Abstract
Schizophrenia is a serious, chronic psychiatric disorder requiring lifelong treatment. Extrapyramidal side effects (EPS) are common adverse reactions to antipsychotic medications. In addition to the dopaminergic system, serotonergic mechanisms, including serotonin (5-HT) receptors, might be involved in EPS development. This study aimed to examine molecular associations of HTR1A, HTR1B, HTR2A, HTR2C and HTR6 gene polymorphisms with acute EPS in 229 male schizophrenia patients, following two weeks of haloperidol monotherapy. The Simpson–Angus Rating Scale for Extrapyramidal Side Effects (SAS), Barnes Akathisia Rating Scale (BARS) and Extrapyramidal Symptom Rating Scale (ESRS) were used to evaluate EPS severity. Genotyping was performed using real-time PCR, following extraction of blood DNA. Significant acute EPS appeared in 48.03% of schizophrenia patients. For the rs13212041 HTR1B gene polymorphism, affecting microRNA regulation of HTR1B gene expression, a higher frequency of TT carriers was found among haloperidol-treated patients with akathisia when compared to the group without akathisia symptoms. In comparison to C-allele carriers, patients carrying the TT genotype had higher akathisia severity, as determined by the SAS, BARS and ESRS scales. These molecular findings suggest potential involvement of 5-HT1B receptors in akathisia development following haloperidol treatment, as well as possible epigenetic mechanisms of serotonergic modulation associated with antipsychotic-induced EPS.
1. Introduction
Schizophrenia is a serious, chronic psychiatric disorder, requiring lifelong treatment [1]. Haloperidol, a highly effective first-generation antipsychotic (FGA), is one of the most prescribed antipsychotics in Europe and the US, and it is often used in clinical trials as a comparator drug [2]. Due to its very strong antagonistic activity on dopamine D2 receptors of the mesolimbic dopamine pathway [3], haloperidol acts as a very potent antipsychotic agent, and it is included on the World Health Organization’s list of essential drugs [4]. However, like other FGAs, it is also associated with the development of both acute and long-term extrapyramidal side effects (EPS) [5], possibly due to blockade of the D2 receptor in the nigrostriatal pathway [6]. Specifically, it has been demonstrated that FGAs bind “tightly” to D2 receptors and dissociate slowly [7], with a D2 receptor occupancy of greater than 80% significantly contributing to the risk of EPS [8]. Other predictors of EPS include younger age, male gender, longer treatment durations, higher dosage, psychiatric diagnoses, such as mood disorder, and previous EPS history [8,9,10,11]. More recently, genetic factors have also been considered, including those related to the metabolism of antipsychotic drugs and free radical scavenging [12,13,14], as well as variants in genes coding for various components of the dopaminergic system [15,16,17].
EPS are well-known and common antipsychotic-induced movement disorders [18]. They include acute EPS, such as akathisia, acute dystonia and parkinsonism, which may occur within days or weeks of initiating treatment, as well as late-onset EPS, such as tardive dyskinesia that develop months or years after the antipsychotic therapy [19]. These serious and debilitating side effects often lead to reduction of patient compliance or even discontinuation of therapy and can present major therapeutic limitations [20]. Newer, second-generation antipsychotics (SGAs) are accompanied by fewer EPS (~15%) when compared to FGAs (50‒75%) [7,21], but are more frequently associated with adverse metabolic effects [6]. Like FGAs, SGAs block D2 receptors; however, they additionally exhibit activity at several serotonin (5-HT) receptors [19,22]. Assorted data suggest an important role of 5-HT neurons and various 5-HT receptors in the modulation of dopaminergic function, and consequently development of EPS following treatment with antipsychotic drugs [23,24,25]. It is possible that molecular determinants of the 5-HT system contribute to the inter-individual differences in development of EPS following treatment with FGAs.
Several studies have reported significant associations between 5-HT receptor gene polymorphisms and the risk of developing EPS [26,27]. Most of these studies have, however, focused on tardive dyskinesia, and only a few investigated the development of acute EPS [28,29]. Therefore, the aim of this study was to investigate the potential relationship of several polymorphisms located in the HTR1A, HTR1B, HTR2A, HTR2C and HTR6 genes, which code for the corresponding 5-HT receptors, with the development of acute EPS, following haloperidol monotherapy. These genetic variants might be clinically useful as pharmacogenetic markers for prediction of the occurrence of acute EPS among patients treated with antipsychotic drugs, as well as for tailoring future genotype-based personalized drug treatments in order to help minimize EPS [30].
2. Results
For this study, 299 male patients with schizophrenia were enrolled, with a mean age of 36.49 ± 10.40 years old. Demographic and clinical features of the subjects enrolled are presented in Table 1. Most of the schizophrenia patients had graduated from high school, were unemployed or retired, as well as being either single or divorced and without children. They were also mostly overweight, with a mean BMI of 26.54 ± 9.18 (Table 1). A relatively high proportion of patients were smokers and drank alcohol, while a significant number of patients had previously consumed one or more illegal psychoactive substances. A considerable number of the patients had also previously attempted suicide. As shown in the Table 1, most of the schizophrenia patients previously received antipsychotic therapy (89.52%), usually a combination of typical and atypical antipsychotics (69.00%), whereas a smaller number of the subjects enrolled were drug naïve (10.48%). A majority of the patients previously met the criteria for complete or partial disease remission, whereas 13.10% of them were considered to be treatment-resistant (Table 1). As demonstrated by their high baseline positive, negative, general psychopathology and total Positive and Negative Syndrome Scale (PANSS) scores, all subjects were admitted to the hospital due to acute exacerbation of schizophrenia and subsequently treated with haloperidol. A total of 66.81% of the schizophrenia patients reported some kind of EPS, and in those patients acute EPS usually developed on the 5th day of haloperidol monotherapy.

Table 1.
Socio-demographic and clinical characteristics of haloperidol-treated schizophrenia patients.
The EPS were evaluated using the Simpson–Angus Rating Scale for Extrapyramidal Side Effects (SAS), the Barnes Akathisia Rating Scale (BARS) and the Extrapyramidal Symptom Rating Scale (ESRS) (Table 2). According to the SAS scale, some EPS were present in 63.32% of patients, with mean total scores of 4.965 ± 5.643; however, in 111 (48.47%) patients, the acute EPS that appeared following haloperidol treatment were significant (defined as a SAS score > 3), whereas 118 (51.53%) patients were in the group without significant acute EPS (SAS score ≤ 3). As shown in Table 2, according to the SAS ratings, the most frequent and severe EPS were tremors, abnormal gait and excessive salivation. The frequency of akathisia, characterized by a feeling of inner restlessness and an inability to stay still [31], as assessed using the SAS scale, was ~23%. This is consistent with the results (~23%) obtained using the BARS scale to rate akathisia and is also in agreement with the reported rates (5–75%) [32,33] and average prevalence (20–35%) [34] of akathisia. The total mean BARS scores of the schizophrenia patients were 1.489 ± 2.989 (Table 2).

