Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis
Abstract
1. Introduction
2. Results
2.1. Immunophenotypic Characterization of Peripheral Blood and Peritoneal Fluid Mononuclear Cells in Patients with Endometriosis and Non-endometriotic Women
2.2. Altered TIM-3 Expression by Peripheral Blood and Peritoneal Fluid Mononuclear Cell Subsets in Patients with Endometriosis and Non-Endometriotic Women
2.3. Differential Expression of Cell Surface Galectin-9 by Peripheral Blood and Peritoneal Fluid Mononuclear Cell Subsets in Patients with Endometriosis and Non-Endometriotic Women
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Patients and Sample Collection
4.3. Lymphocyte Separation, Cryopreservation, and Thawing
4.4. Antibodies
4.5. Immunolabeling of Leukocytes and Flow Cytometric Analysis
4.6. FoxP3 Intracellular Labeling
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TIM | T-cell immunoglobulin and mucin domain |
| Gal | Galectin |
| PB | Peripheral blood |
| PF | Peritoneal fluid |
| NK | Natural Killer cell |
| CD | Cluster of differentiation |
| Treg | Regulatory T cell |
| FoxP3 | Forkhead box P3 |
| Th | T helper cell |
| TCR | T cell receptor |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| IL-1β | Interleukin 1 beta |
| TNFα | Tumor Necrosis Factor alpha |
| PBMC | Peripheral blood mononuclear cell |
| NE | Non-endometriotic |
| E | Endometriosis |
| PTL | Peritoneal fluid leukocytes |
| rASRM | revised American Society of Reproductive Medicine |
| EU | European Union |
| GDPR | General Data Protection Regulation |
| SD | Standard deviation |
| BMI | Body mass index |
| GnRH | Gonadotropin releasing hormone |
| DIE | Deep infiltrative endometriosis |
| NKT | Natural Killer T cell |
| n.s. | Non-significant |
| MFI | Mean Fluorescent Intensity |
| K2EDTA | Dipotassium-Ethylene Diamine Tetraacetic Acid |
| RPMI | Roswell Park Memorial Institute |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| FITC | Fluorescein isothiocyanate |
| PE | Phycoerythrin |
| PerCP | Peridinin-chlorophyll protein |
| APC | Allophycocyanin |
| D-PBS | Dulbecco’s phosphate saline buffer |
| PFA | Paraformaldehyde |
| FACS | Fluorescence-activated cell sorting |
References
