Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy
Abstract
:1. Introduction
2. Targeting Transcription Factors: Transcription Therapy
2.1. Strategies to Target Transcription Factors
2.2. Transcription: A Complex Process That Can Provide Multiple Targets
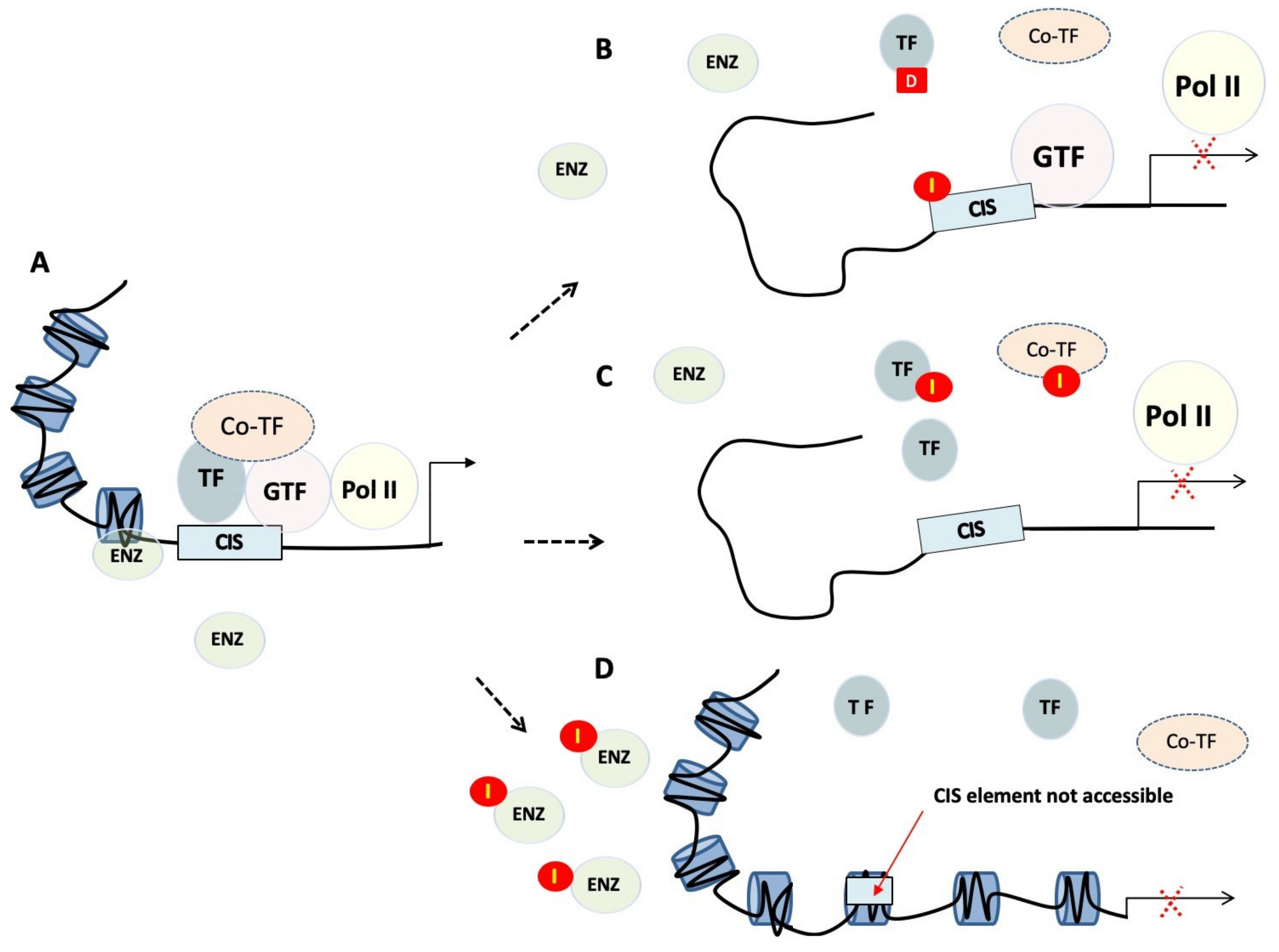
2.2.1. Chromatin Remodelling and Epigenetics
2.2.2. Recruitment of TFs to Cis-regulatory Elements
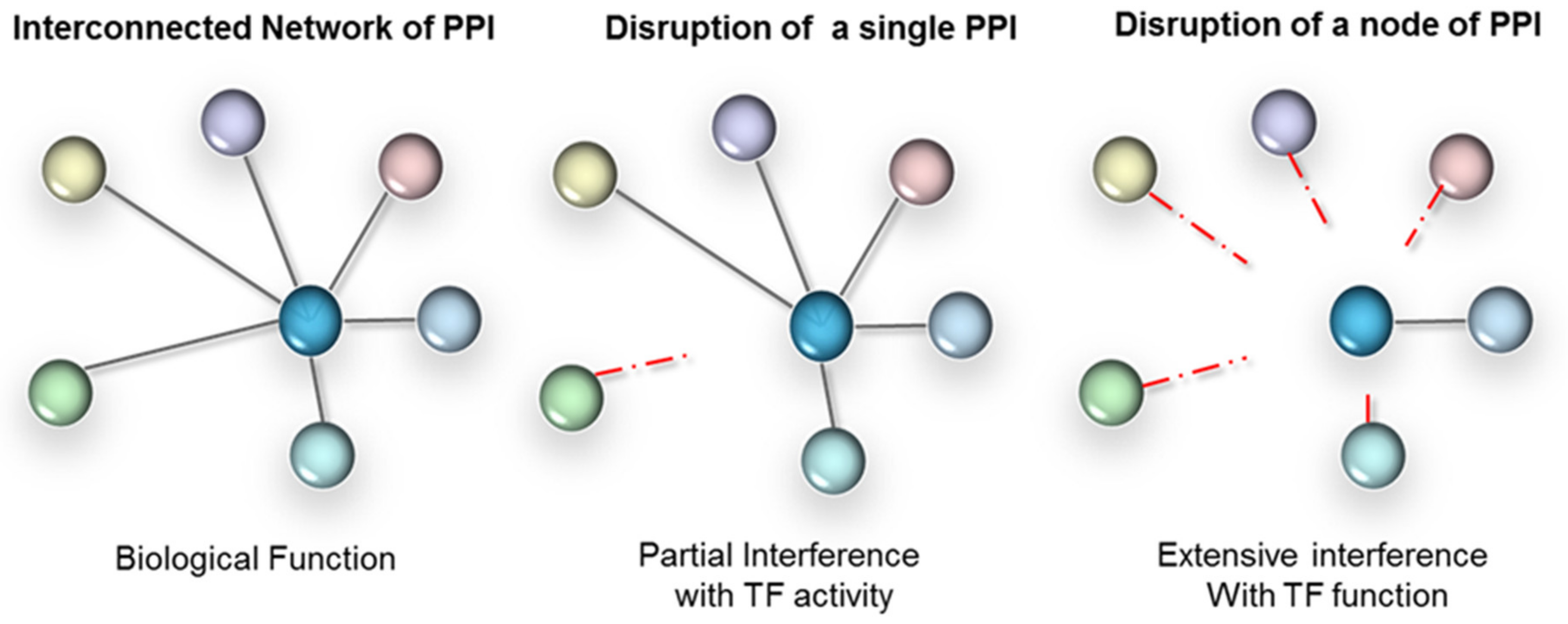
2.2.3. Targeting Protein Complexes
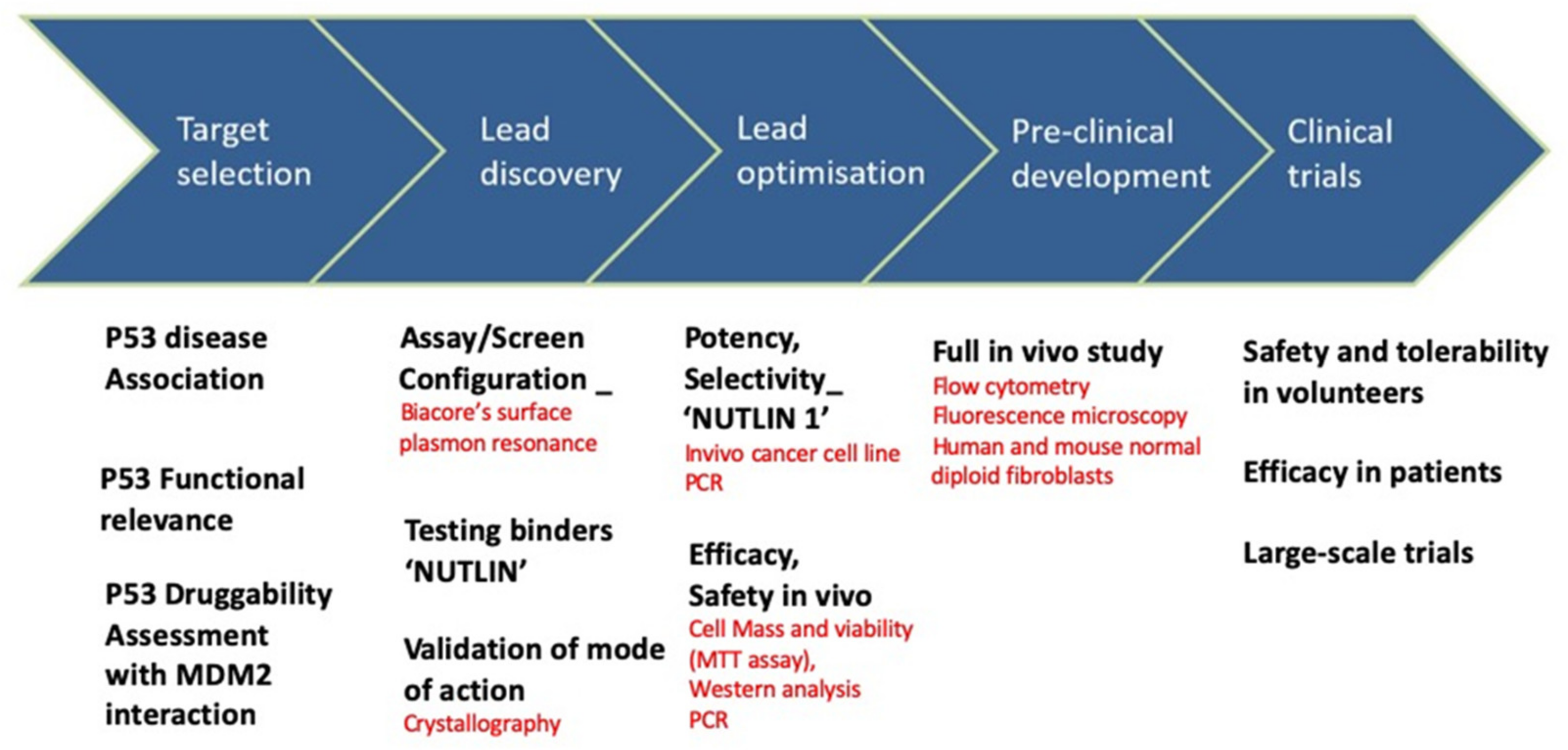
3. Techniques for Target Validation and Drug Screening
3.1. Generalist and Structural Techniques
3.1.1. Mass Spectrometry (MS)
3.1.2. X-ray Crystallography
3.1.3. Nuclear Magnetic Resonance (NMR)
3.2. Protein-Protein Interactions Interrogation Techniques
3.2.1. Surface Plasmon Resonance (SPR)
3.2.2. Isothermal Titration Calorimetry (ITC)
3.2.3. Microscale Thermophoresis (MST)
3.2.4. Affinity Chromatography
3.2.5. Immunoprecipitation
3.2.6. ELISA
3.2.7. Alpha Screen
3.2.8. Förster Resonance Energy Transfer (FRET)
3.3. In Cellulo Techniques
3.3.1. Imaging
3.3.2. Protein-Fragment Complementation Assay (PCA)
3.4. Functional Assays
3.4.1. RNA-seq, ChIP-seq, ChIP-MS
3.4.2. Developmental Models
3.4.3. In Silico Techniques
4. Targeting Transcription Factors: Examples
4.1. p53
4.2. SOX18
4.3. Ets Family (ETS)
Clinical Trials for Transcription Therapy
5. Summary and Perspectives
5.1. Cryo-EM
5.2. Single-Molecule Imaging Techniques
5.3. Molecular Imaging
5.4. Personalized Medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster-Böckler, B.; Bateman, A. Protein interactions in human genetic diseases. Genome Biol. 2008, 9, R9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botstein, D.; Risch, N. Discovering genotypes underlying human phenotypes: Past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 2003, 33, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, P.P. Transcription therapy for cancer. Oncogene 2001, 20, 3116–3127. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Papavassiliou, A.G. Transcription factor therapeutics: Long-shot or lodestone. Curr. Med. Chem. 2005, 12, 691–701. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Ahn, K.S.; Sandur, S.K.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Ichikawa, H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: Modern target but ancient solution. Ann. N. Y. Acad. Sci. 2006, 1091, 151–169. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Young, N.; Hahn, C.N.; Poh, A.; Dong, C.; Wilhelm, D.; Olsson, J.; Muscat, G.E.; Parsons, P.; Gamble, J.R.; Koopman, P. Effect of disrupted SOX18 transcription factor function on tumor growth, vascularization, and endothelial development. J. Natl. Cancer Inst. 2006, 98, 1060–1067. [Google Scholar] [CrossRef]
- Duong, T.; Proulx, S.T.; Luciani, P.; Leroux, J.C.; Detmar, M.; Koopman, P.; Francois, M. Genetic ablation of SOX18 function suppresses tumor lymphangiogenesis and metastasis of melanoma in mice. Cancer Res. 2012, 72, 3105–3114. [Google Scholar] [CrossRef] [Green Version]
- Moellering, R.E.; Cornejo, M.; Davis, T.N.; Del Bianco, C.; Aster, J.C.; Blacklow, S.C.; Kung, A.L.; Gilliland, D.G.; Verdine, G.L.; Bradner, J.E. Direct inhibition of the NOTCH transcription factor complex. Nature 2009, 462, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Green, K.A.; Carroll, J.S. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat. Rev. Cancer 2007, 7, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Coombes, R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2002, 2, 101–112. [Google Scholar] [CrossRef]
- Lupien, M.; Meyer, C.A.; Bailey, S.T.; Eeckhoute, J.; Cook, J.; Westerling, T.; Zhang, X.; Carroll, J.S.; Rhodes, D.R.; Liu, X.S.; et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010, 24, 2219–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuukasjarvi, T.; Kononen, J.; Helin, H.; Holli, K.; Isola, J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 1996, 14, 2584–2589. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Borg, A.; Wolf, D.M.; Oesterreich, S.; Fuqua, S.A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997, 57, 1244–1249. [Google Scholar] [PubMed]
- Filtz, T.M.; Vogel, W.K.; Leid, M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 2014, 35, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.; Cautain, B.; de Pedro, N.; Link, W. Targeting nucleocytoplasmic transport in cancer therapy. Oncotarget 2014, 5, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Tootle, T.L.; Rebay, I. Post-translational modifications influence transcription factor activity: A view from the ETS superfamily. Bioessays 2005, 27, 285–298. [Google Scholar] [CrossRef]
