Analysis of the Activity and Expression of Cyclooxygenases COX1 and COX2 in THP-1 Monocytes and Macrophages Cultured with BiodentineTM Silicate Cement
Abstract
:1. Introduction
2. Results
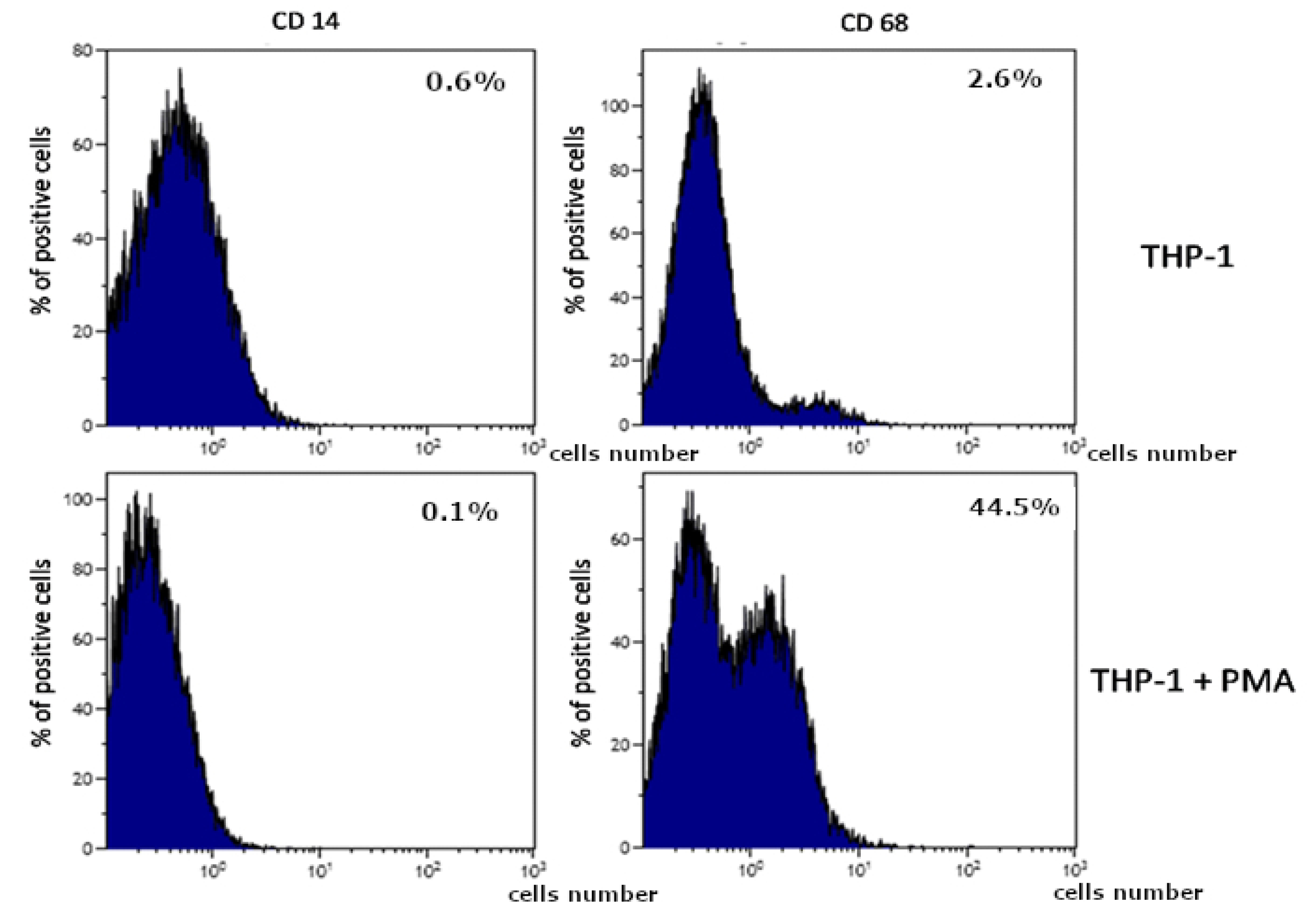
2.1. BiodentineTM -Induced Activation of THP-1 Monocytes
2.2. THP-1 Monocytes and Macrophages Culture Visualization
2.3. Prostaglandin E2 (PGE2) in THP-1 Monocytes and Macrophages
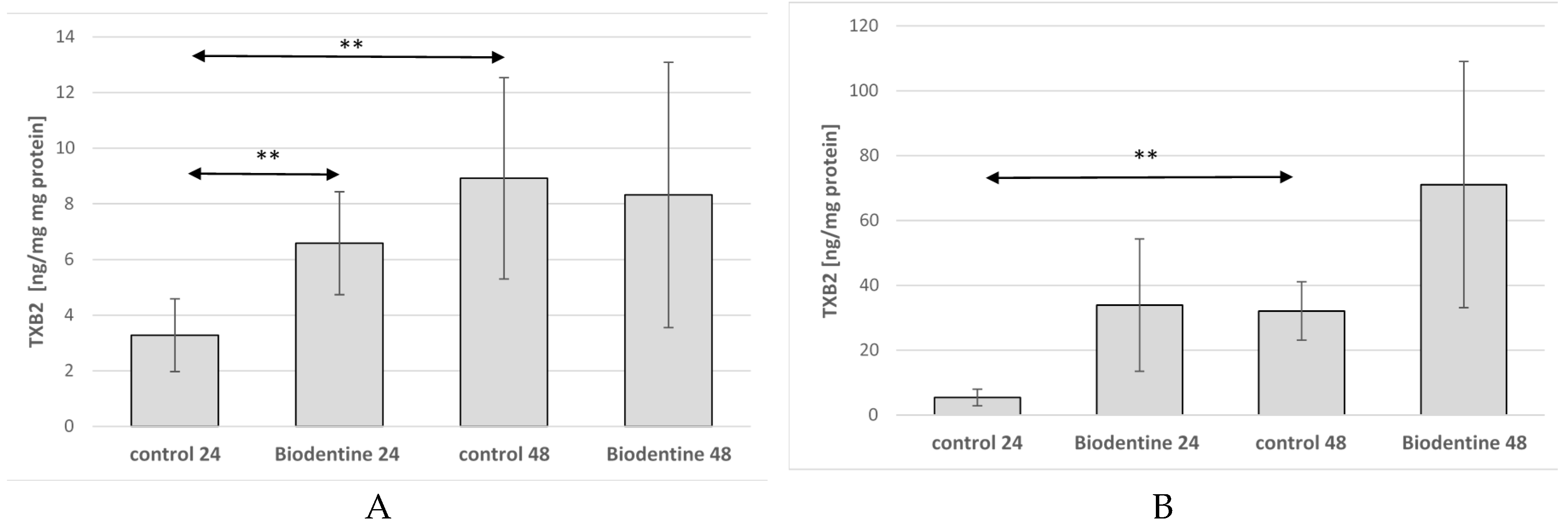
2.4. Thromboxane TXB2 in THP-1 Monocytes and Macrophages
2.5. Cyclooxygenase-1 Expression in Monocytes
2.6. Cyclooxygenase-2 Expression in Macrophages
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Chemical Properties and Preparation of BiodentineTM
4.3. Cell Culture and Treatment
4.4. Verification of BiodentineTM Induced Activation of THP-1 Monocytes and Initiation of Inflammatory Reaction
4.5. The Differentiation of THP-1 Cells into Macrophages. Flow Cytometry Measurement
4.6. Verification of BiodentineTM -Induced Initiation of the Inflammatory Reaction in Macrophages
4.7. Measurements of COX-1 and COX-2 Activity
4.8. Western Blotting Analysis of COX-1 and COX-2 Expression
4.9. Imaging of Cyclooxygenase-1 and Cyclooxygenase-2 Expression
4.10. Protein Concentration
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The use of bioceramics in endodontics—Literature review. Clujul Med. 2016, 89, 470–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emara, R.; Elhennawy, K.; Schwendicke, F. Effects of calcium silicate cements on dental pulp cells: A systematic review. J. Dent. 2018, 77, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Castelo-Baz, P.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50 (Suppl. S2), e63–e72. [Google Scholar] [CrossRef] [Green Version]
- Giraud, T.; Jeanneau, C.; Bergmann, M.; About, I. Tricalcium Silicate Capping Materials Modulate Pulp Healing and Inflammatory Activity in Vitro. J. Endod. 2018, 44, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Paula, A.; Carrilho, E.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Botelho, M.F.; Marto, C.M.; Ferreira, M.M. Direct Pulp Capping: Which is the Most Effective Biomaterial? A Retrospective Clinical Study. Materials (Basel) 2019, 12, 3382. [Google Scholar] [CrossRef] [Green Version]
- Laurent, P.; Camps, J.; About, I. Biodentine (TM) induces TGF-B1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental Pulp Capped with Biodentine and Mineral trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Lipski, M.; Nowicka, A.; Kot, K.; Postek-Stefańska, L.; Wysoczańska-Jankowicz, I.; Borkowski, L.; Andresz, P.; Jarząbek, A.; Grocholewicz, K.; Sobolewska, E.; et al. Factors affecting the outcomes of direct pulp capping using Biodentine. Clin. Oral Investig. 2018, 22, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Da Fonseca, T.S.; Silva, G.F.; Guerreiro-Tanomaru, J.M.; Delfino, M.M.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Biodentine and MTA modulate immunoinflammatory response favoring bone formation in sealing of furcation perforations in rat molars. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus Biodentine: Review of Literature with a Comparative Analysis. J. Clin. Diagn. Res. 2017, 11. [Google Scholar] [CrossRef]