- Barbosa, C.P.; De Souza, Â.M.B.; Bianco, B.; Christofolini, D.M.; Mafra, F.A.; De Lima, G.R. OC-125 immunostaining in endometriotic lesion samples. Arch. Gynecol. Obstet. 2010, 281, 43–47. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Liu, H.; Lang, J.H. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med. Sci. Monit. 2011, 17, 92–99. [Google Scholar]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and immune dysfunction in endometriosis. BioMed Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef]
- Olkowska-Truchanowicz, J.; Bocian, K.; Maksym, R.B.; Białoszewska, A.; Włodarczyk, D.; Baranowski, W.; Ząbek, J.; Korczak-Kowalska, G.; Malejczyk, J. CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum. Reprod. 2013, 28, 119–124. [Google Scholar] [CrossRef]
- Budiu, R.A.; Diaconu, I.; Chrissluis, R.; Dricu, A.; Edwards, R.P.; Vlad, A.M. A conditional mouse model for human MUC1-positive endometriosis shows the presence of anti-MUC1 antibodies and Foxp3+ regulatory T cells. Dis. Model. Mech. 2009, 2, 593–603. [Google Scholar] [CrossRef]
- Barcz, E.; Kamiński, P.; Marianowski, L. Role of cytokines in pathogenesis of endometriosis. Med. Sci. Monit. 2000, 6, 1042–1046. [Google Scholar]
- Mier-Cabrera, J.; Jiménez-Zamudio, L.; García-Latorre, E.; Cruz-Orozco, O.; Hernández-Guerrero, C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG 2011, 118, 6–16. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; de Bruyn, M.; Helfrich, W.; Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med. Res. Rev. 2013, 33 (Suppl. S1), E102–E126. [Google Scholar] [CrossRef] [PubMed]
- Spitzenberger, F.; Graessler, J.; Schroeder, H.E. Molecular and functional characterization of galectin 9 mRNA isoforms in porcine and human cells and tissue. Biochimie 2001, 83, 851–862. [Google Scholar] [CrossRef]
- Heusschen, R.; Freitag, N.; Tirado-González, I.; Barrientos, G.; Moschansky, P.; Muñoz-Fernández, R.; Leno-Durán, E.; Klapp, B.F.; Thijssen, V.L.J.L.; Blois, S.M. Profiling Lgals9 splice variant expression at the fetal-maternal interface: Implications in normal and pathological human pregnancy. Biol. Reprod. 2013, 88, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, Y.; Li, S.; Zhang, Y.; Liu, Y.; Wu, Y.; Chen, Z. Blockade of Tim-3 signaling restores the virus-specific CD8+ T-cell response in patients with chronic hepatitis B. Eur. J. Immunol. 