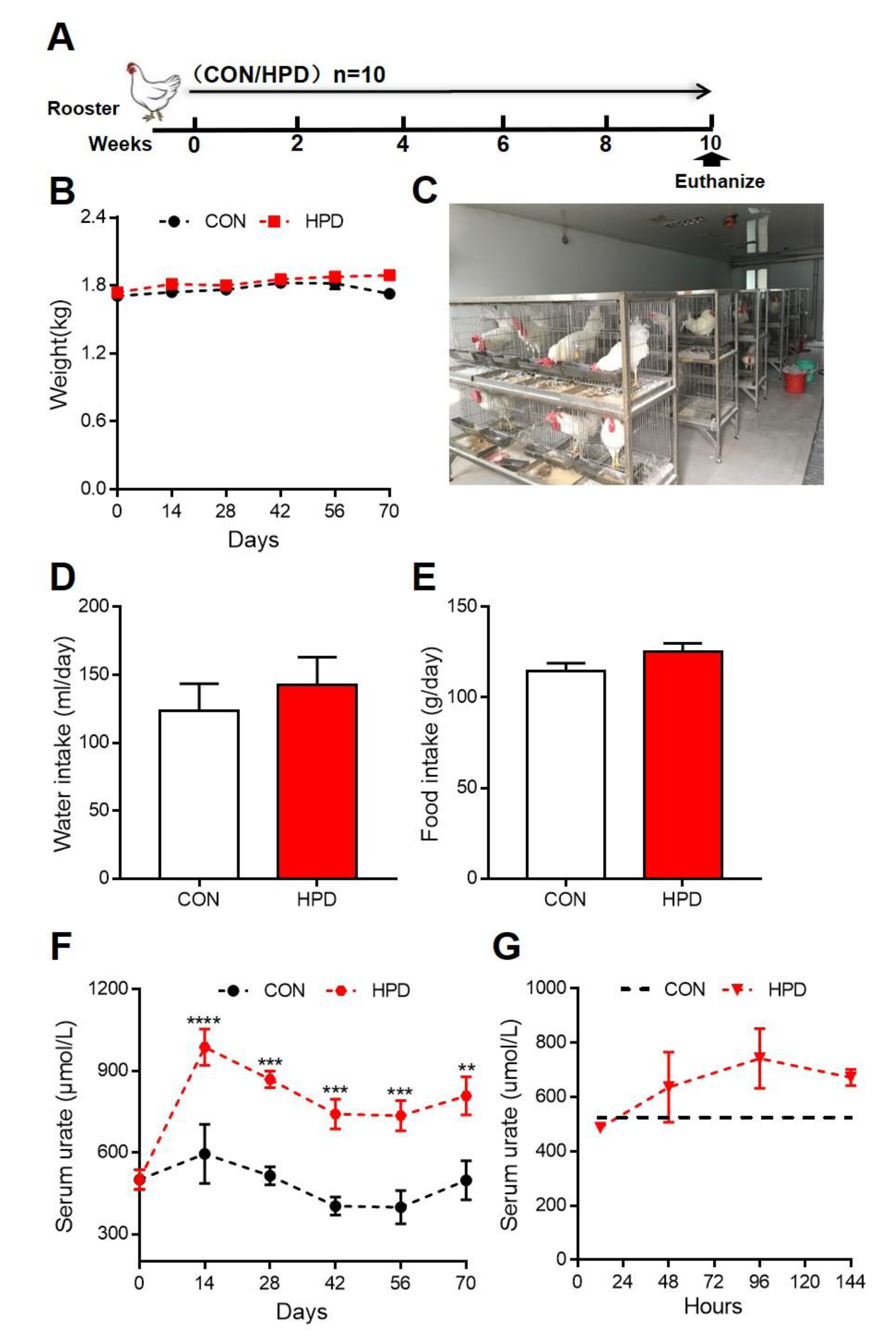
2.2. HPD Increases Serum Levels of Urate in Chickens
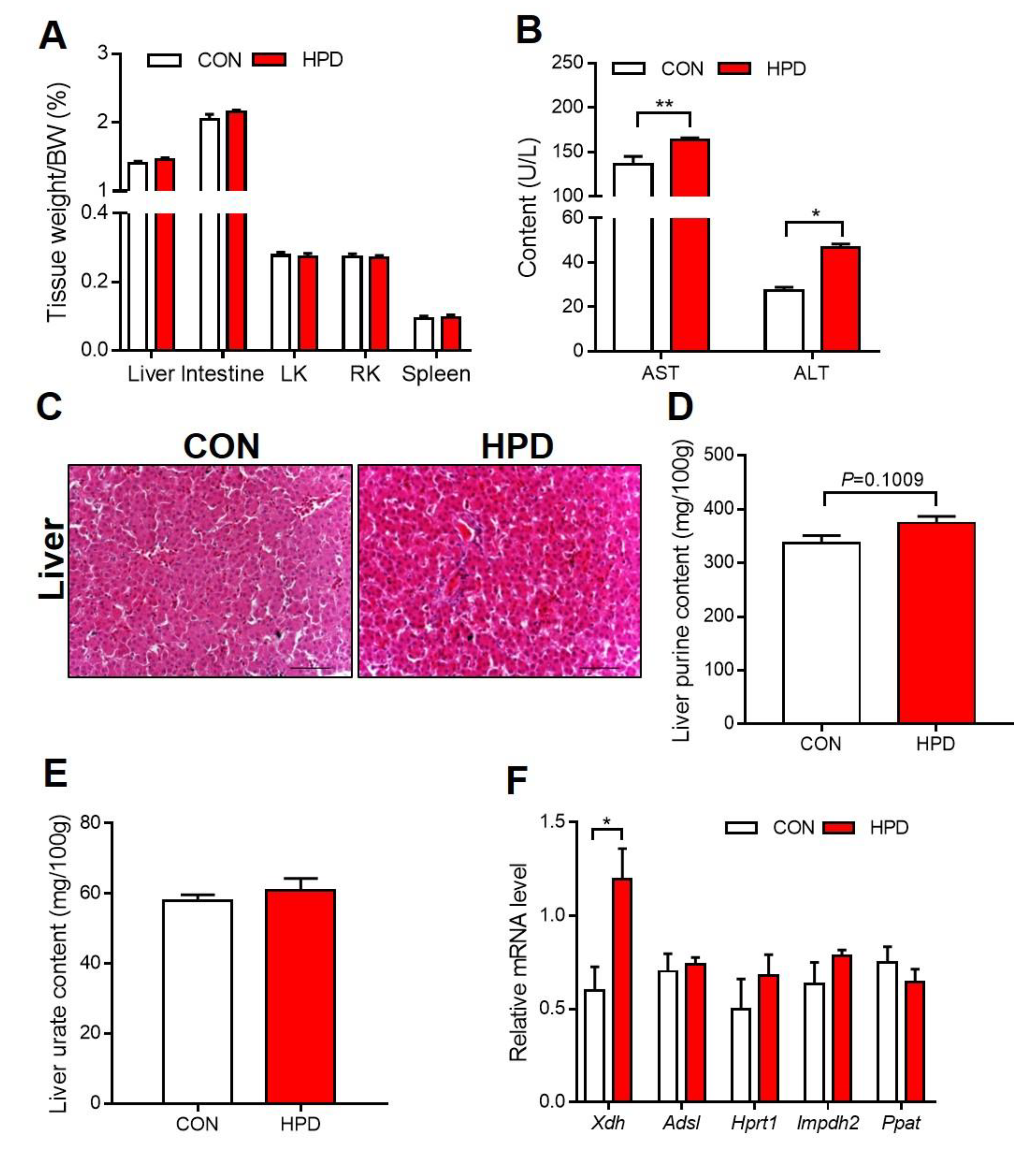
2.3. The Effect of HPD on Biochemical Parameters in Chickens
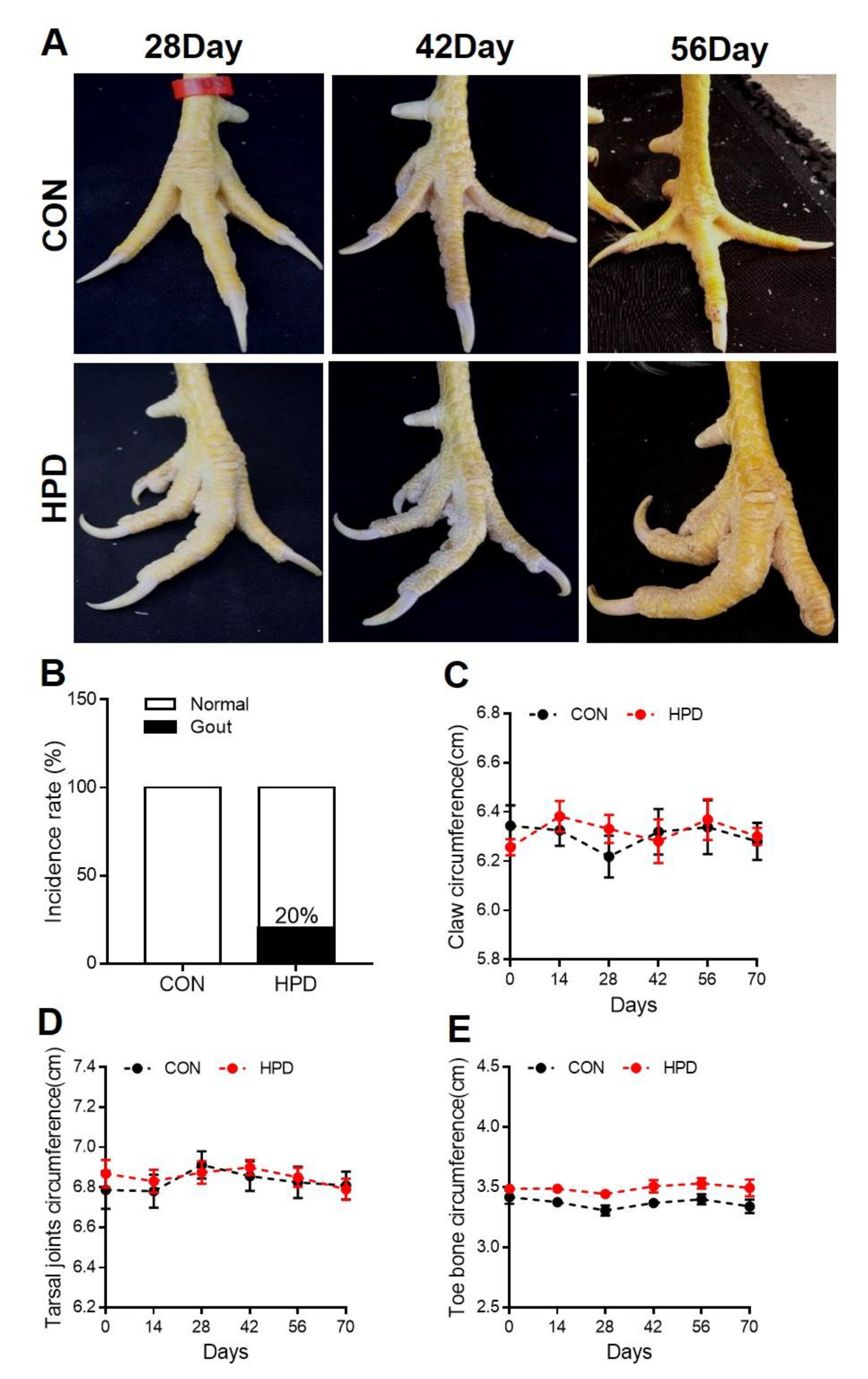
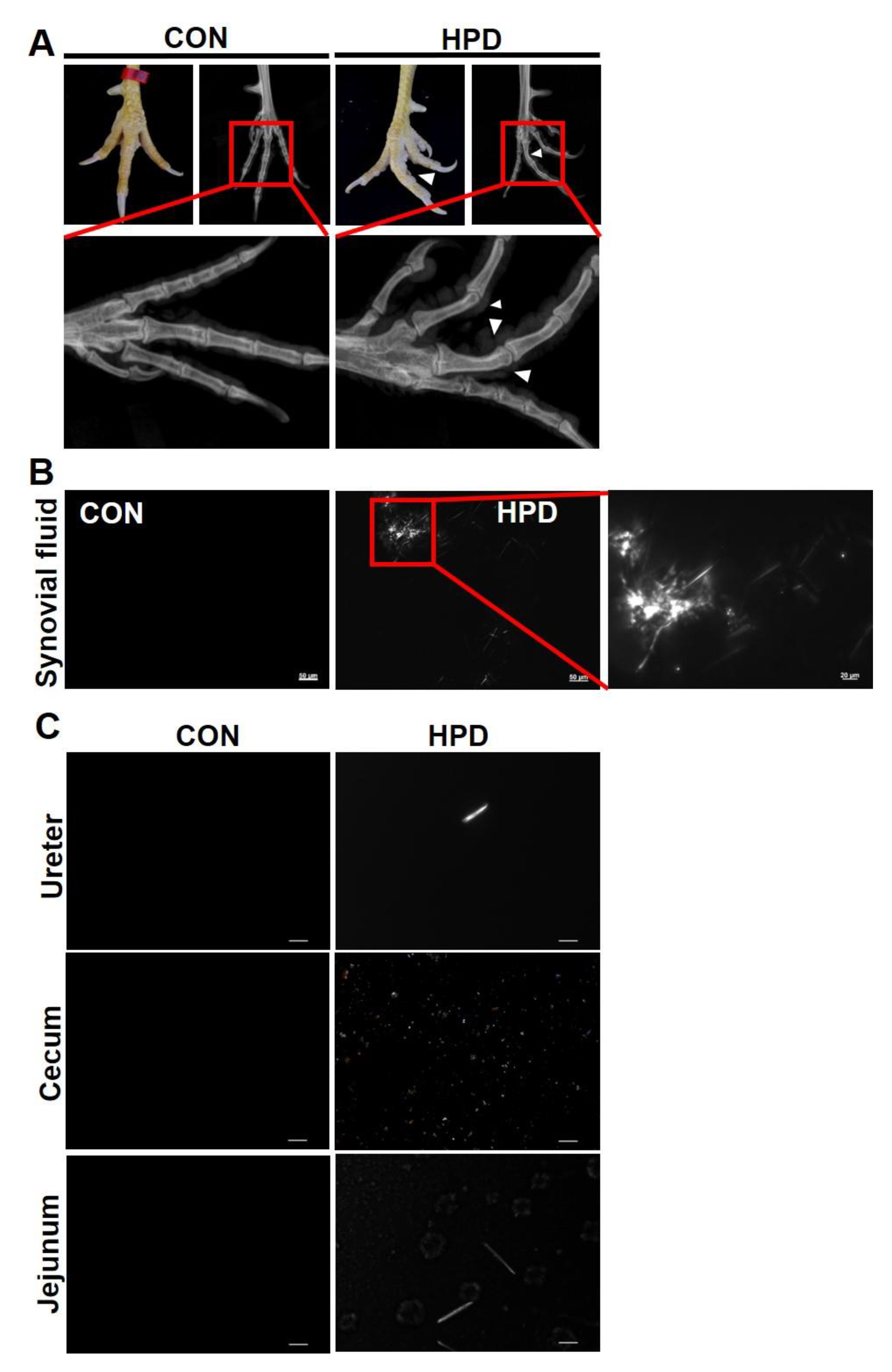
2.4. HPD Induces an Abnormal Claw Morphology in Chickens
2.5. HPD Induces MSU Crystal Production in Synovial Fluid and Other Tissue Fluids
2.6. HPD Increases the Production of Urate in the Liver
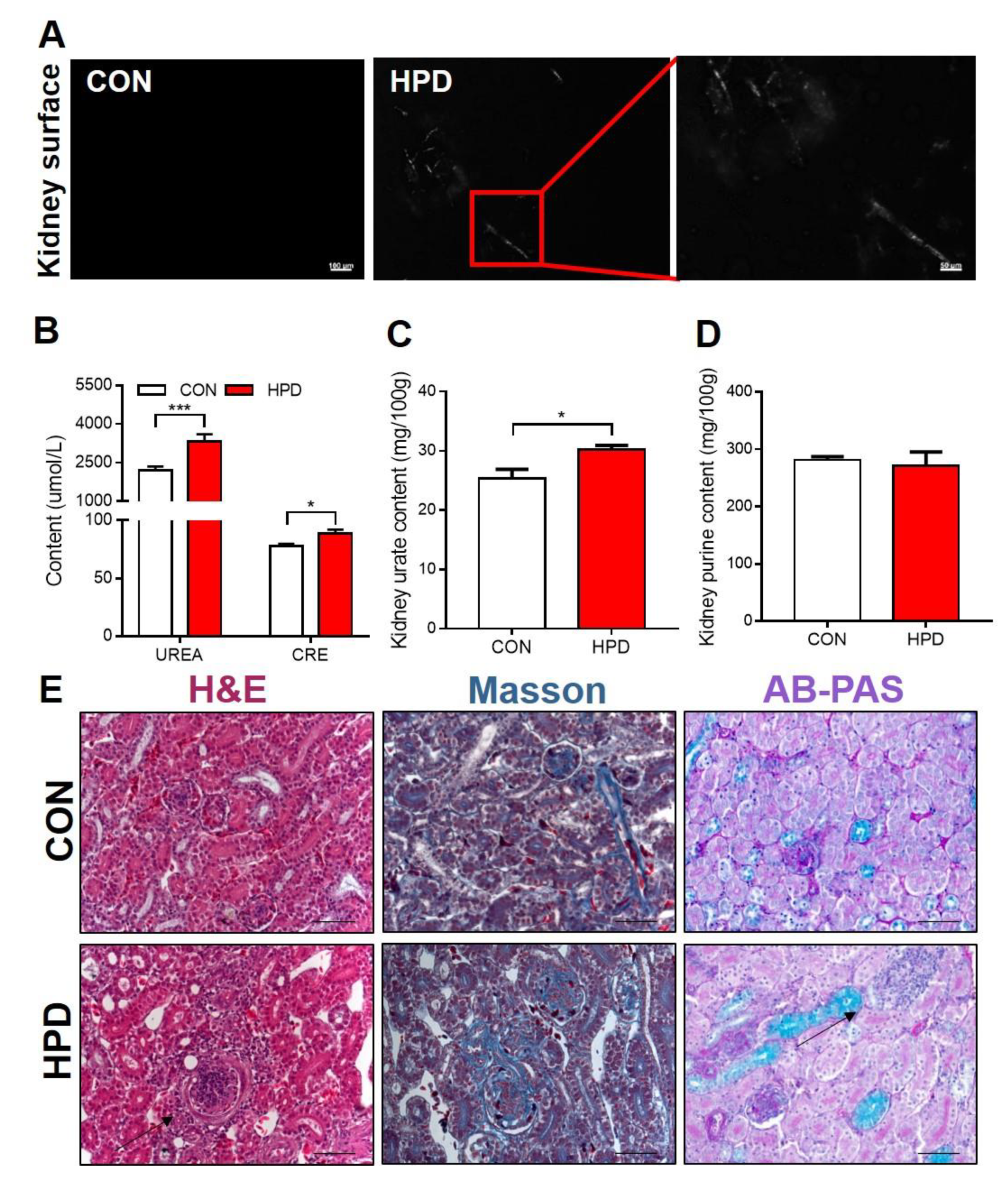
2.7. HPD Induces Renal Injury in Chickens
2.8. Allopurinol and Probenecid Treatment Decreases Serum Urate in Chickens of Hyperuricemia
2.9. Allopurinol and Probenecid Treatment Decreases Gout Incidence Rate in Chickens
3. Discussion
4. Materials and Methods
4.1. Phylogenetic Tree Construction
4.2. Animals and Experimental Design
4.3. Histological Analysis
4.4. Radiographic Imaging
4.5. Biochemical Analysis
4.6. Gene Expression Analysis
4.7. High-Performance Liquid Chromatography Analysis (HPLC) Analysis
4.8. MSU Crystal Detection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, K.C.; Lin, H.Y.; Chou, P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J. Rheumatol. 2000, 27, 1045–1050. [Google Scholar] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H.; Xia, W.; Chang, X.; Wang, M.; An, L. Prevalence and correlates of hyperuricemia in the middle-aged and older adults in China. Sci. Rep. 2018, 8, 4314. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am. J. Med. 2012, 125, 679–687. [Google Scholar] [CrossRef]