- Melnick, A. Predicting the effect of transcription therapy in hematologic malignancies. Leukemia 2005, 19, 1109–1117. [Google Scholar] [CrossRef]
- Rodriguez-Paredes, M.; Esteller, M. A combined epigenetic therapy equals the efficacy of conventional chemotherapy in refractory advanced non-small cell lung cancer. Cancer Discov. 2011, 1, 557–559. [Google Scholar] [CrossRef] [Green Version]
- Rishi, V.; Potter, T.; Laudeman, J.; Reinhart, R.; Silvers, T.; Selby, M.; Stevenson, T.; Krosky, P.; Stephen, A.G.; Acharya, A.; et al. A high-throughput fluorescence-anisotropy screen that identifies small molecule inhibitors of the DNA binding of B-ZIP transcription factors. Anal. Biochem. 2005, 340, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Souissi, I.; Ladam, P.; Cognet, J.A.; Le Coquil, S.; Varin-Blank, N.; Baran-Marszak, F.; Metelev, V.; Fagard, R. A STAT3-inhibitory hairpin decoy oligodeoxynucleotide discriminates between STAT1 and STAT3 and induces death in a human colon carcinoma cell line. Mol. Cancer 2012, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, K.; Pillay, S.; Bin Ahmad, N.R.; Bikadi, Z.; Hazai, E.; Yan, L.; Kolatkar, P.R.; Pervushin, K.; Jauch, R. Identification of a polyoxometalate inhibitor of the DNA binding activity of Sox2. ACS Chem. Biol. 2011, 6, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Thomas, S.M.; Kim, S.; Yeh, J.I.; Ferris, R.L.; Johnson, J.T.; Duvvuri, U.; Lee, J.; Sahu, N.; Joyce, S.; et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: Implications for cancer therapy. Cancer Discov. 2012, 2, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.H.; Hafley, G.; Harrington, R.A.; Peterson, E.D.; Ferguson, J.T.; Lorenz, T.J.; Goyal, A.; Gibson, M.; Mack, M.J.; Gennevois, D.; et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA 2005, 294, 2446–2454. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Klaus, M.; Prokoph, N.; Girbig, M.; Wang, X.; Huang, Y.H.; Srivastava, Y.; Hou, L.; Narasimhan, K.; Kolatkar, P.R.; Francois, M.; et al. Structure and decoy-mediated inhibition of the SOX18/Prox1-DNA interaction. Nucleic Acids Res. 2016, 44, 3922–3935. [Google Scholar] [CrossRef] [Green Version]
- Veitia, R.A. Exploring the molecular etiology of dominant-negative mutations. Plant Cell 2007, 19, 3843–3851. [Google Scholar] [CrossRef] [Green Version]
- Belikov, S.; Berg, O.G.; Wrange, O. Quantification of transcription factor-DNA binding affinity in a living cell. Nucleic Acids Res. 2016, 44, 3045–3058. [Google Scholar] [CrossRef] [Green Version]
- Fordyce, P.M.; Gerber, D.; Tran, D.; Zheng, J.; Li, H.; DeRisi, J.L.; Quake, S.R. De novo identification and biophysical characterization of transcription-factor binding sites with microfluidic affinity analysis. Nat. Biotechnol. 2010, 28, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Maerkl, S.J.; Quake, S.R. A systems approach to measuring the binding energy landscapes of transcription factors. Science 2007, 315, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskatov, J.A.; Meier, J.L.; Puckett, J.W.; Yang, F.; Ramakrishnan, P.; Dervan, P.B. Modulation of NF-kappaB-dependent gene transcription using programmable DNA minor groove binders. Proc. Natl. Acad. Sci. USA 2012, 109, 1023–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickols, N.G.; Jacobs, C.S.; Farkas, M.E.; Dervan, P.B. Modulating hypoxia-inducible transcription by disrupting the HIF-1-DNA interface. ACS Chem. Biol. 2007, 2, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.D.; Dugan, A.; Gestwicki, J.E.; Mapp, A.K. Fine-tuning multiprotein complexes using small molecules. ACS Chem. Biol. 2012, 7, 1311–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkin, M.R.; Tang, Y.; Wells, J.A. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem. Biol. 2014, 21, 1102–1114. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Overman, J.; Francois, M. Pharmacological manipulation of transcription factor protein-protein interactions: Opportunities and obstacles. Cell Regen. 2015, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.A.; McClendon, C.L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 2007, 450, 1001–1009. [Google Scholar] [CrossRef]
- Berg, T. Inhibition of transcription factors with small organic molecules. Curr. Opin. Chem. Biol. 2008, 12, 464–471. [Google Scholar] [CrossRef]
