The Role of Vitamin D in Modulating Mesenchymal Stem Cells and Endothelial Progenitor Cells for Vascular Calcification
Abstract
1. Introduction
2. Mechanism of Vascular Calcification
2.1. Endothelial Injury Causing Vascular Calcification
2.2. The Role of Vascular Smooth Muscle Ccells (VSMCs) in Vascular Calcification
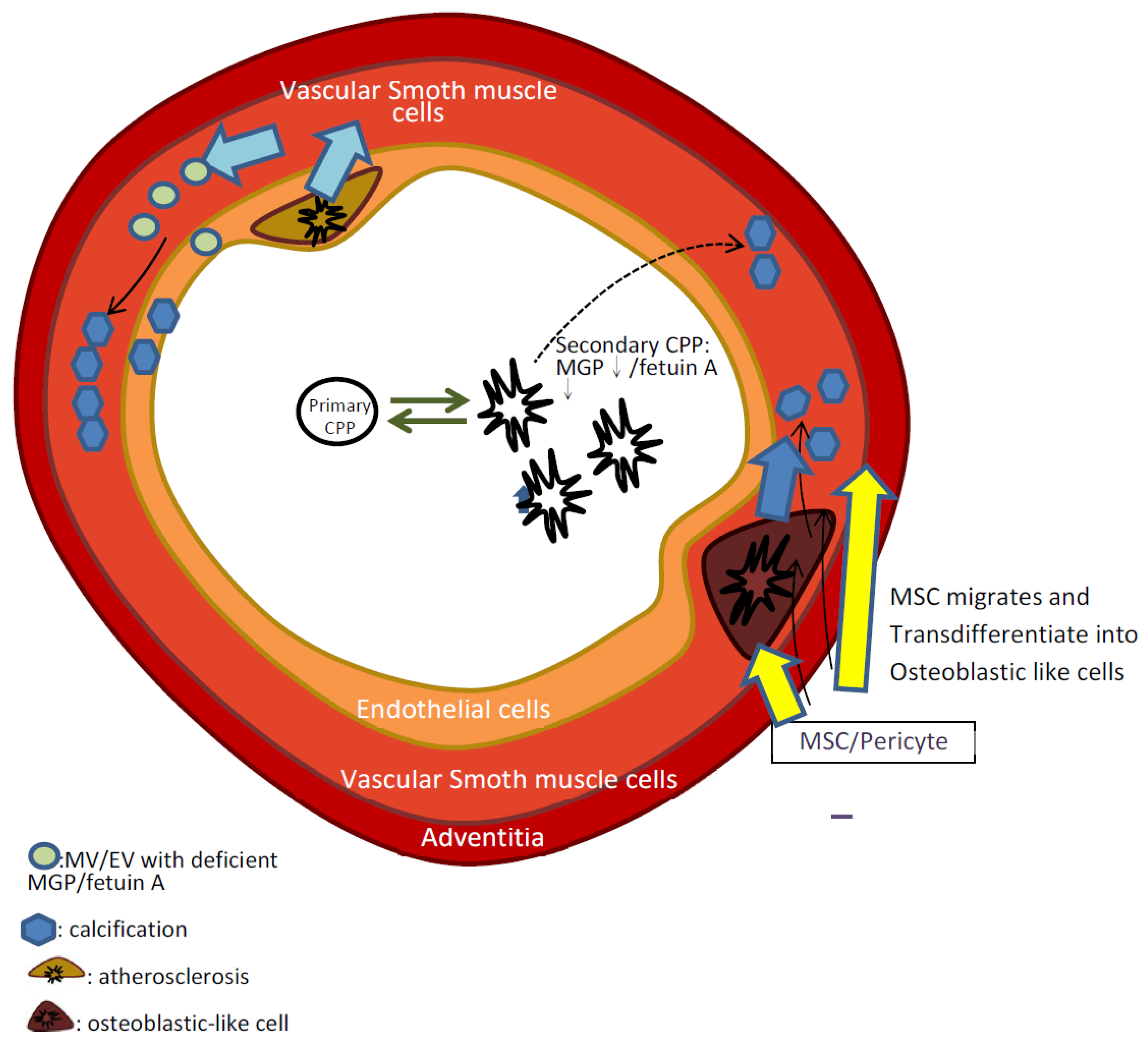
2.3. The Role of Adventitial MSCs and Pericyte in Vascular Calcification
2.4. The Role of Matrix Vesicles/Exosomes and Calciprotein Particles Containing Insufficient Calcification Inhibitors
3. The Role of EPCs, Hematopoietic Progenitor Cells, and MSCs in Vascular Calcification
3.1. EPCs and Arterial Calcification
3.2. Hematopoietic Progenitor Cells and Arterial Calcification
3.3. MSCs and Arterial Calcification
3.4. Extracelluar Vesicles and Calciprotein Particles Stimulated by MSCs
4. Possible Therapeutic Roles of Vitamin D in MSCs and Vascular Calcification
4.1. The Influence of Vitamin D on EPCs in Vascular Calcification
4.2. The Role of Vitamin D and MSCs/Pericytes in Vascular Calcification
4.3. The Role of Vitamin D in Adipose Tissue-Derived Stem Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| αSMA | α-smooth muscle actin |
| Ang | angiotensin |
| BMP | bone morphogenetic protein |
| BMP2 | bone morphogenetic protein2 |
| C/EBP | CCAAT/enhancer binding protein |
| CAD | coronary artery disease |
| CBFA1 | core-binding factor α-1 |
| CD | cluster of differentiation |
| Cdk-2 | Cyclin-dependent kinase 2 |
| CKD | chronic kidney disease |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
| CYP24A1 | Cytochrome P450 family 24 subfamily A member 1 |
| EPCs | endothelial progenitor cells |
| FGF23 | Fibroblast growth factor 23 |
| Gli1 | The Human Glioma-Associated Oncogene Homolog 1 |
| HIF-1-alpha | hypoxia-inducible factor-1-alpha |
| IL-1 | interleukin 1 |
| IL-6 | Interleukin-6 |
| KDR | kinase insert domain receptor |
| LDL | low-density lipoprotein |
| M1 macrophage | classically activated macrophage |
| MAPK | mitogen-activated protein kinase |
| MGP | matrix Gla protein |
| miR | MicroRNA |
| MMP | matrix metalloproteinase |
| MSCs | mesenchymal stem cells |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mechanistic target of Rapamycin complex 1 |
| mTORC2 | mechanistic target of Rapamycin complex 2 |
| MV | Matrix vesicle |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B |
| NG-2 | Neural/glial antigen 2 |
| PDGFR | Platelet-derived growth factor receptors |
| PDGFRβ | Platelet-derived growth factor receptor beta |
| PPAR-ɣ | peroxisome proliferator-activated receptor gamma |
| RAAS | renin-angiotensin-aldosterone system |
| Sca-1 | stem cell antigen 1 |
| SGK-1 | serum- and glucocorticoid-inducible kinase 1 |
| Smad 1/5/8 | Mothers against decapentaplegic homolog 1/5/8 |
| TNF-α | tumor necrosis factor alpha |
| UVB | ultraviolet B |
| VEGF | vascular endothelial growth factor |
| VSMC | vascular smooth muscle cell |
| Wnt | Wingless-INT |
References
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Narula, N.; Dannenberg, A.J.; Olin, J.W.; Bhatt, D.L.; Johnson, K.W.; Nadkarni, G.; Min, J.; Torii, S.; Poojary, P.; Anand, S.S.; et al. Pathology of Peripheral Artery Disease in Patients with Critical Limb Ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163. [Google Scholar] [CrossRef]