Table 2.
Number and percentage (%) of schizophrenia patients with particular acute extrapyramidal side effects (EPS) and its severity (scores), as assessed with the Simpson–Angus Rating Scale for Extrapyramidal Side Effects (SAS), the Barnes Akathisia Rating Scale (BARS) and the Extrapyramidal Symptom Rating Scale (ESRS) scales following haloperidol treatment.
For evaluation using the ESRS scale, some items assessing chronic EPS were excluded from the rating. In the ESRS questionnaire and behavioral scale, 65.50% of patients reported parkinsonism, dystonia or akathisia. The ESRS physician’s examination identified bradykinesia, abnormal gait and posture, as well as rigidity as the most frequent EPS, whereas tremor and rigidity were the most severe EPS based on the highest ESRS scores (Table 2). In concurrence with the SAS and BARS scales, the frequency of akathisia occurrence as assessed by the ESRS scale was ~23%. Although the ESRS clinical global impression detected some symptoms of parkinsonism in ~58% of the subjects, these symptoms were very mild (2.077 ± 1.905 scores), indicating minimal or low-stage parkinsonism (Table 2). The total mean ESRS scores of the schizophrenia patients enrolled was 21.49 ± 21.24. As shown in Table 2, the severity of EPS in schizophrenia patients following haloperidol monotherapy is quite variable, as demonstrated by the high standard deviation (SD) in the BARS, SAS and ESRS scores, and it is probably due to the influence of various environmental as well as genetic factors.
The molecular approach involving gene polymorphisms was studied using real-time PCR, following extraction of DNA from the blood of patients. We focused on the HTR1A, HTR1B, HTR2A, HTR2C and HTR6 gene polymorphisms. The genotype distributions in schizophrenia patients for all of the 5-HT receptor gene polymorphisms tested in the study were in Hardy–Weinberg equilibrium (HWE). As shown in Table 3, no significant differences were observed in the frequency of the genotypes or alleles for any of the 5-HT receptor gene polymorphisms studied between patients with or without significant acute EPS (SAS score >3) following haloperidol monotherapy. However, when we compared the SAS, BARS and ESRS total scores in schizophrenia patients carrying different genotypes or alleles of the 5-HT receptor gene polymorphisms, we found a significant association of the HTR1B rs13212041 polymorphism with the total BARS scores (Table 4). Specifically, the total BARS scores were significantly different between patients carrying various HTR1B rs13212041 genotypes (p = 0.009; Kruskal–Wallis test). Applying of a post-hoc Dunn’s multiple comparisons test demonstrated that haloperidol-treated schizophrenia patients carrying the HTR1B rs13212041 TT genotype had a significantly higher total BARS scores (p = 0.007) than carriers of the CT genotype.

Table 3.
Genotype and allele frequencies of 5-HT receptor gene polymorphisms in schizophrenia patients, subdivided according to the development of significant acute EPS following haloperidol therapy.

Table 4.
SAS, BARS and ESRS total scores in haloperidol-treated schizophrenia patients carrying different genotypes or alleles of 5-HT receptor gene polymorphisms.
Moreover, schizophrenia patients carrying the HTR1B homozygous TT genotype had significantly higher BARS scores (p = 0.002; Mann–Whitney test) than carriers of the C allele (Figure 1). As shown in Table 5, further analysis using the Kruskal–Wallis test revealed significant differences in the scores of all individual items on the BARS scale between haloperidol-treated schizophrenia patients carrying different genotypes of the HTR1B rs13212041 polymorphism. Specifically, Dunn’s multiple comparisons test demonstrated that carriers of the TT genotype had significantly higher scores for each individual item on the BARS score than patients carrying the CT genotype (Table 5).
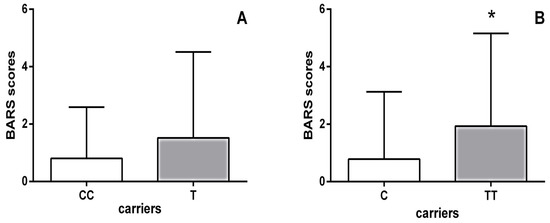
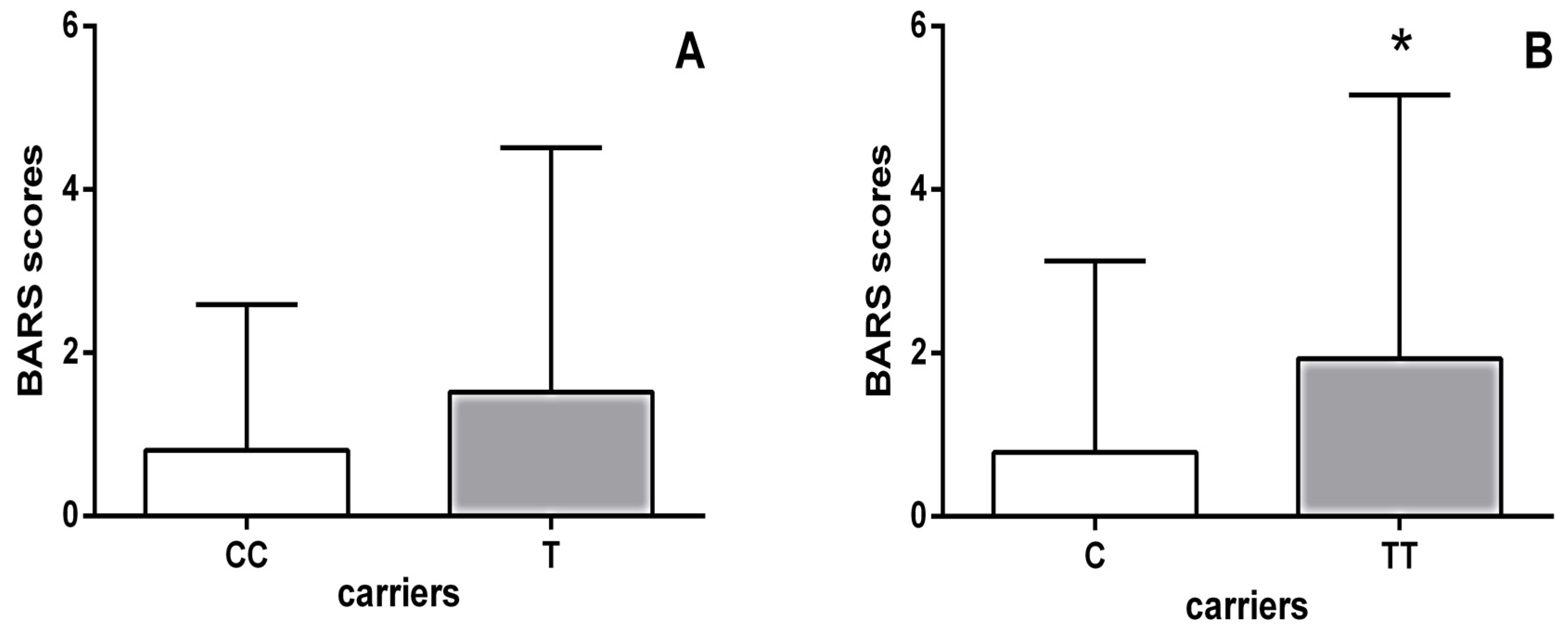
Figure 1.