- Pleskacova, A.; Bartakova, V.; Chalasova, K.; Pacal, L.; Kankova, K.; Tomandl, J. Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus-The Effect on Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2018, 19, 3696. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Fellstrom, B.; Danielson, B.G.; Karlstrom, B.; Lithell, H.; Ljunghall, S.; Vessby, B. The influence of a high dietary intake of purine-rich animal protein on urinary urate excretion and supersaturation in renal stone disease. Clin. Sci. 1983, 64, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Perez-Ruiz, F.; Calabozo, M.; Erauskin, G.G.; Ruibal, A.; Herrero-Beites, A.M. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 2002, 47, 610–613. [Google Scholar] [CrossRef]
- Stiburkova, B.; Pavelcova, K.; Pavlikova, M.; Jesina, P.; Pavelka, K. The impact of dysfunctional variants of ABCG2 on hyperuricemia and gout in pediatric-onset patients. Arthritis Res. Ther. 2019, 21, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, A.; Matsuo, H.; Nakaoka, H.; Nakamura, T.; Nakashima, H.; Takada, Y.; Oikawa, Y.; Takada, T.; Sakiyama, M.; Shimizu, S.; et al. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci. Rep. 2014, 4, 5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, R.L.; Wallace, M.C.; Phipps-Green, A.J.; Topless, R.; Drake, J.M.; Tan, P.; Dalbeth, N.; Merriman, T.R.; Stamp, L.K. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharm. J. 2017, 17, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tin, A.; Marten, J.; Halperin Kuhns, V.L.; Li, Y.; Wuttke, M.; Kirsten, H.; Sieber, K.B.; Qiu, C.; Gorski, M.; Yu, Z.; et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat. Genet. 2019, 51, 1459–1474. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.; Nakaoka, H.; Nakayama, A.; Okada, Y.; Yamamoto, K.; Higashino, T.; Sakiyama, M.; Shimizu, T.; Ooyama, H.; Ooyama, K.; et al. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Ann. Rheum. Dis. 2019, 78, 1430–1437. [Google Scholar] [CrossRef]