- Mizuiri, S.; Nishizawa, Y.; Yamashita, K.; Mizuno, K.; Ishine, M.; Doi, S.; Masaki, T.; Shigemoto, K. Coronary artery calcification score and common iliac artery calcification score in non-dialysis CKD patients. Nephrology 2018, 23, 837–845. [Google Scholar] [CrossRef]
- Mathew, R.O.; Bangalore, S.; Lavelle, M.P.; Pellikka, P.A.; Sidhu, M.S.; Boden, W.E.; Asif, A. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: A review. Kidney Int. 2017, 91, 797–807. [Google Scholar] [CrossRef]
- Lin, J.S.; Evans, C.V.; Johnson, E.; Redmond, N.; Coppola, E.L.; Smith, N. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2018, 320, 281–297. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lu, C.-L.; Zheng, C.-M.; Chen, R.-M.; Lin, Y.-F.; Liu, W.-C.; Yen, T.-H.; Chen, R.; Lu, K.-C. Emerging Role of Vitamins D and K in Modulating Uremic Vascular Calcification: The Aspect of Passive Calcification. Nutrients 2019, 11, 152. [Google Scholar] [CrossRef]
- Gorriz, J.L.; Molina, P.; Cerveron, M.J.; Vila, R.; Bover, J.; Nieto, J.; Barril, G.; Martinez-Castelao, A.; Fernandez, E.; Escudero, V.; et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin. J. Am. Soc. Nephrol. Cjasn 2015, 10, 654–666. [Google Scholar] [CrossRef]
- Yap, Y.S.; Ting, K.T.; Chi, W.C.; Lin, C.H.; Liu, Y.C.; Chuang, W.L. Aortic Arch Calcification Predicts Patency Loss of Arteriovenous Fistula in End-Stage Renal Disease Patients. Sci. Rep. 2016, 6, 24943. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Kramann, R.; Goettsch, C.; Wongboonsin, J.; Iwata, H.; Schneider, R.K.; Kuppe, C.; Kaesler, N.; Chang-Panesso, M.; Machado, F.G.; Gratwohl, S.; et al. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell 2016, 19, 628–642. [Google Scholar] [CrossRef]
- Hou, Y.C.; Liu, W.C. Role of Vitamin D in Uremic Vascular Calcification. BioMed Res. 2017, 2017, 2803579. [Google Scholar] [CrossRef]
- Sun, R.; Huang, J.; Sun, B. Mobilization of endothelial progenitor cells in sepsis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2020, 69, 1–9. [Google Scholar] [CrossRef]
- Wu, C.C.; Hung, S.C.; Kuo, K.L.; Tarng, D.C. Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access. Toxins 2017, 9, 25. [Google Scholar] [CrossRef]
- Yang, S.-W.; Hennessy, R.R.; Khosla, S.; Lennon, R.; Loeffler, D.; Sun, T.; Liu, Z.; Park, K.-H.; Wang, F.-L.; Lerman, L.O.; et al. Circulating osteogenic endothelial progenitor cell counts: New biomarker for the severity of coronary artery disease. Int. J. Cardiol. 2017, 227, 833–839. [Google Scholar] [CrossRef]
- Cianciolo, G.; Capelli, I.; Cappuccilli, M.; Scrivo, A.; Donadei, C.; Marchetti, A.; Rucci, P.; La Manna, G. Is chronic kidney disease-mineral and bone disorder associated with the presence of endothelial progenitor cells with a calcifying phenotype? Clin. Kidney J. 2017, 10, 389–396. [Google Scholar] [CrossRef]
- Dong, Y.; Stallmann-Jorgensen, I.S.; Pollock, N.K.; Harris, R.A.; Keeton, D.; Huang, Y.; Li, K.; Bassali, R.; Guo, D.H.; Thomas, J.; et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J. Clin. Endocrinol. Metab. 2010, 95, 4584–4591. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, A.K.; Lal, A.; Kumar, V.; Singhal, M.; Billot, L.; Gupta, K.L.; Banerjee, D.; Jha, V. A Randomized Trial of Vitamin D Supplementation on Vascular Function in CKD. J. Am. Soc. Nephrol. Jasn 2017, 28, 3100–3108. [Google Scholar] [CrossRef]