2012, 42, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Earl, L.A.; Jacobs, L.; Baum, L.G. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J. Biol. Chem. 2008, 283, 12248–12258. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Koehn, C.D.; Zhang, Z.; Su, K. Galectins in the pathogenesis of rheumatoid arthritis. J. Clin. Cell. Immunol. 2013, 4, 1000164. [Google Scholar]
- Naka, E.L.; Ponciano, V.C.; Cenedeze, M.A.; Pacheco-Silva, A.; Câmara, N.O.S. Detection of the Tim-3 ligand, galectin-9, inside the allograft during a rejection episode. Int. Immunopharmacol. 2009, 9, 658–662. [Google Scholar] [CrossRef]
- Seki, M.; Oomizu, S.; Sakata, K.-M.; Sakata, A.; Arikawa, T.; Watanabe, K.; Ito, K.; Takeshita, K.; Niki, T.; Saita, N.; et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008, 127, 78–88. [Google Scholar] [CrossRef]
- Oomizu, S.; Arikawa, T.; Niki, T.; Kadowaki, T.; Ueno, M.; Nishi, N.; Yamauchi, A.; Hattori, T.; Masaki, T.; Hirashima, M. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS ONE 2012, 7, e48574. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Liu, F.-T.; Hirashima, M.; Anderson, A. An emerging role for galectins in tuning the immune response: Lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 2007, 66, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, W.; Yuan, J.; Wu, K.; Zhou, H.; Zhang, W.; Chen, Z.K. Activation of Tim-3-Galectin-9 pathway improves survival of fully allogeneic skin grafts. Transpl. Immunol. 2008, 19, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Niki, T.; Tsutsui, S.; Hirose, S.; Aradono, S.; Sugimoto, Y.; Takeshita, K.; Nishi, N.; Hirashima, M. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J. Biol. Chem. 2009, 284, 32344–32352. [Google Scholar] [CrossRef] [PubMed]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010, 235, 172–189. [Google Scholar] [CrossRef]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Balogh, A.; Karpati, E.; Mastrolia, S.A.; Staretz-Chacham, O.; Hahn, S.; Erez, O.; Papp, Z.; Kim, C.J. Galectins: Double-edged swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia. J. Pathol. Transl. Med. 2015, 49, 181–208. [Google Scholar] [CrossRef]
- Popovici, R.M.; Krause, M.S.; Germeyer, A.; Strowitzki, T.; von Wolff, M. Galectin-9: A new endometrial epithelial marker for the mid- and late-secretory and decidual phases in humans. J. Clin. Endocrinol. Metab. 2005, 90, 6170–6176. [Google Scholar] [CrossRef]