BARS scores of haloperidol-treated schizophrenia patients subdivided according to their HTR1B rs13212041 polymorphism status: (A) carriers of the homozygous CC genotype (0.800 ± 1.789) vs. carriers of the T allele (1.517 ± 2.996); (B) carriers of the homozygous TT genotype (1.931 ± 3.228) vs. carriers of the C allele (0.785 ± 2.341). * p = 0.002; Mann–Whitney test, TT vs. C carriers.

Table 5.
Scores of individual BARS items in haloperidol-treated schizophrenia patients carrying different genotypes of the HTR1B rs13212041 polymorphism.
Since we have found a significant association of the HTR1B rs13212041 polymorphism with scores on the BARS scale, which is used for rating akathisia, we evaluated akathisia in haloperidol-treated patients with schizophrenia using the SAS and ESRS scales as well. The results obtained by the Kruskal–Wallis test demonstrated that patients carrying the CC, CT and TT genotypes differed significantly (p = 0.008) in severity of akathisia, as assessed using the SAS scale.
Carriers of the HTR1B TT genotype (0.519 ± 0.862) also had significantly higher SAS scores for akathisia when compared to carriers of the CT genotype (0.203 ± 0:619) (p = 0.006; Dunn’s multiple comparisons test), as well as to carriers of C the allele (0.215 ± 0.634) (p = 0.002, Mann–Whitney test). Similarly, when akathisia was evaluated using the ESRS scale, we observed significant differences in the scores between haloperidol-treated schizophrenia patients carrying different HTR1B rs13212041 genotypes (p = 0.011; Kruskal–Wallis test). Further analysis confirmed that the TT carriers (0.661 ± 1.163) had higher ESRS scores for akathisia when compared to the CT carriers (0.222 ± 0.676; p = 0.009; Dunn’s multiple comparisons test), as well as to C-allele carriers (0.221 ± 0.661; p = 0.003; Mann–Whitney test). Additionally, TT carriers were significantly more frequent (p = 0.006, χ2-test) among haloperidol-treated patients with akathisia (81.63%) than in the group without akathisia symptoms (56.52%), as determined by both the SAS and BARS scales. According to the ESRS scale, there were also significantly more schizophrenia patients carrying the TT genotype (p = 0.016; χ2-test) who developed akathisia (79.59%) than those without akathisia symptoms (57.14%) following haloperidol monotherapy.
3. Discussion
To the best of our knowledge, our study is the first to report an association between the HTR1B rs13212041 polymorphism and antipsychotic-induced acute EPS, and more specifically akathisia, in schizophrenia patients. Evaluation with the SAS, BARS and ESRS scales revealed a significantly higher frequency of HTR1B TT carriers among haloperidol-treated patients who developed akathisia than in those that did not, as well as a higher severity of akathisia in patients carrying the TT genotype in comparison to C-allele carriers. This finding confirms the close link between molecular events affecting the 5-HT system of a patient and their genetic susceptibility to develop antipsychotic-induced EPS [35]. The majority of previous pharmacogenetic studies have focused on chronic EPS, such as tardive dyskinesia [26,27,36,37,38], while only a few reported associations of antipsychotic-induced acute EPS, such as parkinsonism and akathisia, with polymorphisms located in the HTR2A and HTR2C genes [28,29,39]. In contrast to those findings, our study did not detect any significant molecular associations between the HTR1A, HTR2A, HTR2C or HTR6 gene polymorphisms and acute EPS following haloperidol monotherapy.
The HTR1B rs13212041 (A1997G) polymorphism is located in the distal 3′-untranslational region (UTR) of HTR1B messenger RNA, and disrupts the binding site for the microRNA, miR-96, consequently influencing the expression of the 5-HT1B receptor [40]. Expression of miR-96 in the brain [41] may be modulated by various environmental factors [40], including antipsychotic drugs. Carriers of the A-allele show reduced HTR1B expression compared to G-allele carriers [40]. Therefore, we can presume that haloperidol-treated schizophrenia patients carrying the TT genotype, who develop akathisia both more frequently and more severely, have lower levels of 5-HT1B receptors than carriers of the C-allele (Figure 2). Such epigenetic mechanisms are supported by the observed associations between DNA methylation patterns in some 5-HT gene promoter regions and response to antipsychotic drugs [42]. Haloperidol was seen to cause an increase in global DNA methylation [43,44], histone 3 phospho-acetylation [45] and expression of various epigenetic modifiers [43]. Moreover, treatment with haloperidol has been associated with altered expression of several miRNAs [43,46,47] and genes [48], some of which may be involved in the development of EPS [49].