- Mallat, Z.; Tedgui, A. Apoptosis in the vasculature: Mechanisms and functional importance. Br. J. Pharmacol. 2000, 130, 947–962. [Google Scholar] [CrossRef]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef]
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 2014, 16, 431. [Google Scholar] [CrossRef]
- Wang, G.; Jacquet, L.; Karamariti, E.; Xu, Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015, 593, 3013–3030. [Google Scholar] [CrossRef]
- Lopes, A.A.; Tong, L.; Thumma, J.; Li, Y.; Fuller, D.S.; Morgenstern, H.; Bommer, J.; Kerr, P.G.; Tentori, F.; Akiba, T.; et al. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Evaluation of possible confounding by nutritional status. Am. J. Kidney Dis. 2012, 60, 90–101. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.Y.; Giachelli, C.M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 2006, 98, 905–912. [Google Scholar] [CrossRef]
- Lang, F.; Ritz, E.; Alesutan, I.; Voelkl, J. Impact of aldosterone on osteoinductive signaling and vascular calcification. Nephron. Physiol. 2014, 128, 40–45. [Google Scholar] [CrossRef]
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Martinez-Moreno, J.M.; Munoz-Castaneda, J.R.; Herencia, C.; Oca, A.M.; Estepa, J.C.; Canalejo, R.; Rodriguez-Ortiz, M.E.; Perez-Martinez, P.; Aguilera-Tejero, E.; Canalejo, A.; et al. In vascular smooth muscle cells paricalcitol prevents phosphate-induced Wnt/beta-catenin activation. Am. J. Physiol. Ren. Physiol. 2012, 303, F1136–F1144. [Google Scholar] [CrossRef]
- Shao, J.-S.; Cheng, S.-L.; Pingsterhaus, J.M.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Investig. 2005, 115, 1210–1220. [Google Scholar] [CrossRef]
- Voelkl, J.; Luong, T.T.; Tuffaha, R.; Musculus, K.; Auer, T.; Lian, X.; Daniel, C.; Zickler, D.; Boehme, B.; Sacherer, M.; et al. SGK1 induces vascular smooth muscle cell calcification through NF-kappaB signaling. J. Clin. Investig. 2018, 128, 3024–3040. [Google Scholar] [CrossRef]
- Voelkl, J.; Lang, F.; Eckardt, K.-U.; Amann, K.; Kuro-O, M.; Pasch, A.; Pieske, B.; Alesutan, I. Signaling pathways involved in vascular smooth muscle cell calcification during hyperphosphatemia. Cell. Mol. Life Sci. Cmls 2019, 76, 2077–2091. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Chatrou, M.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human Vascular Smooth Muscle Cells Undergo Vesicle-Mediated Calcification in Response to Changes in Extracellular Calcium and Phosphate Concentrations: A Potential Mechanism for Accelerated Vascular Calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef]
- Shroff, R.C.; McNair, R.; Skepper, J.N.; Figg, N.; Schurgers, L.J.; Deanfield, J.; Rees, L.; Shanahan, C.M. Chronic Mineral Dysregulation Promotes Vascular Smooth Muscle Cell Adaptation and Extracellular Matrix Calcification. J. Am. Soc. Nephrol. 2010, 21, 103–112. [Google Scholar] [CrossRef]
- Moochhala, S.H. Extracellular pyrophosphate in the kidney: How does it get there and what does it do? Nephron. Physiol. 2012, 120, p33–p38. [Google Scholar] [CrossRef]