- Imaizumi, T.; Kumagai, M.; Sasaki, N.; Kurotaki, H.; Mori, F.; Seki, M.; Nishi, N.; Fujimoto, K.; Tanji, K.; Shibata, T.; et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J. Leukoc. Biol. 2002, 72, 486–491. [Google Scholar]
- Steelman, A.J.; Smith, R.; Welsh, C.J.; Li, J. Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J. Biol. Chem. 2013, 288, 23776–23787. [Google Scholar] [CrossRef]
- Brubel, R.; Bokor, A.; Pohl, A.; Schilli, G.K.; Szereday, L.; Bacher-Szamuel, R.; Rigo, J.; Polgar, B. Serum galectin-9 as a noninvasive biomarker for the detection of endometriosis and pelvic pain or infertility-related gynecologic disorders. Fertil. Steril. 2017, 108, 1016–1025.e2. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D. Endometriosis classification: An update. Curr. Opin. Obstet. Gynecol. 2011, 23, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Khazali, S. Endometriosis classification-the quest for the holy grail? J. Reprod. Infertil. 2016, 17, 67. [Google Scholar] [PubMed]
- Andres, M.P.; Borrelli, G.M.; Abrão, M.S. Endometriosis classification according to pain symptoms: Can the ASRM classification be improved? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obstet. Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Mori, T.; Ito, F.; Koshiba, A.; Takaoka, O.; Kataoka, H.; Maeda, E.; Okimura, H.; Mori, T.; Kitawaki, J. Exacerbation of endometriosis due to regulatory T-Cell dysfunction. J. Clin. Endocrinol. Metab. 2017, 102, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Xu, H.; Finas, D.; Banz, C.; Diedrich, K.; Hornung, D. Is endometriosis associated with systemic subclinical inflammation? Gynecol. Obstet. Investig. 2006, 62, 139–147. [Google Scholar] [CrossRef]
- Oosterlynck, D.J.; Meuleman, C.; Lacquet, F.A.; Waer, M.; Koninckx, P.R. Flow cytometry analysis of lymphocyte subpopulations in peritoneal fluid of women with endometriosis. Am. J. Reprod. Immunol. 1994, 31, 25–31. [Google Scholar] [CrossRef]
- Gallinelli, A.; Chiossi, G.; Giannella, L.; Marsella, T.; Genazzani, A.D.; Volpe, A. Different concentrations of interleukins in the peritoneal fluid of women with endometriosis: Relationships with lymphocyte subsets. Gynecol. Endocrinol. 2004, 18, 144–151. [Google Scholar] [CrossRef]