- Hwang, H.; Vreven, T.; Janin, J.; Weng, Z. Protein-protein docking benchmark version 4.0. Proteins 2010, 78, 3111–3114. [Google Scholar] [CrossRef] [Green Version]
- Fuller, J.C.; Burgoyne, N.J.; Jackson, R.M. Predicting druggable binding sites at the protein-protein interface. Drug Discov. Today 2009, 14, 155–161. [Google Scholar] [CrossRef]
- Azzarito, V.; Long, K.; Murphy, N.S.; Wilson, A.J. Inhibition of alpha-helix-mediated protein-protein interactions using designed molecules. Nat. Chem. 2013, 5, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Graves, B.; Guerlavais, V.; Tovar, C.; Packman, K.; To, K.H.; Olson, K.A.; Kesavan, K.; Gangurde, P.; Mukherjee, A.; et al. Stapled alpha-helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. USA 2013, 110, E3445–E3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, A.L.; Keseru, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef]
- Moreira, I.S.; Fernandes, P.A.; Ramos, M.J. Hot spots—A review of the protein-protein interface determinant amino-acid residues. Proteins 2007, 68, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Basse, M.J.; Betzi, S.; Bourgeas, R.; Bouzidi, S.; Chetrit, B.; Hamon, V.; Morelli, X.; Roche, P. 2P2Idb: A structural database dedicated to orthosteric modulation of protein-protein interactions. Nucleic Acids Res. 2013, 41, D824–D827. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Gestwicki, J.E. Features of protein-protein interactions that translate into potent inhibitors: Topology, surface area and affinity. Expert Rev. Mol. Med. 2012, 14, e16. [Google Scholar] [CrossRef] [Green Version]
- Higueruelo, A.P.; Schreyer, A.; Bickerton, G.R.; Pitt, W.R.; Groom, C.R.; Blundell, T.L. Atomic interactions and profile of small molecules disrupting protein-protein interfaces: The TIMBAL database. Chem. Biol. Drug Des. 2009, 74, 457–467. [Google Scholar] [CrossRef]
- Basse, M.J.; Betzi, S.; Morelli, X.; Roche, P. 2P2Idb v2: Update of a structural database dedicated to orthosteric modulation of protein-protein interactions. Database 2016, 2016. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Vita, M.; Henriksson, M. The Myc oncoprotein as a therapeutic target for human cancer. Semin. Cancer Biol. 2006, 16, 318–330. [Google Scholar] [CrossRef]
- Kiessling, A.; Wiesinger, R.; Sperl, B.; Berg, T. Selective inhibition of c-Myc/Max dimerization by a pyrazolo[1,5-a]pyrimidine. ChemMedChem 2007, 2, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Cohen, S.B.; Desharnais, J.; Sonderegger, C.; Maslyar, D.J.; Goldberg, J.; Boger, D.L.; Vogt, P.K. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 3830–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Vogt, P.K.; Boger, D.L.; Lunec, J. Disruption of the MYC transcriptional function by a small-molecule antagonist of MYC/MAX dimerization. Oncol. Rep. 2008, 19, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Stellas, D.; Szabolcs, M.; Koul, S.; Li, Z.; Polyzos, A.; Anagnostopoulos, C.; Cournia, Z.; Tamvakopoulos, C.; Klinakis, A.; Efstratiadis, A. Therapeutic effects of an anti-Myc drug on mouse pancreatic cancer. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Balling, R.; Zeng, A.-P. Reverse engineering and verification of gene networks: Principles, assumptions, and limitations of present methods and future perspectives. J. Biotechnol. 2009, 144, 190–203. [Google Scholar] [CrossRef]
- Kitano, H. Systems biology: A brief overview. Science 2002, 295, 1662–1664. [Google Scholar] [CrossRef] [Green Version]
- Laubenbacher, R.; Stigler, B. A computational algebra approach to the reverse engineering of gene regulatory networks. J. Theor. Biol. 2004, 229, 523–537. [Google Scholar] [CrossRef] [Green Version]
- Collas, P. The current state of chromatin immunoprecipitation. Mol. Biotechnol. 2010, 45, 87–100. [Google Scholar] [CrossRef]
- Park, P.J. ChIP–seq: Advantages and challenges of a maturing technology. Nat. Rev. Genet. 2009, 10, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.; D’Santos, C.; Serandour, A.A.; Ali, H.R.; Brown, G.D.; Atkins, A.; Rueda, O.M.; Holmes, K.A.; Theodorou, V.; Robinson, J.L.; et al. Endogenous purification reveals GREB1 as a key estrogen receptor regulatory factor. Cell Rep. 2013, 3, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Sierecki, E.; Stevers, L.M.; Giles, N.; Polinkovsky, M.E.; Moustaqil, M.; Mureev, S.; Johnston, W.A.; Dahmer-Heath, M.; Skalamera, D.; Gonda, T.J.; et al. Rapid mapping of interactions between human SNX-BAR proteins measured in vitro by AlphaScreen and single-molecule spectroscopy. Mol. Cell. Proteom. 2014, 13, 2233–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierecki, E.; Polinkovsky, M.; Giles, N.; Moustaqil, M.; Alexandrov, K.; Gambin, Y. Probing the architecture of the Mediator complex (939.3). FASEB J. 2014, 28, 939.3. [Google Scholar]