- Nigwekar, S.U.; Thadhani, R.; Brandenburg, V.M. Calciphylaxis. New Engl. J. Med. 2018, 378, 1704–1714. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; De Maré, A.; De Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751. [Google Scholar] [CrossRef]
- Adijiang, A.; Goto, S.; Uramoto, S.; Nishijima, F.; Niwa, T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transpl. 2008, 23, 1892–1901. [Google Scholar] [CrossRef]
- Valcheva, P.; Cardus, A.; Panizo, S.; Parisi, E.; Bozic, M.; Lopez Novoa, J.M.; Dusso, A.; Fernandez, E.; Valdivielso, J.M. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 2014, 235, 247–255. [Google Scholar] [CrossRef]
- Aoshima, Y.; Mizobuchi, M.; Ogata, H.; Kumata, C.; Nakazawa, A.; Kondo, F.; Ono, N.; Koiwa, F.; Kinugasa, E.; Akizawa, T. Vitamin D receptor activators inhibit vascular smooth muscle cell mineralization induced by phosphate and TNF-alpha. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 1800–1806. [Google Scholar] [CrossRef]
- Chen, S.; Law, C.S.; Gardner, D.G. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J. Steroid Biochem. Mol. Biol. 2010, 118, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; Nishizawa, Y.; Shioi, A.; Morii, H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 1998, 98, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Cardus, A.; Panizo, S.; Parisi, E.; Fernandez, E.; Valdivielso, J.M. Differential effects of vitamin D analogs on vascular calcification. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Bhat, O.M.; Yuan, X.; Camus, S.; Salloum, F.N.; Li, P.L. Abnormal Lysosomal Positioning and Small Extracellular Vesicle Secretion in Arterial Stiffening and Calcification of Mice Lacking Mucolipin 1 Gene. Int. J. Mol. Sci. 2020, 21, 1713. [Google Scholar] [CrossRef]
- Carmo, L.S.; Burdmann, E.A.; Fessel, M.R.; Almeida, Y.E.; Pescatore, L.A.; Farias-Silva, E.; Gamarra, L.F.; Lopes, G.H.; Aloia, T.P.A.; Liberman, M. Expansive Vascular Remodeling and Increased Vascular Calcification Response to Cholecalciferol in a Murine Model of Obesity and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 200–211. [Google Scholar] [CrossRef]
- Corselli, M.; Chen, C.W.; Sun, B.; Yap, S.; Rubin, J.P.; Peault, B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012, 21, 1299–1308. [Google Scholar] [CrossRef]
- Yang, W.J.; Zheng, L.; Wu, X.H.; Huang, Z.Q.; Niu, C.B.; Zhao, H.L.; Leung, T.W.; Wong, L.K.; Chen, X.Y. Postmortem Study Exploring Distribution and Patterns of Intracranial Artery Calcification. Stroke 2018, 49, 2767–2769. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, W.J.; Niu, C.B.; Zhao, H.L.; Wong, K.S.; Leung, T.W.H.; Chen, X.Y. Correlation of Adventitial Vasa Vasorum with Intracranial Atherosclerosis: A Postmortem Study. J. Stroke 2018, 20, 342–349. [Google Scholar] [CrossRef]
- Ijaz, T.; Sun, H.; Pinchuk, I.V.; Milewicz, D.M.; Tilton, R.G.; Brasier, A.R. Deletion of NF-κB/RelA in Angiotensin II-Sensitive Mesenchymal Cells Blocks Aortic Vascular Inflammation and Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1881–1890. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; He, L.; Zhang, H.; Pu, W.; Liu, K.; et al. Arterial Sca1(+) Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell Stem Cell 2020, 26, 81–96. [Google Scholar] [CrossRef]