- Da Fonseca, T.S.; Da Silva, G.F.; Tanomaru, M.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. In Vivo evaluation of the inflammatory response and IL-6 immunoexpression promoted by Biodentine and MTA Angelus. Int. Endod. J. 2016, 49, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.M.; Gomes-Cornélio, A.L.; Soares-Costa, A.; Salles, L.P.; Velayutham, M.; Rossa-Junior, C.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. An assessment of the overexpression of BMP-2 in transfected human osteoblast cells stimulated by mineral trioxide aggregate and Biodentine. Int. Endod. J. 2017. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Nazarian, H.; Khojasteh, A.; Shokouhinejad, N. Gene expression and cytokine release during odontogenic differentiation of human dental pulp stem cells induced by 2 endodontic biomaterials. J. Endod. 2014, 40, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abdulkhader, S.Z. Full Pulpotomy with Biodentine in Symptomatic Young Permanent Teeth with Carious Exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef]
- De Rossi, A.; Silva Gatón-Hernández, P.; Sousa-Neto, M.D.; Nelson-Filho, P.; Silva, R.A.; de Queiroz, A.M. Comparison of pulpal responses to pulpotomy and pulp capping with Biodentine and mineral trioxide aggregate in dogs. J. Endod. 2014, 40, 1362–1369. [Google Scholar] [CrossRef]
- Futami, T.; Fujii, N.; Ohnihi, H.; Taguchi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.; Savelkoul, H.F.J.; Wichers, H.J. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A 2004, 70, 194–205. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 792–802. [Google Scholar] [CrossRef] [Green Version]
- Shirakura, M.; Fujii, N.; Ohnishi, H.; Taguchi, Y.; Ohshima, H.; Nomura, S.; Maeda, T. Tissue response to titanium implantation in the rat maxilla, with special reference to the effects of surface conditions on bone formation. Clin. Oral. Implants Res. 2003, 14, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Auger, M.J.; Ross, J.A. The biology of the macrophage. In The Macrophage; IRL Press: New York, NY, USA, 1992; pp. 3–74. [Google Scholar]
- Van Furth, R. Human monocytes and cytokines. Res. Immunol. 1998, 149, 719–720. [Google Scholar] [CrossRef]
- Gordon, S. The macrophage. Bioessays 1995, 17, 977–986. [Google Scholar] [CrossRef]
- DeWitt, D.L. Prostaglandin endoperoxide synthase: Regulation of enzyme expression. Biochim. Biophys. Acta 1991, 1083, 121–134. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Piotrowska, K.; Chlubek, D. Cyclooxygenase-1 as the main source of proinlammatory factors after sodium orthovanadate treatment. Biol. Trace Elem. Res. 2015, 163, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, I. Distinct functions of COX-1 and COX-2 Prostaglandins other. Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar]