- Shi, Y. A glimpse of structural biology through X-ray crystallography. Cell 2014, 159, 995–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blundell, T.L.; Patel, S. High-throughput X-ray crystallography for drug discovery. Curr. Opin. Pharmacol. 2004, 4, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Tuukkanen, A.T.; Svergun, D.I. Weak protein–ligand interactions studied by small-angle X-ray scattering. FEBS J. 2014, 281, 1974–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, B.; Sayers, Z. Investigating increasingly complex macromolecular systems with small-angle X-ray scattering. IUCrJ 2014, 1, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Serber, Z.; Ledwidge, R.; Miller, S.M.; Dötsch, V. Evaluation of parameters critical to observing proteins inside living escherichia c oli by in-cell NMR spectroscopy. J. Am. Chem. Soc. 2001, 123, 8895–8901. [Google Scholar] [CrossRef]
- Cooper, M.A. Optical biosensors in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef]
- Geschwindner, S.; Carlsson, J.F.; Knecht, W. Application of optical biosensors in small-molecule screening activities. Sensors 2012, 12, 4311–4323. [Google Scholar] [CrossRef] [Green Version]
- Velazquez-Campoy, A.; Freire, E. Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat. Protoc. 2006, 1, 186–191. [Google Scholar] [CrossRef]
- Chaires, J.B. Calorimetry and thermodynamics in drug design. Annu. Rev. Biophys. 2008, 37, 135–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, S.A.; Dijkman, P.M.; Lea, W.A.; van den Bogaart, G.; Jerabek-Willemsen, M.; Lazic, A.; Joseph, J.S.; Srinivasan, P.; Baaske, P.; Simeonov, A.; et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 2013, 59, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Yamaguchi, Y. Affinity chromatography of native and recombinant proteins from receptors for insulin and IGF-I to recombinant single chain antibodies. Front. Endocrinol. 2015, 6, 166. [Google Scholar] [CrossRef] [Green Version]
- Bonifacino, J.S.; Dell’Angelica, E.C.; Springer, T.A. Immunoprecipitation. Curr. Protoc. Mol. Biol. 1999, 48, 10.16.1–10.16.29. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.F.; Kirakossian, H.; Singh, S.; Wu, Z.P.; Irvin, B.R.; Pease, J.S.; Switchenko, A.C.; Irvine, J.D.; Dafforn, A.; Skold, C.N. Luminescent oxygen channeling immunoassay: Measurement of particle binding kinetics by chemiluminescence. Proc. Natl. Acad. Sci. USA 1994, 91, 5426–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierecki, E.; Giles, N.; Polinkovsky, M.; Moustaqil, M.; Alexandrov, K.; Gambin, Y. A cell-free approach to accelerate the study of protein–protein interactions In Vitro. Interface Focus 2013, 3, 20130018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, G.; Illy, C.; Pedro, L.; Roby, P.; Bosse, R. AlphaScreen™ kinase HTS platforms. Curr. Med. Chem. 2004, 11, 721–730. [Google Scholar] [CrossRef]
- Guenat, S.; Rouleau, N.; Bielmann, C.; Bedard, J.; Maurer, F.; Allaman-Pillet, N.; Nicod, P.; Bielefeld-Sevigny, M.; Beckmann, J.S.; Bonny, C.; et al. Homogeneous and nonradioactive high-throughput screening platform for the characterization of kinase inhibitors in cell lysates. J. Biomol. Screen. 2006, 11, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Medintz, I.L.; Hildebrandt, N. FRET-Förster Resonance Energy Transfer: From Theory to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schröder, G.F.; Grubmüller, H. FRETsg: Biomolecular structure model building from multiple FRET experiments. Comput. Phys. Commun. 2004, 158, 150–157. [Google Scholar] [CrossRef]
- Noah, J.W. New developments and emerging trends in high-throughput screening methods for lead compound identification. Int. J. High Throughput Screen. 2010, 1, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Karaman, B.; Sippl, W. Computational Drug Repurposing: Current Trends. Curr. Med. Chem. 2019, 26, 5389–5409. [Google Scholar] [CrossRef] [PubMed]