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 2015, 11, 649–662. [Google Scholar] [CrossRef]
- Kedar, E.; Simkin, P.A. A perspective on diet and gout. Adv. Chronic Kidney Disease 2012, 19, 392–397. [Google Scholar] [CrossRef]
- Fustin, J.M.; Doi, M.; Yamada, H.; Komatsu, R.; Shimba, S.; Okamura, H. Rhythmic nucleotide synthesis in the liver: Temporal segregation of metabolites. Cell Rep. 2012, 1, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lario, B.; Macarron-Vicente, J. Uric acid and evolution. Rheumatology 2010, 49, 2010–2015. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.R.; Yang, L.; Sebetso, G.; Allen, R.; Doan, T.H.; Blundell, R.; Lui, E.Y.; Morrow, C.A.; Fraser, J.A. Characterization of the complete uric acid degradation pathway in the fungal pathogen Cryptococcus neoformans. PLoS ONE 2013, 8, e64292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hille, R.; Nishino, T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Abu-Remaileh, M.; Kanarek, N.; Freinkman, E.; Gao, X.; Louissaint, A., Jr.; Lewis, C.A.; Sabatini, D.M. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell 2017, 169, 258–272. [Google Scholar] [CrossRef] [Green Version]
- Simoyi, M.F.; Falkenstein, E.; Van Dyke, K.; Blemings, K.P.; Klandorf, H. Allantoin, the oxidation product of uric acid is present in chicken and turkey plasma. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 135, 325–335. [Google Scholar] [CrossRef]
- Paganoni, S.; Schwarzschild, M.A. Urate as a Marker of Risk and Progression of Neurodegenerative Disease. Neurotherapeutics 2017, 14, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Salway, J.G. The Krebs Uric Acid Cycle: A Forgotten Krebs Cycle. Trends Biochem. Sci. 2018, 43, 847–849. [Google Scholar] [CrossRef]