- Font-Nieves, M.; Sans-Fons, G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Perdomo, A.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regualtion of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. JBC 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Iadarola, M.; Yang, H.Y.; Dionne, R.A. Expression of COX-1and COX-2 in a clinical model of acute inflammation. J. Pain 2007, 8, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Hohg, H.; Jang, B.C. Transcriptional and translational regulation of COX- 2 in a clinical model of acute inflammation. Int. J. Mol. Med. 2012, 30, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Dinoi, T.; Marzo, G.; Campanella, V.; Giuca, M.R.; Gatto, R.; Pasini, M. Clinical and radiographic evaluation of biodentine versus calcium hydroxide in primary teeth pulpotomies: A retrospective study. BMC Oral Health 2018, 18, 54. [Google Scholar] [CrossRef]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, P.; Nowicka, A.; Lipski, M.; Dura, W.; Droździk, A.; Woźniak, K. A Comparative Chemical Study of Calcium Silicate-Containing and Epoxy Resin-Based Root Canal Sealers. Biomed. Res. Int. 2016, 2016, 9808432. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Ormeño, A.; Cabrera, C.; Cabezas, R.; Silva, C.I.; Ramírez, V.; Mercade, M. Direct Pulp Capping with Calcium Hydroxide, Mineral Trioxide Aggregate and Biodentine in Permanent Young Teeth with Caries: A Randomized Clinical Trial. J. Endod. 2017, 43, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Jalan, A.L.; Warhadpande, M.M.; Dakshindas, D.M. A comparison of human dental pulp response to calcium hydroxide and Biodentine as direct pulp-capping agents. J. Conserv. Dent. 2017, 20, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.M.; Shen, Y.; Wang, Z.J.; Li, L.; Zheng, Y.F.; Häkkinen, L.; Haapasalo, M. In vitro cytotoxicity evaluation of a novel root repair material. J. Endod. 2013, 39, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef]
- Sinkar, R.C.; Patil, S.S.; Jogad, N.P.; Gade, V.J. Comparison of sealing ability of ProRoot MTA, RetroMTA and Biodentine as furcation repair materials: An ultraviolet spectrophotometric analysis. J. Conserv. Dent. 2015, 18, 445–448. [Google Scholar] [CrossRef]
- Guneser, M.B.; Akbulut, M.B.; Eldeniz, A.U. Effect of various endodontic irrigants on the push-out bond strength of biodentine and conventional root perforation repairmaterials. J. Endod. 2013, 39, 380–384. [Google Scholar] [CrossRef]
- Koubi, G.; Colon, P.; Franquin, J.C.; Hartmann, A.; Richard, G.; Faure, M.O.; Lambert, G. Clinical evaluation of the performance and safety of a new dentine substitute, biodentine, in the restoration of posterior teeth—A prospective study. Clin. Oral Investig. 2013, 17, 243–249. [Google Scholar] [CrossRef] [Green Version]