- Michnick, S.W.; Ear, P.H.; Manderson, E.N.; Remy, I.; Stefan, E. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat. Rev. Drug Discov. 2007, 6, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, L.; Di, L.-J. Applications of Protein Fragment Complementation Assays for Analyzing Biomolecular Interactions and Biochemical Networks in Living Cells. J. Proteome Res. 2019, 18, 2987–2998. [Google Scholar] [CrossRef] [PubMed]
- Wiens, M.D.; Campbell, R.E. Surveying the landscape of optogenetic methods for detection of protein-protein interactions. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1415. [Google Scholar] [CrossRef] [PubMed]
- Massoud, T.F.; Paulmurugan, R.; Gambhir, S.S. A molecularly engineered split reporter for imaging protein-protein interactions with positron emission tomography. Nat. Med. 2010, 16, 921–926. [Google Scholar] [CrossRef]
- Pedelacq, J.-D.; Cabantous, S. Development and Applications of Superfolder and Split Fluorescent Protein Detection Systems in Biology. Int. J. Mol. Sci. 2019, 20, 3479. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.S.; Schwinn, M.K.; Hall, M.P.; Zimmerman, K.; Otto, P.; Lubben, T.H.; Butler, B.L.; Binkowski, B.F.; Machleidt, T.; Kirkland, T.A.; et al. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 2016, 11, 400–408. [Google Scholar] [CrossRef]
- Kerppola, T.K. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 2008, 37, 465–487. [Google Scholar] [CrossRef] [Green Version]
- Paulmurugan, R.; Gambhir, S.S. Firefly Luciferase Enzyme Fragment Complementation for Imaging in Cells and Living Animals. Anal. Chem. 2005, 77, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Sung, M.K.; Huh, W.K. Bimolecular fluorescence complementation analysis system for In Vivo detection of protein-protein interaction in Saccharomyces cerevisiae. Yeast 2007, 24, 767–775. [Google Scholar] [CrossRef]
- Schutze, K.; Harter, K.; Chaban, C. Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol. Biol. 2009, 479, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.; Vasudevan, L.; Crans, R.A.J.; Saini, D.K.; Stove, C.P. Luminescence- and Fluorescence-Based Complementation Assays to Screen for GPCR Oligomerization: Current State of the Art. Int. J. Mol. Sci. 2019, 20, 2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, J.; Watanabe, T.; Seki, T.; Karasawa, S.; Izumikawa, M.; Seki, T.; Iemura, S.I.; Natsume, T.; Nomura, N.; Goshima, N.; et al. Novel In Vitro protein fragment complementation assay applicable to high-throughput screening in a 1536-well format. J. Biomol. Screen. 2009, 14, 970–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poe, J.A.; Vollmer, L.; Vogt, A.; Smithgall, T.E. Development and validation of a high-content bimolecular fluorescence complementation assay for small-molecule inhibitors of HIV-1 Nef dimerization. J. Biomol. Screen. 2014, 19, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.N.; Lo, Y.K.; Lin, Y.S.; Tang, T.K.; Hsu, C.H.; Hsu, J.T.; Lee, A.Y. Identification of Novel Cdc7 Kinase Inhibitors as Anti-Cancer Agents that Target the Interaction with Dbf4 by the Fragment Complementation and Drug Repositioning Approach. EBioMedicine 2018, 36, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Hudry, B.; Viala, S.; Graba, Y.; Merabet, S. Visualization of protein interactions in living Drosophila embryos by the bimolecular fluorescence complementation assay. BMC Biol. 2011, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Kerppola, T.K. Visualization of the Genomic Loci That Are Bound by Specific Multiprotein Complexes by Bimolecular Fluorescence Complementation Analysis on Drosophila Polytene Chromosomes. Methods Enzymol. 2017, 589, 429–455. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Bandilla, P.; von Reutern, M.; Schnepf, M.; Rieder, S.; Unnerstall, U.; Gaul, U. True equilibrium measurement of transcription factor-DNA binding affinities using automated polarization microscopy. Nat. Commun. 2018, 9, 1605. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.D.; Nemčko, F.; Woodfin, A.R.; Wienerroither, S.; Vlasova, A.; Schleiffer, A.; Pagani, M.; Rath, M.; Stark, A. A high-throughput method to identify trans-activation domains within transcription factor sequences. EMBO J. 2018, 37, e98896. [Google Scholar] [CrossRef]
- Glick, Y.; Orenstein, Y.; Chen, D.; Avrahami, D.; Zor, T.; Shamir, R.; Gerber, D. Integrated microfluidic approach for quantitative high-throughput measurements of transcription factor binding affinities. Nucleic Acids Res. 2015, 44, e51. [Google Scholar] [CrossRef] [Green Version]