- Tinajero, M.G.; Gotlieb, A.I. Recent Developments in Vascular Adventitial Pathobiology: The Dynamic Adventitia as a Complex Regulator of Vascular Disease. Am. J. Pathol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, R.; Chevre, R.; Rodriguez, C.; Ordonez, A.; Martinez-Gonzalez, J.; Andres, V.; Mendez-Ferrer, S. Nestin(+) cells direct inflammatory cell migration in atherosclerosis. Nat. Commun. 2016, 7, 12706. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Vezzani, B.; Khan, N.; Su, J.; Xu, L.; Yan, G.; Liu, Y.; Li, R.; Gaur, A.; Diao, Z.; et al. CD10 expression identifies a subset of human perivascular progenitor cells with high proliferation and calcification potentials. Stem Cells 2019. [Google Scholar] [CrossRef]
- Tigges, U.; Komatsu, M.; Stallcup, W.B. Adventitial pericyte progenitor/mesenchymal stem cells participate in the restenotic response to arterial injury. J. Vasc. Res. 2013, 50, 134–144. [Google Scholar] [CrossRef]
- Folestad, E.; Kunath, A.; Wagsater, D. PDGF-C and PDGF-D signaling in vascular diseases and animal models. Mol. Asp. Med. 2018, 62, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sohnel, O.; Grases, F. Supersaturation of body fluids, plasma and urine, with respect to biological hydroxyapatite. Urol. Res. 2011, 39, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andia, J.B.; Roman-Garcia, P.; Hruska, K. The connections between vascular calcification and bone health. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. Biomed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Li, Q.; Pfeifer, A.; Werner, N. Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease. JACC Basic Transl. Sci. 2017, 2, 790–807. [Google Scholar] [CrossRef]
- Gui, T.; Zhou, G.; Sun, Y.; Shimokado, A.; Itoh, S.; Oikawa, K.; Muragaki, Y. MicroRNAs that target Ca(2+) transporters are involved in vascular smooth muscle cell calcification. Lab. Investig. A J. Tech. Methods Pathol. 2012, 92, 1250–1259. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, X.; Shen, Y.; Chen, L.; Xu, C.; Zhao, H.; Wu, Y.; Zhang, Q.; Zhong, J.; Tang, Z.; et al. MicroRNA-32 promotes calcification in vascular smooth muscle cells: Implications as a novel marker for coronary artery calcification. PLoS ONE 2017, 12, e0174138. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Huang, F.; Feng, W.; Chen, J.; Zhang, H.; Wang, J.; Luo, P.; Huang, H. Exosomes, the message transporters in vascular calcification. J. Cell. Mol. Med. 2018, 22, 4024–4033. [Google Scholar] [CrossRef]
- Herrmann, M.; Schafer, C.; Heiss, A.; Graber, S.; Kinkeldey, A.; Buscher, A.; Schmitt, M.M.; Bornemann, J.; Nimmerjahn, F.; Herrmann, M.; et al. Clearance of fetuin-A--containing calciprotein particles is mediated by scavenger receptor-A. Circ. Res. 2012, 111, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Heiss, A.; Schafer, C.; Ketteler, M. Fetuin-A regulation of calcified matrix metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Block, G.A.; Bachtler, M.; Smith, E.R.; Jahnen-Dechent, W.; Arampatzis, S.; Chertow, G.M.; Parfrey, P.; Ma, X.; Floege, J. Blood Calcification Propensity, Cardiovascular Events, and Survival in Patients Receiving Hemodialysis in the EVOLVE Trial. Clin. J. Am. Soc. Nephrol. Cjasn 2017, 12, 315–322. [Google Scholar] [CrossRef]