- Dias, J.A.; Podgaec, S.; de Oliveira, R.M.; Carnevale Marin, M.L.; Baracat, E.C.; Abrão, M.S. Patients with endometriosis of the rectosigmoid have a higher percentage of natural killer cells in peripheral blood. J. Minim. Invasive Gynecol. 2012, 19, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.Z.; Cuenca, V.; Stitzel, A.; Tice, D. Immune rosettes of T and B lymphocytes in infertile women with endometriosis. J. Reprod. Med. 1987, 32, 194–197. [Google Scholar]
- Iwasaki, K.; Makino, T.; Maruyama, T.; Matsubayashi, H.; Nozawa, S.; Yokokura, T. Leukocyte subpopulations and natural killer activity in endometriosis. Int. J. Fertil. Menopausal Stud. 1993, 38, 229–234. [Google Scholar] [PubMed]
- Szyllo, K.; Tchorzewski, H.; Banasik, M.; Glowacka, E.; Lewkowicz, P.; Kamer-Bartosinska, A. The involvement of T lymphocytes in the pathogenesis of endometriotic tissues overgrowth in women with endometriosis. Mediat. Inflamm. 2003, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Yamamoto, K.; Fujishita, A.; Muto, H.; Koshiba, A.; Kuroboshi, H.; Saito, S.; Teramukai, S.; Nakashima, M.; Kitawaki, J. Differential levels of regulatory T cells and T-helper-17 cells in women with early and advanced endometriosis. J. Clin. Endocrinol. Metab. 2019, 104, 4715–4729. [Google Scholar] [CrossRef] [PubMed]
- Gogacz, M.; Winkler, I.; Bojarska-Junak, A.; Tabarkiewicz, J.; Semczuk, A.; Rechberger, T.; Adamiak, A. T regulatory lymphocytes in patients with endometriosis. Mol. Med. Rep. 2014, 10, 1072–1076. [Google Scholar] [CrossRef]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef]
- Shaco-Levy, R.; Sharabi, S.; Benharroch, D.; Piura, B.; Sion-Vardy, N. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 226–232. [Google Scholar] [CrossRef]
- Vergetaki, A.; Jeschke, U.; Vrekoussis, T.; Taliouri, E.; Sabatini, L.; Papakonstanti, E.A.; Makrigiannakis, A. Galectin-1 overexpression in endometriosis and its regulation by neuropeptides (CRH, UCN) indicating its important role in reproduction and inflammation. PLoS ONE 2014, 9, e114229. [Google Scholar] [CrossRef]
- Noël, J.-C.; Chapron, C.; Borghese, B.; Fayt, I.; Anaf, V. Galectin-3 is overexpressed in various forms of endometriosis. Appl. Immunohistochem. Mol. Morphol. AIMM 2011, 19, 253–257. [Google Scholar] [CrossRef]