- Bischof, J.; Duffraisse, M.; Furger, E.; Ajuria, L.; Giraud, G.; Vanderperre, S.; Paul, R.; Björklund, M.; Ahr, D.; Ahmed, A.W.; et al. Generation of a versatile BiFC ORFeome library for analyzing protein–protein interactions in live Drosophila. eLife 2018, 7, e38853. [Google Scholar] [CrossRef]
- Lepur, A.; Kovačević, L.; Belužić, R.; Vugrek, O. Combining Unique Multiplex Gateway Cloning and Bimolecular Fluorescence Complementation (BiFC) for High-Throughput Screening of Protein–Protein Interactions. J. Biomol. Screen. 2016, 21, 1100–1111. [Google Scholar] [CrossRef] [Green Version]
- Remy, I.; Campbell-Valois, F.; Michnick, S.W. Detection of protein–protein interactions using a simple survival protein-fragment complementation assay based on the enzyme dihydrofolate reductase. Nat. Protoc. 2007, 2, 2120. [Google Scholar] [CrossRef]
- Moustaqil, M.; Bhumkar, A.; Gonzalez, L.; Raoul, L.; Hunter, D.J.; Carrive, P.; Sierecki, E.; Gambin, Y. A Split-Luciferase Reporter Recognizing GFP and mCherry Tags to Facilitate Studies of Protein–Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2681. [Google Scholar] [CrossRef] [Green Version]
- Dedon, P.C.; Soults, J.A.; Allis, C.D.; Gorovsky, M.A. A simplified formaldehyde fixation and immunoprecipitation technique for studying protein-DNA interactions. Anal. Biochem. 1991, 197, 83–90. [Google Scholar] [CrossRef]
- Euskirchen, G.M.; Rozowsky, J.S.; Wei, C.L.; Lee, W.H.; Zhang, Z.D.; Hartman, S.; Emanuelsson, O.; Stolc, V.; Weissman, S.; Gerstein, M.B.; et al. Mapping of transcription factor binding regions in mammalian cells by ChIP: Comparison of array-and sequencing-based technologies. Genome Res. 2007, 17, 898–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.P.; VerMilyea, M.D.; Turner, B.M. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat. Genet. 2006, 38, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.D.; Denisenko, O.; Bomsztyk, K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat. Protoc. 2006, 1, 179. [Google Scholar] [CrossRef]
- Dainese, R.; Gardeux, V.; Llimos, G.; Alpern, D.; Jiang, J.Y.; Meireles-Filho, A.C.A.; Deplancke, B. A highly parallel, automated platform enabling individual or sequential ChIP of histone marks and transcription factors. bioRxiv 2019, 728634. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [Green Version]
- Suckling, K. Animal research: Too much faith in models clouds judgement. Nature 2008, 455, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philos. Ethics Humanit. Med. 2009, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786. [Google Scholar] [CrossRef] [PubMed]
- Wendler, A.; Wehling, M. The translatability of animal models for clinical development: Biomarkers and disease models. Curr. Opin. Pharmacol. 2010, 10, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; Shepard, K.A.; DeWitt, N.D. Human disease modeling with induced pluripotent stem cells. Curr. Opin. Genet. Dev. 2012, 22, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Scheer, N.; Wolf, C.R. Genetically humanized mouse models of drug metabolizing enzymes and transporters and their applications. Xenobiotica 2014, 44, 96–108. [Google Scholar] [CrossRef]
- Li, A.P.; Segall, M. Early ADME/Tox studies and in silico screening. Drug Discov. Today 2002, 7, 25–27. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.-H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H.; De Groot, M. Can the Internet help to meet the challenges in ADME and e-ADME? SAR QSAR Environ. Res. 2002, 13, 391–401. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H. High-throughput and in silico techniques in drug metabolism and pharmacokinetics. Curr. Opin. Drug Discov. Dev. 2002, 5, 33. [Google Scholar]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finlay, C.A. The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol. Cell. Biol. 1993, 13, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In Vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Wegner, M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. [Google Scholar] [CrossRef]
- Eom, B.W.; Jo, M.J.; Kook, M.C.; Ryu, K.W.; Choi, I.J.; Nam, B.H.; Kim, Y.W.; Lee, J.H. The lymphangiogenic factor SOX 18: A key indicator to stage gastric tumor progression. Int. J. Cancer 2012, 131, 41–48. [Google Scholar] [CrossRef]
- Overman, J.; Fontaine, F.; Moustaqil, M.; Mittal, D.; Sierecki, E.; Sacilotto, N.; Zuegg, J.; Robertson, A.A.; Holmes, K.; Salim, A.A.; et al. Pharmacological targeting of the transcription factor SOX18 delays breast cancer in mice. eLife 2017, 6. [Google Scholar] [CrossRef]