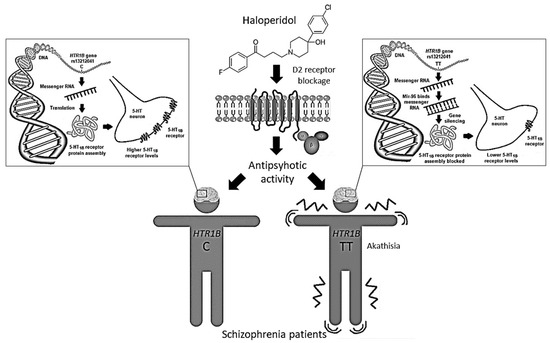
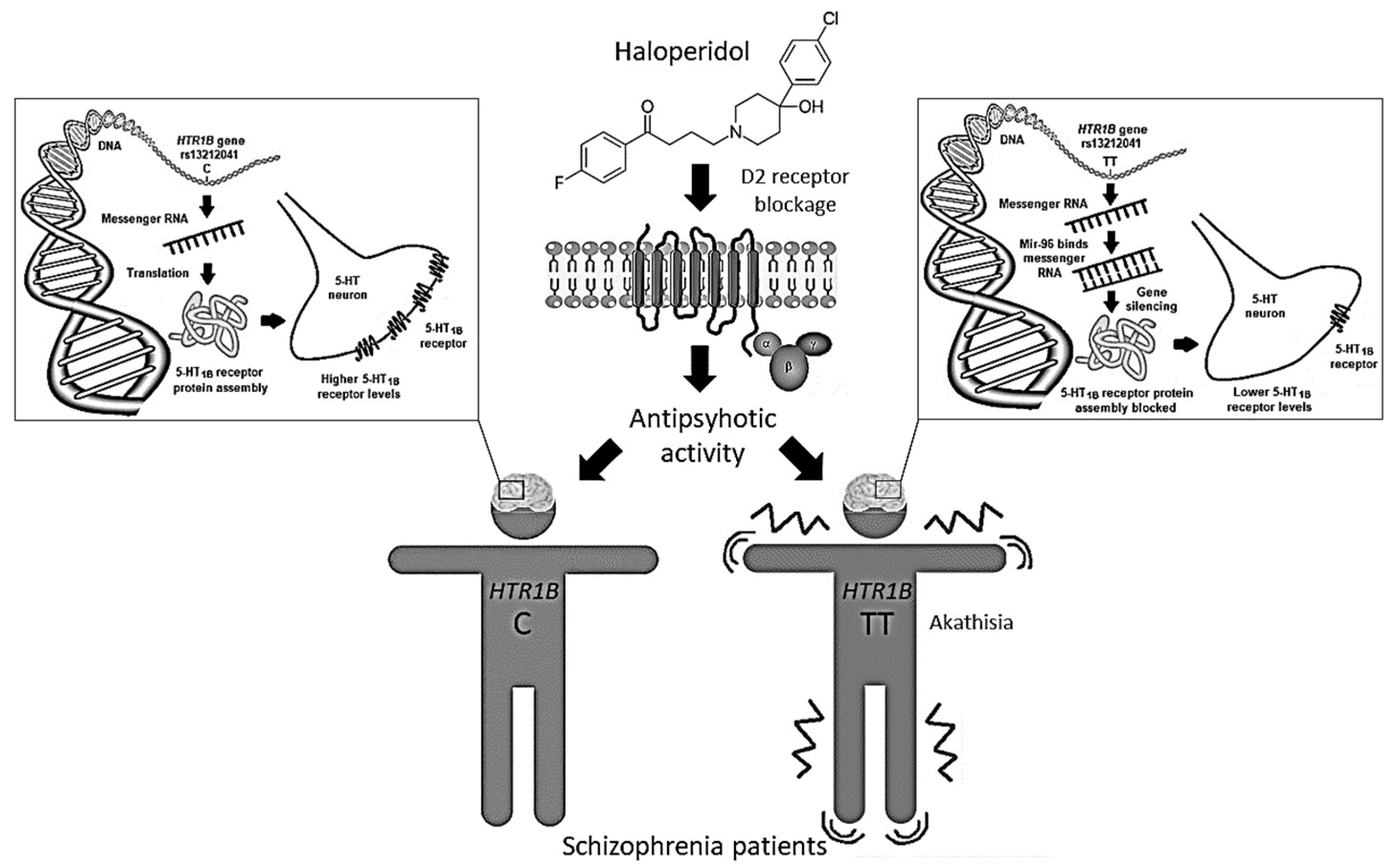
Figure 2.
The rs13212041 HTR1B gene polymorphism located in the distal 3′-UTR of messenger RNA disrupts the binding site for the microRNA, miR-96, influencing 5-HT1B receptor expression. Haloperidol-treated schizophrenia patients carrying the HTR1B TT genotype, who have lower levels of 5-HT1B receptors, develop akathisia more frequently and severely than carriers of the C-allele.
The molecular mechanisms by which 5-HT1B receptors might play a role in the development of haloperidol-induced akathisia are elusive. The majority of current antipsychotics act as antagonists at 5-HT1B receptors, and usually demonstrate inverse agonist properties [50,51]. Therefore, the different potencies of individual antipsychotics at 5-HT1B sites, in comparison to D2 receptors, could influence their individual propensity to induce EPS. Data regarding the role of 5-HT1B receptors on striatal dopamine release are contradictory [23,52,53]. Instead, 5-HT1B receptors, expressed by striatal cells, would modulate the impact of nigrostriatal dopamine specifically on dopamine-receptive cells of the striatum, independently of the net effect on dopamine efflux [24,54]. This is in line with the findings that 5-HT1B receptor stimulation diminishes the dyskinesia induced by dopamine receptor agonists [55,56]. 5-HT1B receptors act as inhibitory autoreceptors or heteroreceptors on both serotonergic and non-serotonergic neurons, and modulate 5-HT activity [57]. Therefore, the HTR1B rs13212041 polymorphism, by influencing 5-HT1B receptor expression, can affect 5-HT neurotransmission, as well as development of antipsychotic-induced EPS.
In our study, all patients were treated with haloperidol as a monotherapy for two weeks, to exclude drug–drug interactions. However, we did not measure haloperidol plasma concentration, nor perform CYP2D6 genotyping. Haloperidol pharmacokinetics is primarily influenced by the metabolic capacity of the genetically regulated CYP2D6 enzyme [12]. Since antipsychotic dose is a well-known risk factor for EPS, it is possible that patients who carry genotypes associated with poor CYP2D6 metabolism are at an increased risk of haloperidol-induced EPS [13]. In addition to haloperidol, all patients received diazepam as a concomitant medication for the treatment of agitation, insomnia and anxiety. We cannot, therefore, completely rule out a possible effect of diazepam on haloperidol-induced EPS [58,59]. Although it has been demonstrated that men and women with schizophrenia differ in their treatment response and antipsychotic side effects [60,61], our study enrolled only male schizophrenia patients. As a previous study also found significant gender differences in allele frequencies of the HRT1B polymorphism (rs1778258) [62], future studies investigating associations between 5-HT receptor gene variants and EPS should include, as well as compare, male and female patients with schizophrenia. The study of Xia et al. [62] additionally observed different HRT1B allelic distributions between schizophrenia patients and healthy control individuals of Han Chinese descent. However, as far as we are aware, this association has not been reported in Caucasian subjects, suggesting ethnicity-related differences. Hence, in addition to an appropriate sample size and statistical power, a significant advantage of the present study is that it involved an ethnically homogenous group of middle-aged male schizophrenia patients in the acute episode of illness. Nevertheless, the lack of healthy control subjects in our study limits its interpretation. Another study limitation is a lack of replication of our findings in an independent sample.