- De Sousa Reis, M.; Scarparo, R.K.; Steier, L.; de Figueiredo, J.A.P. Periradicular inflammatory response, bone resorption and cementum repair after sealing of furcation perforation with mineral trioxide aggregate (MTA Angelus™) or Biodentine™. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.; Laranjo, M.; Marto, C.M.; Abrantes, A.M.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E. Biodentine™ Boosts, WhiteProRoot®MTA Increases and Life® Suppresses Odontoblast Activity. Materials (Basel) 2019, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Chlubek, D. In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E₂ (PGE₂) and Thromboxane B₂ (TXB₂) Concentration in THP-1 Macrophages. Int. J. Mol. Sci. 2017, 18, 2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarette, R. Titanium surface characteristics, includimg topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, L.N.; Jiao, K.; Wang, T.D.; Zhang, W.; Camilleri, J.; Bergeron, B.E.; Feng, H.L.; Mao, J.; Chen, J.H.; Pashley, D.H.; et al. A review of the bioactivity of hydraulic calcium silicate cements. J. Dent. 2014, 42, 517–533. [Google Scholar] [CrossRef] [Green Version]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro model for immunomodulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Auwerx, J. The human leukemia cell line, THP1: A multifacetted model for study of monocyte-macrophage differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef]








| Preparation | Producer | Composition |
|---|---|---|
| BiodentineTM | Septodont France | Powder: |
| tricalcium and dicalcium silicate | ||
| (3CaO·SiO2 and 2CaO·SiO2) | ||
| calcium carbonate (CaCO3) | ||
| Zirconium dioxide (ZrO2) | ||
| Liquid: | ||
| 10% calcium chloride (CaCl2·2H2O) | ||
| water | ||
| polycarboxylate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barczak, K.; Palczewska-Komsa, M.; Nowicka, A.; Chlubek, D.; Buczkowska-Radlińska, J. Analysis of the Activity and Expression of Cyclooxygenases COX1 and COX2 in THP-1 Monocytes and Macrophages Cultured with BiodentineTM Silicate Cement. Int. J. Mol. Sci. 2020, 21, 2237. https://doi.org/10.3390/ijms21062237
Barczak K, Palczewska-Komsa M, Nowicka A, Chlubek D, Buczkowska-Radlińska J. Analysis of the Activity and Expression of Cyclooxygenases COX1 and COX2 in THP-1 Monocytes and Macrophages Cultured with BiodentineTM Silicate Cement. International Journal of Molecular Sciences. 2020; 21(6):2237. https://doi.org/10.3390/ijms21062237
Chicago/Turabian StyleBarczak, Katarzyna, Mirona Palczewska-Komsa, Alicja Nowicka, Dariusz Chlubek, and Jadwiga Buczkowska-Radlińska. 2020. "Analysis of the Activity and Expression of Cyclooxygenases COX1 and COX2 in THP-1 Monocytes and Macrophages Cultured with BiodentineTM Silicate Cement" International Journal of Molecular Sciences 21, no. 6: 2237. https://doi.org/10.3390/ijms21062237