- Madireddi, S.; Eun, S.-Y.; Lee, S.-W.; Nemčovičová, I.; Mehta, A.K.; Zajonc, D.M.; Nishi, N.; Niki, T.; Hirashima, M.; Croft, M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J. Exp. Med. 2014, 211, 1433–1448. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Szigeti, B.; Farkas, N.; Szereday, L. The importance of the PD-1/PD-L1 pathway at the maternal-fetal interface. BMC Pregnancy Childbirth 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Meggyes, M.; Bogar, B.; Schmitz, N.; Barakonyi, A.; Varnagy, A.; Farkas, B.; Tamas, P.; Bodis, J.; Szekeres-Bartho, J.; et al. Involvement of Galectin-9/TIM-3 pathway in the systemic inflammatory response in early-onset preeclampsia. PLoS ONE 2013, 8, 71811. [Google Scholar] [CrossRef] [PubMed]
- Kashio, Y.; Nakamura, K.; Abedin, M.J.; Seki, M.; Nishi, N.; Yoshida, N.; Nakamura, T.; Hirashima, M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 2003, 170, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Gautron, A.S.; Dominguez-Villar, M.; de Marcken, M.; Hafler, D.A. Enhanced suppressor function of TIM-3+FoxP3+ regulatory T cells. Eur. J. Immunol. 2014, 44, 2703–2711. [Google Scholar] [CrossRef]
- Pedros, C.; Duguet, F.; Saoudi, A.; Chabod, M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 974–995. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X. Foxp3 instability helps ttregs distinguish self and non-self. Front. Immunol. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Ju, Y.; Hou, N.; Meng, J.; Wang, X.; Zhang, X.; Zhao, D.; Liu, Y.; Zhu, F.; Zhang, L.; Sun, W.; et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J. Hepatol. 2010, 52, 322–329. [Google Scholar] [CrossRef]
- Jost, S.; Moreno-Nieves, U.Y.; Garcia-Beltran, W.F.; Rands, K.; Reardon, J.; Toth, I.; Piechocka-Trocha, A.; Altfeld, M.; Addo, M.M. Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection. Retrovirology 2013, 10, 74. [Google Scholar] [CrossRef]
- Szereday, L.; Meggyes, M.; Berki, T.; Miseta, A.; Farkas, N.; Gervain, J.; Par, A.; Par, G. Direct-acting antiviral treatment downregulates immune checkpoint inhibitor expression in patients with chronic hepatitis C. Clin. Exp. Med. 2020. (published online, ahead of print). [Google Scholar] [CrossRef]