Although akathisia occurs more frequently following the use of high-potency FGAs, such as haloperidol (15–40%), its development has been also observed with certain SGAs [63,64]. Since antipsychotic drug type has been identified as a risk factor for akathisia [31], further studies should test a wider range of antipsychotics for the association observed between the HRT1B polymorphism and akathisia. Our results could be of further importance if we consider that akathisia is not limited to antipsychotic medication. Antidepressants, especially selective serotonin reuptake inhibitors (SSRI) [65], monoamine oxidase inhibitors (MAOI) [66] and tricyclic antidepressants (TCA) [67], have also been associated with akathisia. Therefore, if confirmed, the HTR1B rs13212041 polymorphism could be a pharmacogenetic predictor of akathisia, to allow better selection of pharmacotherapy and reduction of EPS, resulting in better patient compliance and quality of life. As severe akathisia symptoms can lead to poor adherence to medications, exacerbation of psychiatric symptoms as well as aggression, violence and suicide [68], it is not surprising that interventions aimed at modulating 5-HT transmission have gathered increasing attention for treatment of akathisia [32,69]. The 5-HT2A/C receptor antagonists, mianserin [70], mirtazapine [71], ritanserin [72] and cyproheptadine [73], have all shown some efficacy against acute akathisia. The results of our study suggest that 5-HT1B receptor agonists, such as zolmitriptan, might also be effective as akathisia treatment [74]. Further research is needed, however, in order to verify our finding that the HTR1B gene polymorphism is a molecular determinant for developing akathisia, and to further expand our understanding of individual patient susceptibility to EPS induced by various medications.
4. Materials and Methods
4.1. Subjects and Clinical Evaluation
The study enrolled 229 male patients with schizophrenia recruited from the Psychiatric Hospital Popovaca and the Department of Psychiatry, University Hospital Centre Zagreb, Croatia. The subjects were all admitted to the hospitals due to acute schizophrenia exacerbation. The diagnosis of schizophrenia was made based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria [75]. The severity of schizophrenia symptoms was evaluated by experienced psychiatrists using the Positive and Negative Syndrome Scale (PANSS) [76]. The study exclusion criteria were serious somatic illnesses, neurologic disorders and a history of drug use during the previous 6 months. All of the patients enrolled had been without previous antipsychotic medication for at least 48 h. Most of the subjects had not taken antipsychotics for several months and some of them were drug naïve. Patients were treated with haloperidol (15 mg/day, orally or intramuscularly) for two weeks, and adjuvant diazepam therapy (40 mg daily) was introduced in the case of agitation, insomnia and anxiety.
The Simpson–Angus Rating Scale for Extrapyramidal Side Effects (SAS) [77], the Barnes Akathisia Rating Scale (BARS) [78] and the Extrapyramidal Symptom Rating Scale (ESRS) [79] were used to evaluate the severity of EPS during treatment with haloperidol in patients with schizophrenia. As in previous studies [16,29], EPS were defined as significant when SAS scores were > 3, and patients were subsequently subdivided into those with significant acute EPS (SAS score > 3) and those without significant acute EPS (SAS score < 3) following haloperidol monotherapy.
The following items were excluded from the ESRS scale: from part I “Parkinsonism, Dystonia, Dyskinesia and Akathisia—Questionnaire and Behavioral Scale, item 10 (Abnormal involuntary movements (dyskinesia) of extremities or trunk) and item 11 (Abnormal involuntary movements (dyskinesia) of tongue, jaw, lips or face); from part III “Dystonia: Physician´s examination”: item 2 (Non-acute or chronic or tardive dystonia); and all of part IV “Dyskinetic movements: Physician´s examination” and of part V “Clinical global impression of severity of Dyskinesia”.
This study was approved by the ethics committees of the Psychiatric Hospital Popovaca and of the University Hospital Centre Zagreb, and was carried out in accordance with the Declaration of Helsinki, 1996 (and its amendments). All participants were Caucasians living in Croatia. Only patients who provided signed informed consent were included in the study.
4.2. Blood Collection and Genotyping
Samples of blood (4 mL) from patients with schizophrenia were collected using a plastic syringe containing 1 mL anticoagulant (acid citrate dextrose). Genomic DNA was isolated from peripheral blood leukocytes by a standard salting-out method [80]. Genotyping was performed according to the manufacturer’s protocol (Applied Biosystems), using TaqMan SNP Genotyping Assays and TaqMan Genotyping Master Mix. TaqMan allele-specific polymerase chain reaction (PCR) was conducted on ABI Prism 7000 Sequencing Detection System apparatus. Briefly, 20 ng of genomic DNA was amplified in a 10 µL reaction volume, using these PCR reaction conditions: 40 cycles at 92 °C for 15 s and 60 °C for 60 s. The HTR1A rs6295, HTR1B rs13212041, HTR2A rs6313, HTR2C rs3813929 and HTR6 rs1805054 polymorphisms were analyzed (Table 6).

Table 6.
Details of the 5-HT receptor gene polymorphisms analyzed in the study.
4.3. Data Analyses
Statistical analyses were performed with GraphPad Prism version 4.00 for Windows (GraphPad Software, Inc., San Diego, CA, USA). The data are expressed as number (n) and percentage (%) or as mean ± SD. Normality of distribution was assessed with the D’Agostino–Pearson omnibus normality test. Since the data was found not to be normally distributed, the Mann–Whitney U-test was used for comparison of two groups, while the Kruskal–Wallis test and post-hoc Dunn’s multiple comparison test were used for analysis of three groups. Possible deviations from HWE were tested using the goodness of fit χ2-test. Genotype and allele frequencies were evaluated by a χ2-test of independence or Fisher exact test, respectively. The results were corrected for multiple testing (5 polymorphisms) using Bonferroni correction, and the p-value for significance was set to 0.01. G*Power 3 Software was used for conducting power analyses, i.e., to determine a priori sample size and to post hoc compute the power achieved. For analyses with a χ2-test (with α = 0.01; power (1−β) = 0.80 and a small effect size (ω = 0.25)), for df = 2, the total desired sample size was 223, and for df = 1 (Fisher exact test), the total desired sample size was 187. For the F test (Kruskal–Wallis test) involving three groups (with α = 0.01; power = 0.80; a small effect size = 0.25), the total desired sample size was 227. For the t-test (Mann–Whitney test) (with α = 0.01; power = 0.80; median effect size = 0.50), total desired sample size was 228. As the actual total sample size was 229, the power analysis confirmed the appropriate sample size and thus statistical power of the study.