- Koutsos, E.A.; Smith, J.; Woods, L.W.; Klasing, K.C. Adult cockatiels (Nymphicus hollandicus) metabolically adapt to high protein diets. J. Nutr. 2001, 131, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Huang, K.; Tang, J. Clinicopathology of gout in growing layers induced by high calcium and high protein diets. Br. Poult. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef]
- Ding, X.; Li, M.; Peng, C.; Wang, Z.; Qian, S.; Ma, Y.; Fang, T.; Feng, S.; Li, Y.; Wang, X.; et al. Uric acid transporters BCRP and MRP4 involved in chickens uric acid excretion. BMC Vet. Res. 2019, 15, 180. [Google Scholar] [CrossRef] [Green Version]
- Carro, M.D.; Falkenstein, E.; Radke, W.J.; Klandorf, H. Effects of allopurinol on uric acid concentrations, xanthine oxidoreductase activity and oxidative stress in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 12–17. [Google Scholar] [CrossRef]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2015, 74, 1789–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.X.; Liu, Y.L.; Yang, Y.; Zhang, D.M.; Kong, L.D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. 2015, 747, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiang, Q.; Gu, Y.; Jia, S.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Bao, X.; Yu, B.; et al. A dietary pattern rich in animal organ, seafood and processed meat products is associated with newly diagnosed hyperuricaemia in Chinese adults: A propensity score-matched case-control study. Br. J. Nutr. 2018, 119, 1177–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, C.; Choi, H.; Chaisson, C.; Hunter, D.; Niu, J.; Neogi, T. Purine-rich foods intake and recurrent gout attacks. Ann. Rheum. Dis. 2012, 71, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Sattui, S.E.; Gaffo, A.L. Treatment of hyperuricemia in gout: Current therapeutic options, latest developments and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 145–159. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Macias-Kauffer, L.; Fernandez-Lopez, J.C.; Villalobos-Comparan, M.; Martinez-Aguilar, M.M.; de la Cruz-Montoya, A.; Ramirez-Salazar, E.G.; Villamil-Ramirez, H.; Quiterio, M.; Ramirez-Palacios, P.; et al. Influence of Genetic and Non-Genetic Risk Factors for Serum Uric Acid Levels and Hyperuricemia in Mexicans. Nutrients 2019, 11, 1336. [Google Scholar] [CrossRef] [Green Version]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: Meta-analysis of population based cohorts. BMJ 2018, 363, k3951. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Dalbeth, N.; Yin, H.; Li, C.; Merriman, T.R.; Wei, W.H. Mouse models for human hyperuricaemia: A critical review. Nat. Rev. Rheumatol. 2019, 15, 413–426. [Google Scholar] [CrossRef]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.J.; Titte, S.; Cade, J.R.; Rideout, B.A.; Oliver, W.J. Uric acid, evolution and primitive cultures. Semin. Nephrol. 2005, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spitsin, S.V.; Scott, G.S.; Mikheeva, T.; Zborek, A.; Kean, R.B.; Brimer, C.M.; Koprowski, H.; Hooper, D.C. Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic. Biol. Med. 2002, 33, 1363–1371. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Kang, D.H.; Feng, L.; Nakagawa, T.; Kanellis, J.; Lan, H.; Mazzali, M.; Johnson, R.J. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002, 40, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Ramazzina, I.; Folli, C.; Secchi, A.; Berni, R.; Percudani, R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat. Chem. Biol. 2006, 2, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Costa-Bauza, A.; Grases, F.; Calvo, P.; Rodriguez, A.; Prieto, R.M. Effect of Consumption of Cocoa-Derived Products on Uric Acid Crystallization in Urine of Healthy Volunteers. Nutrients 2018, 10, 1516. [Google Scholar] [CrossRef] [Green Version]