- Motamedi, M.; Shahbaz, S.; Fu, L.; Dunsmore, G.; Xu, L.; Harrington, R.; Houston, S.; Elahi, S. Galectin-9 expression defines a subpopulation of nk cells with impaired cytotoxic effector molecules but enhanced IFN-γ production, dichotomous to TIGIT, in HIV-1 infection. ImmunoHorizons 2019, 3, 531–546. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Liang, S.; Potter, J.; Jiang, X.; Mao, H.-Q.; Li, Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J. Immunol. 2013, 190, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Morishita, A.; Niki, T.; Hara, J.; Sato, M.; Tani, J.; Miyoshi, H.; Yoneyama, H.; Masaki, T.; Hattori, T.; et al. Galectin-9 prolongs the survival of septic mice by expanding Tim-3-expressing natural killer T cells and PDCA-1+ CD11c+ macrophages. Crit. Care 2013, 17, R284. [Google Scholar] [CrossRef] [PubMed]
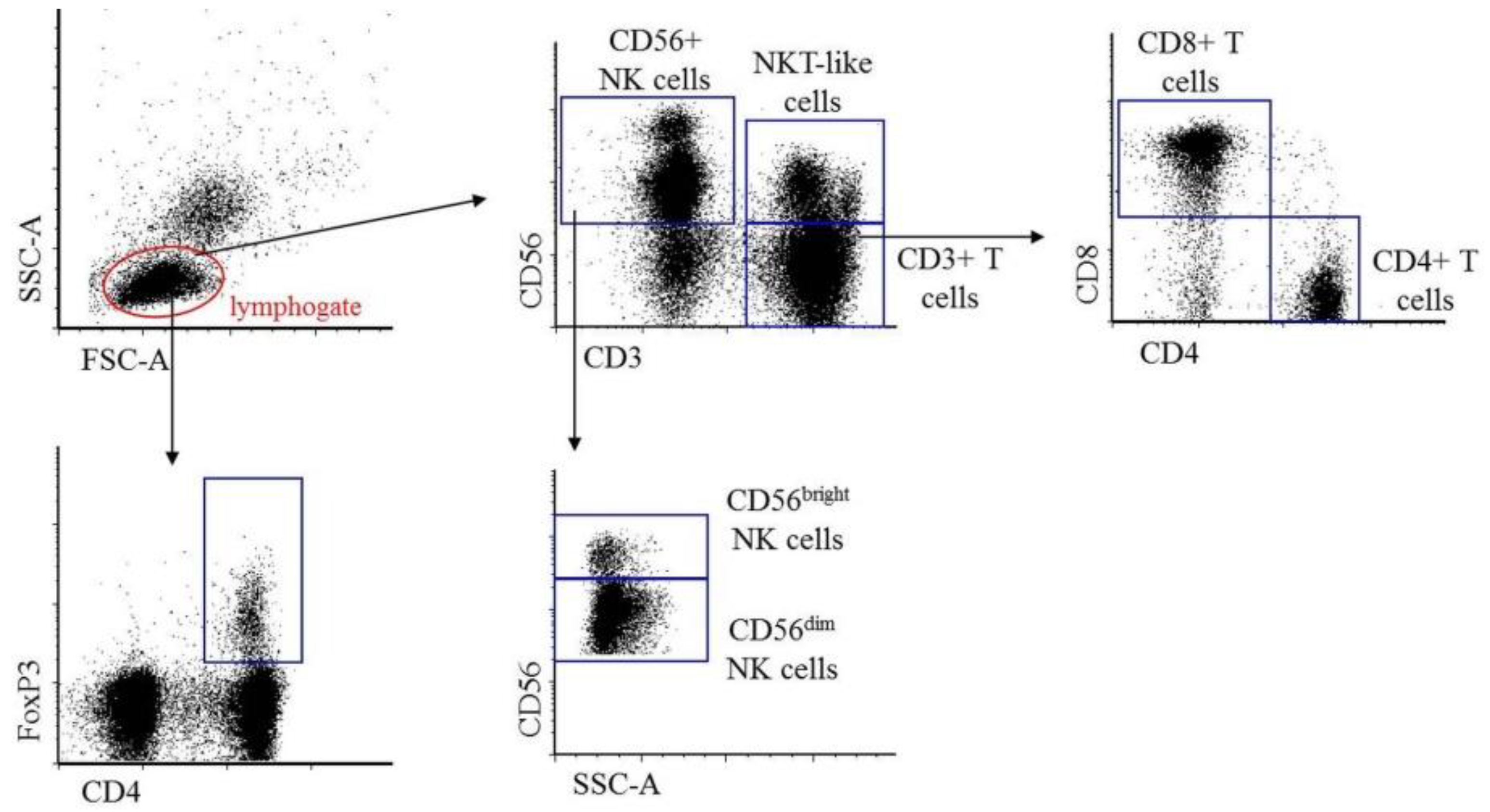

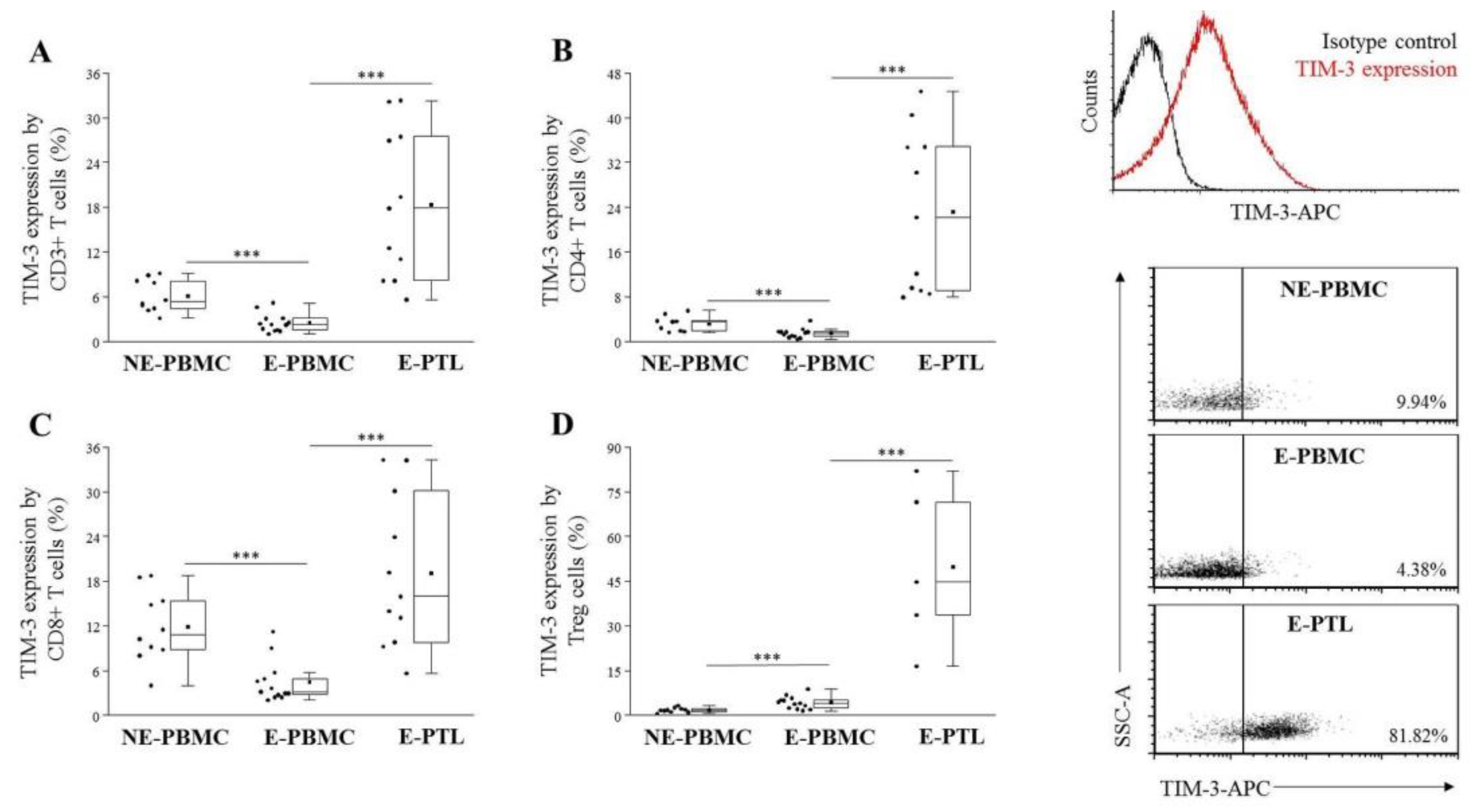

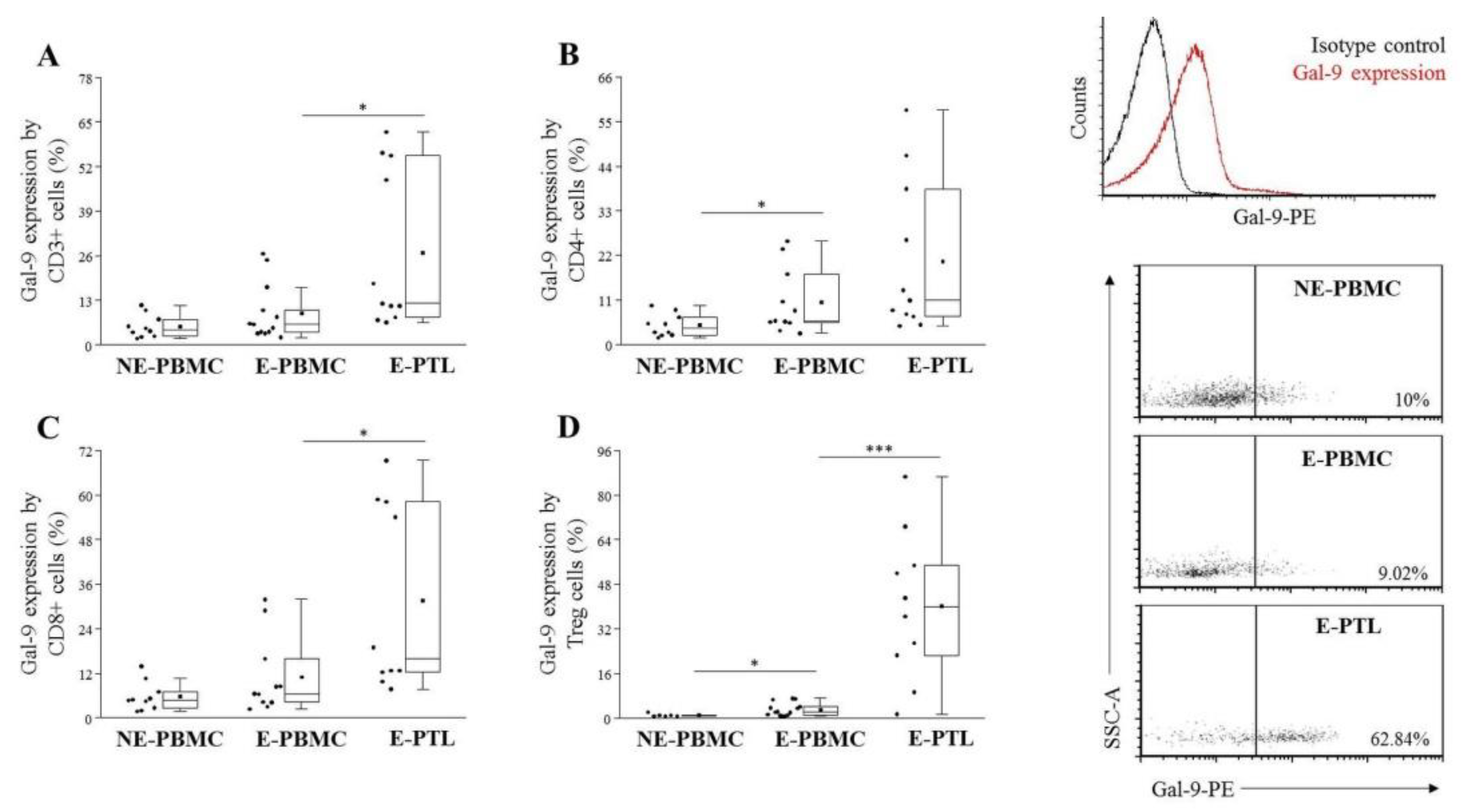
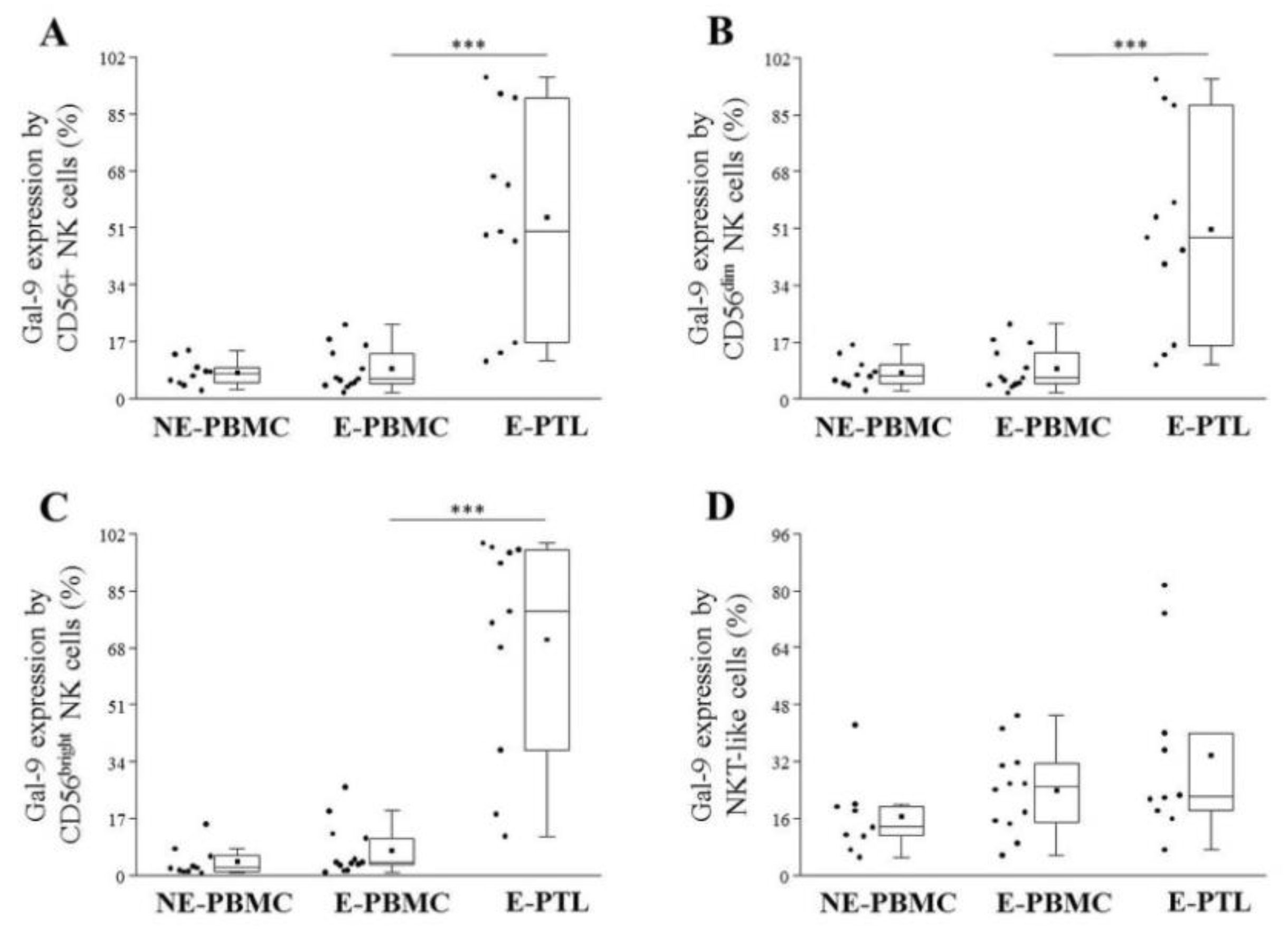
| E-PBMC (n = 12) | E-PTL (n = 11) | p-Value | |
|---|---|---|---|
| Mean age (years ± SD) | 31.92 ± 4.337 | 29.00 ± 4.313 | 0.1218 |
| Age ranges at diagnosis (years) | 26–40 | 24–38 | - |
| Mean BMI (kg/m2 ± SD) | 24.11 ± 4.895 | 21.09 ± 2.880 | 0.1009 |
| Gynecological History – n (%) | |||
| Normal cycle length (25–35 days) | 4 (33.33) | 3 (27.27) | - |
| Irregular cycle | 4 (33.33) | 4 (36.36) | - |
| Suppressed cycle (GnRH analogue) | 0 (0.00) | 1 (9.09) | - |
| Fertility Data – n (%) | |||
| Previous normal pregnancy | 4 (33.33) | 0 (0.00) | - |
| Previous pathological pregnancy | 2 (16.67) | 1 (9.09) | - |
| Infertility | 7 (58.33) | 9 (81.82) | - |
| Endometriosis-Related Data – n (%) | |||
| Previous laparoscopic intervention | 8 (66.67) | 8 (72.73) | - |
| Pharmacologic treatment | 3 (25.00) | 4 (36.36) | - |
| Pelvic pain | 8 (66.67) | 5 (45.45) | - |
| Dysmenorrhea | 9 (75.00) | 9 (81.82) | - |
| Dyspareunia | 5 (41.67) | 6 (54.55) | - |
| Dyschesia | 8 (66.67) | 8 (72.73) | - |
| Dysuria | 1 (8.33) | 1 (9.09) | - |
| Type of Endometriosis – n (%) | |||
| Peritoneal | 1 (8.33) | 3 (27.27) | - |
| Ovarian endometriosis | 2 (16.67) | 2 (18.18) | - |
| Deep infiltrating endometriosis (DIE) | 3 (25.00) | 2 (18.18) | - |
| Combined (DIE + other) | 6 (50.00) | 4 (36.36) | - |
| Other Associated Diseases – n (%) | |||