5. Conclusions
To the best of our knowledge, this is the first study to report an association of the HTR1B rs13212041 polymorphism with antipsychotic-induced akathisia. Our results demonstrate that homozygous patients with schizophrenia who carry the TT genotype are more prone to develop akathisia and experience higher akathisia severity following haloperidol therapy than carriers of the C-allele. These molecular findings indicate the potential involvement of 5-HT1B receptors in the development of akathisia in haloperidol-treated patients. As the rs13212041 polymorphism affects microRNA regulation of HTR1B gene expression, these data might suggest a role for epigenetic mechanisms in 5-HT modulation associated with antipsychotic-induced EPS. Further studies, including a larger number of subjects, should test a wider range of antipsychotics for association between the HRT1B polymorphism and akathisia, and should also include and compare male and female patients with schizophrenia. If confirmed, such pharmacogenetic predictors of EPS could be helpful toward a better selection of medication in order to reduce EPS, resulting in better patient compliance and quality of life. For schizophrenia patients for whom haloperidol remains an important treatment option, 5-HT1B receptor agonists might represent a useful therapeutic approach for management of akathisia.
Author Contributions
Conceptualization, D.S.S., D.M.-S. and N.P.; project administration, D.S.S., D.M.-S., A.M.-P. and N.P.; investigation, M.G., M.Z., M.S. and M.N.P.; validation, D.S.S., M.S. and A.M.-P.; formal analysis, M.G. and D.S.S.; writing—original draft preparation, M.G. and D.S.S.; writing—review and editing, D.S.S., M.Z., A.M.-P., D.M.-S. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Nicholas J. Bradshaw for editing the English language.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| BARS | Barnes Akathisia Rating Scale |
| BMI | Body mass index |
| DSM-IV | Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition |
| EPS | Extrapyramidal side effects |
| ESRS | Extrapyramidal Symptom Rating Scale |
| FGAs | Typical or first-generation antipsychotics |
| 5-HT | 5-hydroxytryptamine; Serotonin |
| HWE | Hardy–Weinberg equilibrium |
| PANSS | Positive and Negative Syndrome Scale |
| PCR | Polymerase chain reaction |
| SAS | Simpson–Angus Rating Scale for Extrapyramidal Side Effects |
| SGAs | Atypical or second-generation antipsychotics |
| WHO | World Health Organization |
References
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Dold, M.; Samara, M.T.; Li, C.; Tardy, M.; Leucht, S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst. Rev. 2015, 1, CD009831. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Dopamine D2 receptor occupancy and clinical effects: A systematic review and pooled analysis. J. Clin. Psychopharm. 2011, 31, 497–502. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Model List of Essential Medicines, 21st List, 2019; License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Seeman, P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J. Psychiatry Neurosci. 2000, 25, 161–166. [Google Scholar] [PubMed]
- Farde, L.; Nordstrom, A.L.; Wiesel, F.A.; Pauli, S.; Halldin, C.; Sedvall, G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch. Gen. Psychiatry 1992, 49, 538–544. [Google Scholar] [CrossRef]
- Lewis, R. Typical and atypical antipsychotics in adolescent schizophrenia: Efficacy, tolerability, and differential sensitivity to extrapyramidal symptoms. Can. J. Psychiatry 1998, 43, 596–604. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Konarski, J.Z. Tolerability profiles of atypical antipsychotics in the treatment of bipolar disorders. J. Clin. Psychiatry 2005, 66, 28–36. [Google Scholar]
- Muscettola, G.; Barbato, G.; Pampallona, S.; Casiello, M.; Bollini, P. Extrapyramidal syndromes in neuroleptic-treated patients: Prevalence, risk factors, and association with tardive dyskinesia. J. Clin. Psychopharmacol. 1999, 19, 203–208. [Google Scholar] [CrossRef]
- Brockmoller, J.; Kirchheiner, J.; Schmider, J.; Walter, S.; Sachse, C.; Muller- Oerlinghausen, B.; Roots, I. The impact of the CYP2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin. Pharm. Ther. 2002, 72, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Crescenti, A.; Mas, S.; Gasso, P.; Parellada, E.; Bernardo, M.; Lafuente, A. CYP2D6*3, *4, *5 and *6 polymorphisms and antipsychotic-induced extrapyramidal side-effects in patients receiving antipsychotic therapy. Clin. Exper. Pharm. Physiol. 2008, 35, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.R.; Van Harten, P.N.; Van Os, J. Antipsychotic-induced tardive dyskinesia and polymorphic variations in COMT, DRD2, CYP1A2 and MnSOD genes: A meta-analysis of pharmacogenetic interactions. Mol. Psychiatry 2008, 13, 544–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lafuente, A.; Bernardo, M.; Mas, S.; Crescenti, A.; Aparici, M.; Gasso, P.; Deulofeu, R.; Mane, A.; Catalan, R.; Carne, X. Polymorphism of dopamine D2 receptor (TaqIA, TaqIB, and-141C Ins/ Del) and dopamine degradation enzyme (COMT G158A, A-278G) genes and extrapyramidal symptoms in patients with schizophrenia and bipolar disorders. Psychiatry Res. 2008, 161, 131–141. [Google Scholar] [CrossRef]
- Zivković, M.; Mihaljević-Peles, A.; Bozina, N.; Saqud, M.; Nikolac-Perkovic, M.; Vuksan-Cusa, B.; Muck-Seler, D. The association study of polymorphisms in DAT, DRD2, and COMT genes and acute extrapyramidal adverse effects in male schizophrenic patients treated with haloperidol. J. Clin. Psychopharmacol. 2013, 33, 593–599. [Google Scholar] [CrossRef]
- Güzey, C.; Scordo, M.G.; Spina, E.; Landsem, V.M.; Spigset, O. Antipsychotic-induced extrapyramidal symptoms in patients with schizophrenia: Associations with dopamine and serotonin receptor and transporter polymorphisms. Eur. J. Clin. Pharm. 2007, 63, 233–241. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Hooten, W.M. Extrapyramidal Symptoms (EPS). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534115/ (accessed on 28 March 2020).