- Zebrowska, E.; Maciejczyk, M.; Zendzian-Piotrowska, M.; Zalewska, A.; Chabowski, A. High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. Int. J. Mol. Sci. 2019, 20, 1547. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef] [Green Version]


| Ingredient | CON | HPD |
|---|---|---|
| Corn (8.7%) | 55.07 | 38.55 |
| Soyabean Meal (GB2) | 26.81 | 48.07 |
| Rice Bran (GB2) | 3.00 | 2.10 |
| Wheat Bran (GB1) | 3.00 | 2.10 |
| NaCl | 0.35 | 0.25 |
| CaHPO4 | 1.57 | 1.10 |
| Limestone | 9.37 | 7.26 |
| dl-Methionine | 0.10 | 0.07 |
| Choline | 0.10 | 0.07 |
| Compound Vitamin Premix | 0.03 | 0.02 |
| Trace Element Premix | 0.30 | 0.21 |
| Betaine | 0.30 | 0.21 |
| Crude Protein (%) | 19.11 | 34.88 |
| Lysine (%) | 0.94 | 0.66 |
| Methionine (%) | 0.35 | 0.25 |
| Methionine + Cystine (%) | 0.66 | 0.46 |
| Calcium (%) | 3.81 | 2.95 |
| Availed Phosphorus (%) | 0.36 | 0.25 |
| Metabolic Energy (Kcal/kg) | 2650 | 2650 |
| Parameter | CON | HPD |
|---|---|---|
| TC (mmol/L) | 3.14 ± 0.43 | 2.45 ± 0.41 a |
| TG (mmol/L) | 1 ± 0.23 | 0.69 ± 0.19 a |
| HDL-C (mmol/L) | 1.77 ± 0.3 | 1.56 ± 0.29 |
| LDL-C (mmol/L) | 0.72 ± 0.08 | 0.49 ± 0.09 a |
| VLDLc (mmol/L) | 0.65 ± 0.16 | 0.4 ± 0.08 a |
| ApoA1 (g/dL) | 0.11 ± 0.02 | 0.12 ± 0.02 |
| ApoB (g/dL) | 0.22 ± 0.04 | 0.23 ± 0.03 |
| GLU (mmol/L) | 8.72 ± 1.14 | 8.8 ± 1.23 |
| LDH (U/L) | 745.42 ± 46.02 | 609.27 ± 98.52 |
| TP (g/L) | 51.55 ± 4.73 | 51.2 ± 2.64 |
| ALB (g/L) | 17 ± 0.78 | 17.38 ± 0.64 |
| ALP (U/L) | 691.62 ± 72.07 | 722.83 ± 95.45 |
| TB (μmol/L) | 12.54 ± 2.72 | 5.8 ± 1.47 a |
| DB (μmol/L) | 2.83 ± 0.67 | 1.7 ± 0.39 a |
| P (mmol/L) | 1.3 ± 0.18 | 1.27 ± 0.1 |