| Autoimmunity | 0 (0.00) | 1 (9.09) | - |
| Insulin resistance | 1 (8.33) | 2 (18.18) | - |
| NE-PBMC | E-PBMC | p-Value | E-PBMC | E-PTL | p-Value | |
|---|---|---|---|---|---|---|
| CD3 | 63.85 ± 8.49 | 53.52 ± 11.43 | <0.03 | 53.52 ± 11.43 | 57.09 ± 4.10 | n.s. |
| CD4 | 37.09 ± 6.50 | 26.45 ± 8.83 | <0.01 | 26.45 ± 8.83 | 14.90 ± 4.66 | <0.001 |
| CD8 | 20.78 ± 7.27 | 18.73 ± 3.33 | n.s. | 18.73 ± 3.33 | 32.11 ± 9.90 | <0.01 |
| Treg | 3.41 ± 1.20 | 1.56 ± 0.53 | <0.001 | 1.56 ± 0.53 | 1.03 ± 0.47 | <0.03 |
| CD4/CD8 | 2.03 ± 0.84 | 1.45 ± 0.61 | n.s. | 2.03 ± 0.84 | 0.49 ± 0.16 | <0.01 |
| CD4/Treg | 11.88 ± 3.86 | 18.01 ± 5.88 | n.s. | 11.88 ± 3.86 | 20.10 ± 13.16 | n.s. |
| CD56+ NK | 15.34 ± 7.20 | 26.40 ± 10.99 | <0.02 | 26.40 ± 10.99 | 24.07 ± 14.06 | n.s. |
| CD56dim NK | 14.01 ± 6.75 | 25.10 ± 10.85 | <0.01 | 25.10 ± 10.85 | 19.03 ± 12.80 | n.s. |
| CD56bright NK | 1.36 ± 0.69 | 1.60 ± 0.91 | n.s. | 1.60 ± 0.91 | 5.30 ± 4.09 | <0.01 |
| NKT-like | 8.57 ± 3.58 | 6.38 ± 4.61 | n.s. | 6.38 ± 4.61 | 12.72 ± 4.48 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meggyes, M.; Szereday, L.; Bohonyi, N.; Koppan, M.; Szegedi, S.; Marics-Kutas, A.; Marton, M.; Totsimon, A.; Polgar, B. Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis. Int. J. Mol. Sci. 2020, 21, 2343. https://doi.org/10.3390/ijms21072343
Meggyes M, Szereday L, Bohonyi N, Koppan M, Szegedi S, Marics-Kutas A, Marton M, Totsimon A, Polgar B. Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis. International Journal of Molecular Sciences. 2020; 21(7):2343. https://doi.org/10.3390/ijms21072343
Chicago/Turabian StyleMeggyes, Matyas, Laszlo Szereday, Noemi Bohonyi, Miklos Koppan, Sarolta Szegedi, Anna Marics-Kutas, Mirjam Marton, Anett Totsimon, and Beata Polgar. 2020. "Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis" International Journal of Molecular Sciences 21, no. 7: 2343. https://doi.org/10.3390/ijms21072343
APA StyleMeggyes, M., Szereday, L., Bohonyi, N., Koppan, M., Szegedi, S., Marics-Kutas, A., Marton, M., Totsimon, A., & Polgar, B. (2020). Different Expression Pattern of TIM-3 and Galectin-9 Molecules by Peripheral and Peritoneal Lymphocytes in Women with and without Endometriosis. International Journal of Molecular Sciences, 21(7), 2343. https://doi.org/10.3390/ijms21072343