- Givens, C.J. Adverse Drug Reactions Associated with Antipsychotics, Antidepressants, Mood Stabilizers, and Stimulants. Nurs. Clin. North. Am. 2016, 51, 309–321. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Litman, R.E.; Pickar, D. Adverse effects of antipsychotic drugs. Drug Saf. 1993, 9, 429–436. [Google Scholar] [CrossRef]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatment of schizophrenia; a critical review of pharmacology and mechanism of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Svob Strac, D.; Sole, M.; Unzeta, M.; Tipton, K.F.; Mück-Šeler, D.; Bolea, I.; Della Corte, L.; Nikolac Perkovic, M.; Pivac, N.; et al. Monoaminergic and Histaminergic Strategies and Treatments in Brain Diseases. Front. Neurosci. 2016, 10, 541. [Google Scholar] [CrossRef]
- Alex, K.D.; Pehek, E.A. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharm 2007, 113, 296–320. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog. Neurobiol. 2017, 151, 175–236. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, G.; De Deurwaerdere, P. New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharm 2016, 157, 125–162. [Google Scholar] [CrossRef] [PubMed]
- Lerer, B.; Segman, R.H.; Tan, E.C.; Basile, V.S.; Cavallaro, R.; Aschauer, H.N.; Strous, R.; Chong, S.A.; Heresco-Levy, U.; Verga, M.; et al. Combined analysis of 635 patients confirms an age-related association of the serotonin 2A receptor gene with tardive dyskinesia and specificity for the non-orofacial subtype. Int. J. Neuropsychopharmacol. 2005, 8, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Zhang, X.B.; Sha, W.W.; Zhang, X.B.; Reynolds, G.P. Association of a polymorphism in the promoter region of the serotonin 5-HT2C receptor gene with tardive dyskinesia in patients with schizophrenia. Mol. Psychiatry 2002, 7, 670–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunes, A.; Scordo, M.G.; Jaanson, P.; Dahl, M.L. Serotonin and dopamine receptor gene polymorphisms and the risk of extrapyramidal side effects in perphenazinetreated schizophrenic patients. Psychopharmacol. (Berl) 2007, 190, 479–484. [Google Scholar] [CrossRef]
- Mas, S.; Gassó, P.; Lafuente, A.; Bioque, M.; Lobo, A.; Gonzàlez-Pinto, A.; Olmeda, M.S.; Corripio, I.; Llerena, A.; Cabrera, B.; et al. Pharmacogenetic study of antipsychotic induced acute extrapyramidal symptoms in a first episode psychosis cohort: Role of dopamine, serotonin and glutamate candidate genes. Pharm. J. 2016, 16, 439–445. [Google Scholar] [CrossRef]
- Zhang, J.P.; Malhotra, A.K. Pharmacogenetics and antipsychotics: Therapeutic efficacy and side effects prediction. Expert Opin. Drug Metab. Toxicol. 2011, 7, 9–37. [Google Scholar] [CrossRef]
- Miller, C.H.; Fleischhacker, W.W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000, 22, 73–81. [Google Scholar] [CrossRef]
- Dayalu, P.; Chou, K.L. Antipsychotic-induced extrapyramidal symptoms and their management. Expert Opin. Pharm. 2008, 9, 1451–1462. [Google Scholar] [CrossRef]
- Salem, H.; Nagpal, C.; Pigott, T.; Teixeira, A.L. Revisiting Antipsychotic-induced Akathisia: Current Issues and Prospective Challenges. Curr. Neuropharmacol. 2017, 15, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Caroff, S.N.; Mann, S.C.; Campbell, E.C.; Sullivan, K.A. Movement disorders associated with atypical antipsychotic drugs. J. Clin. Psychiatry 2002, 63, 12–19. [Google Scholar] [PubMed]
- Ohno, Y.; Shimizu, S.; Tokudome, K. Pathophysiological roles of serotonergic system in regulating extrapyramidal motor functions. Biol. Pharm. Bull. 2013, 36, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Dahl, M.L.; Spina, E.; Scordo, M.G. Further evidence for the association between 5-HT2C receptor gene polymorphisms and extrapyramidal side effects in male schizophrenic patients. Eur. J. Clin. Pharm. 2008, 64, 477–482. [Google Scholar] [CrossRef]
- Al-Janabi, I.; Arranz, M.J.; Blakemore, A.I.; Saiz, P.A.; Susce, M.T.; Glasser, P.E.; Clark, D.; De Leon, J. Association study of serotonergic gene variants with antipsychotic-induced adverse reactions. Psychiatr. Genet. 2009, 19, 305–311. [Google Scholar] [CrossRef]
- Al Hadithy, A.F.; Ivanova, S.A.; Pechlivanoglou, P.; Semke, A.; Fedorenko, O.; Kornetova, E.; Ryadovaya, L.; Brouwers, J.R.; Wilffert, B.; Bruggeman, R.; et al. Tardive dyskinesia and DRD3, HTR2A and HTR2C gene polymorphisms in Russian psychiatric inpatients from Siberia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 475–481. [Google Scholar] [CrossRef]
- Knol, W.; Van Marum, R.J.; Jansen, P.A.; Strengman, E.; Al Hadithy, A.F.; Wilffert, B.; Schobben, A.F.; Ophoff, R.A.; Egberts, T.C. Genetic variation and the risk of haloperidol-related parkinsonism in elderly patients: A candidate gene approach. J. Clin. Psychopharmacol. 2013, 33, 405–410. [Google Scholar] [CrossRef]
- Jensen, K.P.; Covault, J.; Conner, T.S.; Tennen, H.; Kranzler, H.R.; Furneaux, H.M. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol. Psychiatry 2009, 14, 381–389. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef]
- Tang, H.; Dalton, C.F.; Srisawat, U.; Zhang, Z.J.; Reynolds, G.P. Methylation at a transcription factor-binding site on the 5-HT1A receptor gene correlates with negative symptom treatment response in first episode schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 645–649. [Google Scholar] [CrossRef]
- Swathy, B.; Banerjee, M. Haloperidol induces pharmacoepigenetic response by modulating miRNA expression, global DNA methylation and expression profiles of methylation maintenance genes and genes involved in neurotransmission in neuronal cells. PLoS ONE 2017, 12, e0184209. [Google Scholar] [CrossRef] [PubMed]
- Melas, P.A.; Rogdaki, M.; Osby, U.; Schalling, M.; Lavebratt, C.; Ekstrom, T.J. Epigenetic aberrations in leukocytes of patients with schizophrenia: Association of global DNA methylation with antipsychotic drug treatment and disease onset. Faseb. J. 2012, 26, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Schroeder, F.A.; Youngs, R.M.; Schmidt, T.W.; Ferris, C.; Konradi, C.; Akbarian, S. Dopamine D2-like antagonists induce chromatin remodeling in striatal neurons through cyclic AMP-protein kinase A and NMDA receptor signaling. J. Neurochem. 2004, 90, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, D.M.; Liu, B.; Duncan, C.E.; Beveridge, N.J.; Tooney, P.A.; Schofield, P.R.; Cairns, M.J. Gene-microRNA interactions associated with antipsychotic mechanisms and the metabolic side effects of olanzapine. Psychopharmacol (Berl) 2013, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.O.; Jeffries, C.D.; Jarskog, L.F.; Thomson, J.M.; Woods, K.; Newman, M.A.; Parker, J.S.; Jin, J.; Hammond, S.M. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007, 8, R27. [Google Scholar] [CrossRef] [PubMed]