| Ca (mmol/L) | 2.9 ± 0.09 | 2.95 ± 0.02 |
| K (mmol/L) | 5.18 ± 0.48 | 5.1 ± 0.76 |
| Na (mmol/L) | 145.17 ± 5.34 | 141.67 ± 3.88 |
| Cl (mmol/L) | 106.33 ± 4.08 | 109.67 ± 4.76 |
| Parameter | CON | HPD | HPD + Allo | HPD + Prob |
|---|---|---|---|---|
| TC (mmol/L) | 3.58 ± 0.41 | 2.45 ± 0.31 a | 3.04 ± 0.67 | 3.04 ± 0.94 |
| TG (mmol/L) | 1.11 ± 0.47 | 0.58 ± 0.12 a | 0.61 ± 0.15 a | 0.62 ± 0.25 a |
| ALT(U/L) | 6.01 ± 1.11 | 5.51 ± 1.24 | 4.56 ± 0.92 a | 4.42 ± 0.68 a |
| AST(U/L) | 520 ± 169.54 | 649.72 ± 301.58 | 466.07 ± 127.14 | 356.27 ± 92.24 b |
| XOR(U/L) | 5.15 ± 1.46 | 7.26 ± 1.99 a | 5.82 ± 1.02 b | 6.79 ± 2.51 b |
| GLU (mmol/L) | 5.3 ± 1.31 | 2.43 ± 1.13 a | 4.2 ± 2.34 | 6.49 ± 1.66 b |
| γ-GT (U/L) | 6.24 ± 1.93 | 6.27 ± 1.55 | 7.24 ± 2.57 | 7.17 ± 1.57 |
| UREA (mmol/L) | 0.27 ± 0.09 | 0.26 ± 0.07 | 0.28 ± 0.06 | 0.26 ± 0.04 |
| CREA (μmol/L) | 17.53 ± 3.16 | 19.18 ± 3.65 | 18.48 ± 2.7 | 22.19 ± 4.28 |
| Ingredient | CON | HPD | HPD + Allo | HPD + Prob |
|---|---|---|---|---|
| Corn (GB2) | 65.00 | 27.00 | 27.00 | 27.00 |
| Fish Meal | 9.71 | 10.00 | 10.00 | 10.00 |
| Beer Yeast | 19.61 | 65.52 | 65.52 | 65.52 |
| Soya Oil | 2.39 | 5.00 | 5.00 | 5.00 |
| L-Lysine | 0.04 | 0.00 | 0.00 | 0.00 |
| DL-Methionine | 0.10 | 0.00 | 0.00 | 0.00 |
| Stone Powder | 1.39 | 1.10 | 1.10 | 1.10 |
| CaHPO4·2H2O | 0.06 | 0.00 | 0.00 | 0.00 |
| Compound Premix | 0.30 | 0.30 | 0.30 | 0.30 |
| Zeolite Powder | 1.29 | 0.00 | 0.00 | 0.00 |
| Crude Protein (%) | 20.00 | 35.00 | 35.00 | 35.00 |
| L-Lysine (%) | 1.25 | 2.45 | 2.45 | 2.45 |
| DL-Methionine (%) | 0.55 | 0.67 | 0.67 | 0.67 |
| Calcium (%) | 0.95 | 0.95 | 0.95 | 0.95 |
| Available Phosphorus (%) | 0.45 | 0.61 | 0.61 | 0.61 |
| Salinity (%) | 0.35 | 0.40 | 0.40 | 0.40 |
| Methionine + cysteine (%) | 0.80 | 1.10 | 1.10 | 1.10 |