- Chen, W.; Anokhina, V.; Dieudonne, G.; Abramowitz, M.K.; Kashyap, R.; Yan, C.; Wu, T.T.; de Mesy Bentley, K.L.; Miller, B.L.; Bushinsky, D.A. Patients with advanced chronic kidney disease and vascular calcification have a large hydrodynamic radius of secondary calciprotein particles. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2019, 34, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Tartaglione, L.; Rotondi, S.; Bover, J.; Goldsmith, D.; Pasquali, M. News on biomarkers in CKD-MBD. Semin. Nephrol. 2014, 34, 598–611. [Google Scholar] [CrossRef]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef]
- Wuyts, J.; Dhondt, A. The role of vitamin K in vascular calcification of patients with chronic kidney disease. Acta Clin. Belg. 2016, 71, 462–467. [Google Scholar] [CrossRef]
- Wen, L.; Chen, J.; Duan, L.; Li, S. Vitamin Kdependent proteins involved in bone and cardiovascular health (Review). Mol. Med. Rep. 2018, 18, 3–15. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Santos, L.; Macedo, A.L.; Matos, A.A.; Silva, A.P.; Neves, P.L.; Staes, A.; Gevaert, K.; Morais, R.; Vermeer, C.; et al. Chronic Kidney Disease Circulating Calciprotein Particles and Extracellular Vesicles Promote Vascular Calcification: A Role for GRP (Gla-Rich Protein). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 575–587. [Google Scholar] [CrossRef]
- Silaghi, C.N.; Ilyes, T.; Filip, V.P.; Farcas, M.; van Ballegooijen, A.J.; Craciun, A.M. Vitamin K Dependent Proteins in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1571. [Google Scholar] [CrossRef]
- Poon, C.C.; Li, R.W.; Seto, S.W.; Kong, S.K.; Ho, H.P.; Hoi, M.P.; Lee, S.M.; Ngai, S.M.; Chan, S.W.; Leung, G.P.; et al. In vitro vitamin K(2) and 1alpha,25-dihydroxyvitamin D(3) combination enhances osteoblasts anabolism of diabetic mice. Eur. J. Pharmacol. 2015, 767, 30–40. [Google Scholar] [CrossRef]
- O’Connor, E.; Molgaard, C.; Michaelsen, K.F.; Jakobsen, J.; Lamberg-Allardt, C.J.; Cashman, K.D. Serum percentage undercarboxylated osteocalcin, a sensitive measure of vitamin K status, and its relationship to bone health indices in Danish girls. Br. J. Nutr. 2007, 97, 661–666. [Google Scholar] [CrossRef]
- Miyake, N.; Hoshi, K.; Sano, Y.; Kikuchi, K.; Tadano, K.; Koshihara, Y. 1,25-Dihydroxyvitamin D3 promotes vitamin K2 metabolism in human osteoblasts. Osteoporos. Int. A J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2001, 12, 680–687. [Google Scholar] [CrossRef]
- Briasoulis, A.; Tousoulis, D.; Antoniades, C.; Papageorgiou, N.; Stefanadis, C. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovasc. Ther. 2011, 29, 125–139. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Zou, J.; Ying, Y.; Luo, Z. Dual Roles of the AMP-Activated Protein Kinase Pathway in Angiogenesis. Cells 2019, 8, 752. [Google Scholar] [CrossRef]
- Schipani, E.; Wu, C.; Rankin, E.B.; Giaccia, A.J. Regulation of Bone Marrow Angiogenesis by Osteoblasts during Bone Development and Homeostasis. Front. Endocrinol. 2013, 4, 85. [Google Scholar] [CrossRef]