- Moustaqil, M.; Fontaine, F.; Overman, J.; McCann, A.; Bailey, T.L.; Rudolffi Soto, P.; Bhumkar, A.; Giles, N.; Hunter, D.J.; Gambin, Y.; et al. Homodimerization regulates an endothelial specific signature of the SOX18 transcription factor. Nucleic Acids Res. 2018, 46, 11381–11395. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Overman, J.; Moustaqil, M.; Mamidyala, S.; Salim, A.; Narasimhan, K.; Prokoph, N.; Robertson, A.A.; Lua, L.; Alexandrov, K.; et al. Small-Molecule Inhibitors of the SOX18 Transcription Factor. Cell Chem. Biol. 2017, 24, 346–359. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.R.; Goodall, S.; Prokop, J.W.; Howard, C.B.; Moustaqil, M.; Kumble, S.; Rasicci, D.T.; Osborne, G.W.; Gambin, Y.; Sierecki, E.; et al. Functional domain analysis of SOX18 transcription factor using a single-chain variable fragment-based approach. mAbs 2018, 10, 596–606. [Google Scholar] [CrossRef]
- Wu, Y.; Duesberg, P. Avian erythroblastosis virus E26: Only one (myb) of two cell-derived coding regions is necessary for oncogenicity. Proc. Natl. Acad. Sci. USA 1994, 91, 4039–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.L.; Schaffner, A.E.; Hofmeister, J.K.; Hartman, M.; Wei, G.; Forsthoefel, D.; Hume, D.A.; Ostrowski, M.C. ets-2 Is a Target for an Akt (Protein Kinase B)/Jun N-Terminal Kinase Signaling Pathway in Macrophages ofmotheaten-viable Mutant Mice. Mol. Cell. Biol. 2000, 20, 8026–8034. [Google Scholar] [CrossRef] [PubMed]
- Bassuk, A.G.; Leiden, J.M. The role of Ets transcription factors in the development and function of the mammalian immune system. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 64, pp. 65–104. [Google Scholar]
- Oikawa, T.; Yamada, T. Molecular biology of the Ets family of transcription factors. Gene 2003, 303, 11–34. [Google Scholar] [CrossRef]
- Oikawa, T. ETS transcription factors: Possible targets for cancer therapy. Cancer Sci. 2004, 95, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Leyh, B. The impact of tumor stroma on drug response in breast cancer. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 20–38. [Google Scholar]
- Pourtier-Manzanedo, A.; Vercamer, C.; Van Belle, E.; Mattot, V.; Mouquet, F.; Vandenbunder, B. Expression of an Ets-1 dominant-negative mutant perturbs normal and tumor angiogenesis in a mouse ear model. Oncogene 2003, 22, 1795–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, X.; Wang, S.C.; Xia, W. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat. Med. 2000, 6, 189–195. [Google Scholar] [CrossRef]
- Kitange, G.; Shibata, S.; Tokunaga, Y.; Yagi, N.; Yasunaga, A.; Kishikawa, M.; Naito, S. Ets-1 transcription factor-mediated urokinase-type plasminogen activator expression and invasion in glioma cells stimulated by serum and basic fibroblast growth factors. Lab. Investig. J. Tech. Methods Pathol. 1999, 79, 407–416. [Google Scholar]
- Song, E.; Lee, S.K.; Wang, J.; Ince, N.; Ouyang, N.; Min, J.; Chen, J.; Shankar, P.; Lieberman, J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003, 9, 347–351. [Google Scholar] [CrossRef]
- Lambert, M.; Jambon, S.; Depauw, S.; David-Cordonnier, M.-H. Targeting transcription factors for cancer treatment. Molecules 2018, 23, 1479. [Google Scholar] [CrossRef] [Green Version]
- Petrylak, D.P.; De Wit, R.; Chi, K.N.; Drakaki, A.; Sternberg, C.N.; Nishiyama, H.; Castellano, D.; Hussain, S.A.; Fléchon, A.; Bamias, A.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): Overall survival and updated results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2020, 21, 105–120. [Google Scholar] [CrossRef]
- Crunkhorn, S. Heart failure drug effective in medulloblastoma. Nat. Rev. Drug Discov. 2018, 17, 864. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Reid, R.H.; Bushnell, D.L.; Quick, D.P.; Ell, P.J. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 637–643. [Google Scholar] [CrossRef]
- Henderson, R.; Baldwin, J.M.; Ceska, T.A.; Zemlin, F.; Beckmann, E.A.; Downing, K.H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 1990, 213, 899–929. [Google Scholar] [CrossRef]
- Ge, P.; Zhou, Z.H. Hydrogen-bonding networks and RNA bases revealed by cryo electron microscopy suggest a triggering mechanism for calcium switches. Proc. Natl. Acad. Sci. USA 2011, 108, 9637–9642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.C.; McMullan, G.; Scheres, S.H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 2015, 40, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Walker, M.; Siebert, C.A.; Muench, S.P.; Ranson, N.A. An introduction to sample preparation and imaging by cryo-electron microscopy for structural biology. Methods 2016, 100, 3–15. [Google Scholar] [CrossRef]