| Allopurinol (%) | 0.00 | 0.00 | 0.09 | 0.00 |
| Probenecid (%) | 0.00 | 0.00 | 0.00 | 0.05 |
| Metabolic Energy (Kcal/kg) | 3000 | 2910 | 2910 | 2910 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product Length (bp) |
|---|---|---|---|
| Xdh | GAGGGATTTACTCTACGGCGA | AGCCTGATTCAGAAACGGGAC | 174 |
| Adsl | CAAAGCTGCAGCCATCATTCACCT | GAGCTTGGGCAGCAGCAAGTTAAA | 106 |
| Hprt1 | TGGACTTGTTCTGCATACCCAA | TCATCCCCACCAATGACTTTTA | 307 |
| Impdh2 | GCTGGTTGGCATCATCTCAT | GCCGGTACTTGTCATCTTCG | 320 |
| Ppat | CGACACTGCTGACTGGGTA | TACTGGAATAAGGCGACCA | 151 |
| Gapdh | CATGGTTGACACCCATCACAA | GGTGCTAAGCGTGTTATCATCTCA | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, F.; Zheng, A.; Xu, P.; Wang, J.; Xue, T.; Dai, S.; Pan, S.; Guo, Y.; Xie, X.; Li, L.; et al. High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout. Int. J. Mol. Sci. 2020, 21, 2147. https://doi.org/10.3390/ijms21062147
Hong F, Zheng A, Xu P, Wang J, Xue T, Dai S, Pan S, Guo Y, Xie X, Li L, et al. High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout. International Journal of Molecular Sciences. 2020; 21(6):2147. https://doi.org/10.3390/ijms21062147
Chicago/Turabian StyleHong, Fan, Aijuan Zheng, Pengfei Xu, Jialin Wang, Tingting Xue, Shu Dai, Shijia Pan, Yuan Guo, Xinlu Xie, Letong Li, and et al. 2020. "High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout" International Journal of Molecular Sciences 21, no. 6: 2147. https://doi.org/10.3390/ijms21062147
APA StyleHong, F., Zheng, A., Xu, P., Wang, J., Xue, T., Dai, S., Pan, S., Guo, Y., Xie, X., Li, L., Qiao, X., Liu, G., & Zhai, Y. (2020). High-Protein Diet Induces Hyperuricemia in a New Animal Model for Studying Human Gout. International Journal of Molecular Sciences, 21(6), 2147. https://doi.org/10.3390/ijms21062147