- Cianciolo, G.; La Manna, G.; Della Bella, E.; Cappuccilli, M.L.; Angelini, M.L.; Dormi, A.; Capelli, I.; Laterza, C.; Costa, R.; Alviano, F.; et al. Effect of vitamin D receptor activator therapy on vitamin D receptor and osteocalcin expression in circulating endothelial progenitor cells of hemodialysis patients. Blood Purif. 2013, 35, 187–195. [Google Scholar] [CrossRef]
- Lu, C.-L.; Leu, J.-G.; Liu, W.-C.; Zheng, C.-M.; Lin, Y.-F.; Shyu, J.-F.; Wu, C.-C.; Lu, K.-C. Endothelial Progenitor Cells Predict Long-Term Mortality in Hemodialysis Patients. Int. J. Med. Sci. 2016, 13, 240–247. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; Speer, T.; Fliser, D. Endothelial progenitor cells in chronic kidney disease. Nephrol. Dial. Transplant. 2009, 25, 341–346. [Google Scholar] [CrossRef]
- Dutta, P.; Courties, G.; Wei, Y.; Leuschner, F.; Gorbatov, R.; Robbins, C.S.; Iwamoto, Y.; Thompson, B.; Carlson, A.L.; Heidt, T.; et al. Myocardial infarction accelerates atherosclerosis. Nature 2012, 487, 325–329. [Google Scholar] [CrossRef]
- Heidt, T.; Sager, H.B.; Courties, G.; Dutta, P.; Iwamoto, Y.; Zaltsman, A.; von Zur Muhlen, C.; Bode, C.; Fricchione, G.L.; Denninger, J.; et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014, 20, 754–758. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.; Fandrich, F.; Varoga, D.; Wruck, C.J.; Mentlein, R.; Helfenstein, A.; Hoseas, D.; Dressel, S.; Tillmann, B.; et al. Programmable cells of monocytic origin (PCMO): A source of peripheral blood stem cells that generate collagen type II-producing chondrocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 304–313. [Google Scholar] [CrossRef]
- Doehring, L.C.; Heeger, C.; Aherrahrou, Z.; Kaczmarek, P.M.; Erdmann, J.; Schunkert, H.; Ehlers, E.M. Myeloid CD34+CD13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. Am. J. Pathol. 2010, 177, 473–480. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, J.W.; Cho, H.J.; Lee, C.S.; Kim, H.S. Identification of Adult Mesodermal Progenitor Cells and Hierarchy in Atherosclerotic Vascular Calcification. Stem Cells 2018, 36, 1075–1096. [Google Scholar] [CrossRef]
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H.; McAlpine, C.S.; Cremer, S.; Hoyer, F.F.; Ji, F.; et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef]
- Miranville, A.; Heeschen, C.; Sengenes, C.; Curat, C.A.; Busse, R.; Bouloumie, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef]
- Hu, Y.; Davison, F.; Ludewig, B.; Erdel, M.; Mayr, M.; Url, M.; Dietrich, H.; Xu, Q. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation 2002, 106, 1834–1839. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; Andre, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.Q.; Zhu, Y.; Han, X.Q.; Liu, N. Exosomes Derived From Mesenchymal Stromal Cells Pretreated With Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front. Endocrinol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, S.; Guo, W.; Diao, Z.; Wang, L.; Han, X.; Guo, W.; Liu, W. Bone marrow mesenchymal stem cell-derived exosomes alleviate high phosphorus-induced vascular smooth muscle cells calcification by modifying microRNA profiles. Funct. Integr. Genom. 2019, 19, 633–643. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Zhao, R.; Zhang, K.; Midgley, A.C.; Kong, D.; Li, Z.; Zhao, Q. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials 2019, 204, 13–24. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Amini, H.; Heydarpour, P.; Amini Chermahini, F.; Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2019, 122, 67–90. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A D-Lightful health perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef]