- Merk, A.; Bartesaghi, A.; Banerjee, S.; Falconieri, V.; Rao, P.; Davis, M.I.; Pragani, R.; Boxer, M.B.; Earl, L.A.; Milne, J.L.; et al. Breaking cryo-EM resolution barriers to facilitate drug discovery. Cell 2016, 165, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lavis, L.D.; Betzig, E. Imaging live-cell dynamics and structure at the single-molecule level. Mol. Cell 2015, 58, 644–659. [Google Scholar] [CrossRef] [Green Version]
- Ji, N.; Van Oudenaarden, A. Single molecule fluorescent in situ hybridization (smFISH) of C.Elegans worms and embryos. WormBook 2012. [Google Scholar] [CrossRef] [Green Version]
- Raj, A.; Van Den Bogaard, P.; Rifkin, S.A.; Van Oudenaarden, A.; Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 2008, 5, 877–879. [Google Scholar] [CrossRef] [Green Version]
- Abranches, E.; Guedes, A.M.; Moravec, M.; Maamar, H.; Svoboda, P.; Raj, A.; Henrique, D. Stochastic NANOG fluctuations allow mouse embryonic stem cells to explore pluripotency. Development 2014, 141, 2770–2779. [Google Scholar] [CrossRef] [Green Version]
- Bartman, C.R.; Hsu, S.C.; Hsiung, C.C.-S.; Raj, A.; Blobel, G.A. Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Mol. Cell 2016, 62, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Larson, D.R.; Fritzsch, C.; Sun, L.; Meng, X.; Lawrence, D.S.; Singer, R.H. Direct observation of frequency modulated transcription in single cells using light activation. eLife 2013, 2, e00750. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culver, J.; Akers, W.; Achilefu, S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008, 49, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Möller, A.; Souvatzoglou, M.; Delso, G.; Bundschuh, R.A.; Chefd’hotel, C.; Ziegler, S.I.; Navab, N.; Schwaiger, M.; Nekolla, S.G. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: Evaluation with PET/CT data. J. Nucl. Med. 2009, 50, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, R.A.; Hoffman, E.A.; Sinak, L.J.; Harris, L.D.; Ritman, E.L. High-speed three-dimensional x-ray computed tomography: The dynamic spatial reconstructor. Proc. IEEE 1983, 71, 308–319. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Razansky, D. Adding fifth dimension to optoacoustic imaging: Volumetric time-resolved spectrally enriched tomography. Light Sci. Appl. 2014, 3, e137. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Liang, H.; Butte, A.J.; Eng, C.; Nussinov, R. Personal mutanomes meet modern oncology drug discovery and precision health. Pharmacol. Rev. 2019, 71, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [Green Version]

| Drug | Target | National Clinical Trial (NCT) |
|---|---|---|
| NCT02407080 | ||
| RG7388/idasanutlin | p53/MDM3 | NCT03287245 |
| NCT02828930 | ||
| NCT03362723 | ||
| NCT03107780 | ||
| AMG232 | p53/MDM2 | NCT02016729 |
| NCT01723020 | ||
| NCT02110355 | ||
| NCT00955812 | ||
| OPB-31122 | STAT3 | NCT00511082 |
| NCT01406574 | ||
| NCT00657176 | ||
| NCT01423903 | ||
| OPB-51602 | STAT3 | NCT01344876 |
| NCT01184807 | ||
| NCT01867073 | ||
| ET743 | DNA | NCT01692678 NCT01343277 |
| NCT00070109 | ||
| NCT01453283 | ||
| BC- 2059/ Tegavivint | TBLl/CTNNBl | NCT03459469 |
| E-7386 | CREB/CTNNBl | NCT03833700 |
| NCT03264664 | ||
| NCT01711034 | ||
| OPB-111077 | STAT3 SH2 interactor | NCT03197714 |
| NCT03158324 | ||
| MK6482 | HIFl complex | NCT04195750 |
| TK216 | EWS-FLI/RHA | NCT02657005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustaqil, M.; Gambin, Y.; Sierecki, E. Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy. Int. J. Mol. Sci. 2020, 21, 2301. https://doi.org/10.3390/ijms21072301
Moustaqil M, Gambin Y, Sierecki E. Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy. International Journal of Molecular Sciences. 2020; 21(7):2301. https://doi.org/10.3390/ijms21072301
Chicago/Turabian StyleMoustaqil, Mehdi, Yann Gambin, and Emma Sierecki. 2020. "Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy" International Journal of Molecular Sciences 21, no. 7: 2301. https://doi.org/10.3390/ijms21072301
APA StyleMoustaqil, M., Gambin, Y., & Sierecki, E. (2020). Biophysical Techniques for Target Validation and Drug Discovery in Transcription-Targeted Therapy. International Journal of Molecular Sciences, 21(7), 2301. https://doi.org/10.3390/ijms21072301