- Fehér, L.Z.; Kálmán, J.; Puskás, L.G.; Gyülvészi, G.; Kitajka, K.; Penke, B.; Palotás, M.; Samarova, E.I.; Molnár, J.; Zvara, A.; et al. Impact of haloperidol and risperidone on gene expression profile in the rat cortex. Neurochem. Int. 2005, 47, 271–280. [Google Scholar] [CrossRef]
- MacGibbon, G.; Lawlor, P.; Bravo, R.; Dragunow, M. Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res. Mol. Brain Res. 1994, 23, 21–32. [Google Scholar] [CrossRef]
- Audinot, V.; Newman-Tancredi, A.; Cussac, D.; Millan, M.J. Inverse agonist properties of antipsychotic agents at cloned, human (h) serotonin (5-HT)(1B) and h5-HT(1D) receptors. Neuropsychopharmacology 2001, 25, 410–422. [Google Scholar] [CrossRef]
- De Deurwaerdere, P.; Bharatiya, R.; Chagraoui, A.; Di Giovanni, G. Constitutive activity of 5-HT receptors: Factual analysis. Neuropharmacology 2020, 107967. [Google Scholar] [CrossRef]
- Bonhomme, N.; De Deurwaerdere, P.; Le Moal, M.; Spampinato, U. Evidence for 5-HT4 receptor subtype involvement in the enhancement of striatal dopamine release induced by serotonin: A microdialysis study in the halothane-anesthetized rat. Neuropharmacology 1995, 34, 269–279. [Google Scholar] [CrossRef]
- Navailles, S.; De Deurwaerdere, P. Presynaptic control of serotonin on striatal dopamine function. Psychopharmacol. (Berl). 2011, 213, 213–242. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.J.; Segu, L.; Hen, R. 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: Converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol. Pharmacol. 1997, 51, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Al-Barghouthy, G.; Pearce, R.K.; Smith, L.; Hagan, J.J.; Jenner, P. Effect of 5-HT1B/D receptor agonist and antagonist administration on motor function in haloperidol and MPTP-treated common marmosets. Pharm. Biochem. Behav. 2004, 79, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Jaunarajs, K.L.; Dupre, K.B.; Steiniger, A.; Klioueva, A.; Moore, A.; Kelly, C.; Bishop, C. Serotonin 1B receptor stimulation reduces D1 receptor agonist-induced dyskinesia. Neuroreport 2009, 20, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Rainier-Pope, C.R. Treatment with diazepam of children with drug-induced extrapyramidal symptoms. S. Afr. Med. J. 1979, 55, 328–330. [Google Scholar] [PubMed]
- Director, K.L.; Muniz, C.E. Diazepam in the treatment of extrapyramidal symptoms: A case report. J. Clin. Psychiatry 1982, 43, 160–161. [Google Scholar] [PubMed]
- Aichhorn, W.; Gasser, M.; Weiss, E.M.; Adlassnig, C.; Marksteiner, J. Gender Differences in Pharmacokinetics and Side Effects of Second Generation Antipsychotic Drugs. Curr. Neuropharmacol. 2005, 3, 73–85. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Wang, G.; Yang, J.; Wang, C. Why sex differences in schizophrenia? J. Transl. Neurosci. (Beijing). 2016, 1, 37–42. [Google Scholar] [CrossRef]
- Xia, X.; Ding, M.; Xuan, J.F.; Xing, J.X.; Pang, H.; Wang, B.J.; Yao, J. Polymorphisms in the human serotonin receptor 1B (HTR1B) gene are associated with schizophrenia: A case control study. BMC Psychiatry 2018, 18, 303. [Google Scholar] [CrossRef]
- Kumar, R.; Sachdev, P. Akathisia and second-generation antipsychotic drugs. Curr. Opin. Psychiatry 2009, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P. Cariprazine: New dopamine biased agonist for neuropsychiatric disorders. Drugs Today (Barc). 2016, 52, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, J.M.; Caley, C.F. Extrapyramidal reactions associated with serotonergic antidepressants. Ann. Pharm. 2015, 49, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.D.; Pace, W.D.; Libby, A.M.; West, D.R.; Valuck, R.J. Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: A retrospective US claims study. Clin 2012, 34, 113–123. [Google Scholar] [CrossRef]
- Madhusoodanan, S.; Alexeenko, L.; Sanders, R.; Brenner, R. Extrapyramidal symptoms associated with antidepressants: A review of the literature and an analysis of spontaneous reports. Ann. Clin. Psychiatry 2010, 22, 148–156. [Google Scholar] [PubMed]
- Cem Atbasoglu, E.C.; Schultz, S.K.; Andreasen, N.C. The relationship of akathisia with suicidality and depersonalization among patients with schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 336–341. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Shardorodsky, M.; Fuchs, C.; Schneidman, M.; Weizman, A. Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist mianserin. Double-blind, placebo-controlled study. Br. J. Psychiatry 1999, 174, 238–242. [Google Scholar] [CrossRef]
- Praharaj, S.K.; Kongasseri, S.; Behere, R.V.; Sharma, P.S. Mirtazapine for antipsychotic induced acute akathisia: A systematic review and meta-analysis of randomized placebo-controlled trials. Adv. Psychopharmacol. 2015, 5, 307–313. [Google Scholar] [CrossRef]
- Miller, C.H.; Hummer, M.; Pycha, R.; Fleischhacker, W.W. The effect of ritanserin on treatment resistant neuroleptic induced akathisia: Case reports. Pro. G Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 247–251. [Google Scholar] [CrossRef]
- Fischel, T.; Hermesh, H.; Aizenberg, D.; Zemishlany, Z.; Munitz, H.; Benjamini, Y.; Weizman, A. Cyproheptadine versus propranolol for the treatment of acute neuroleptic-induced akathisia: A comparative double-blind study. J. Clin. Psychopharmacol. 2001, 21, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Avital, A.; Gross-Isseroff, R.; Stryjer, R.; Hermesh, H.; Weizman, A.; Shiloh, R. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: A comparative double-blind study. Eur. Neuropsychopharmacol. 2009, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Press: Washington, DC, USA, 1994. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.M.; Angus, J.W.S. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. 1970, 212, 11–19. [Google Scholar] [CrossRef]
- Barnes, T.R.E. A rating scale for drug-induced akathisia. Br. J. Psychiatry 1989, 154, 672–676. [Google Scholar] [CrossRef]
- Chouinard, G.; Ross-Chouinard, A.; Annable, L.; Jones, B. The extrapyramidal symptom rating scale. Can. J. Neurol. Sci. 1980, 7, 233–244. [Google Scholar]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).