- Gonzalez, E.A.; Sachdeva, A.; Oliver, D.A.; Martin, K.J. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am. J. Nephrol. 2004, 24, 503–510. [Google Scholar] [CrossRef]
- LaClair, R.E.; Hellman, R.N.; Karp, S.L.; Kraus, M.; Ofner, S.; Li, Q.; Graves, K.L.; Moe, S.M. Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am. J. Kidney Dis. 2005, 45, 1026–1033. [Google Scholar] [CrossRef]
- Banerjee, S.; Basu, S.; Sengupta, J. Vitamin D in nephrotic syndrome remission: A case-control study. Pediatric Nephrol. 2013, 28, 1983–1989. [Google Scholar] [CrossRef]
- Dusso, A.S.; Tokumoto, M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: A downward spiral in kidney disease. Kidney Int. 2011, 79, 715–729. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Maruyama, T.; Wakino, S.; Tokuyama, H.; Hashiguchi, A.; Tada, S.; Homma, K.; Monkawa, T.; Thomas, J.; Miyashita, K.; et al. A case of severe osteomalacia caused by Tubulointerstitial nephritis with Fanconi syndrome in asymptomotic primary biliary cirrhosis. Bmc Nephrol. 2015, 16, 187. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Wang, L.; Gao, Y. Upregulation of nuclear factor-kappaB activity mediates CYP24 expression and reactive oxygen species production in indoxyl sulfate-induced chronic kidney disease. Nephrology 2016, 21, 774–781. [Google Scholar] [CrossRef]
- Michaud, J.; Naud, J.; Ouimet, D.; Demers, C.; Petit, J.L.; Leblond, F.A.; Bonnardeaux, A.; Gascon-Barre, M.; Pichette, V. Reduced hepatic synthesis of calcidiol in uremia. J. Am. Soc. Nephrol. 2010, 21, 1488–1497. [Google Scholar] [CrossRef]
- Harinarayan, C.V. Vitamin D and diabetes mellitus. Hormones 2014, 13, 163–181. [Google Scholar] [CrossRef]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in Chronic Kidney Disease and Cardiovascular Disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef]
- Beckman, M.J.; Tadikonda, P.; Werner, E.; Prahl, J.; Yamada, S.; DeLuca, H.F. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry 1996, 35, 8465–8472. [Google Scholar] [CrossRef]
- Shimada, T.; Yamazaki, Y.; Takahashi, M.; Hasegawa, H.; Urakawa, I.; Oshima, T.; Ono, K.; Kakitani, M.; Tomizuka, K.; Fujita, T.; et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Ren. Physiol. 2005, 289, F1088–F1095. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, 42816. [Google Scholar] [CrossRef]
- Zhou, S.; Glowacki, J. Chronic kidney disease and vitamin D metabolism in human bone marrow-derived MSCs. Ann. New York Acad. Sci. 2017, 1402, 43–55. [Google Scholar] [CrossRef]
- Meng, F.; Bertucci, C.; Gao, Y.; Li, J.; Luu, S.; LeBoff, M.S.; Glowacki, J.; Zhou, S. Fibroblast growth factor 23 counters vitamin D metabolism and action in human mesenchymal stem cells. J. Steroid Biochem. Mol. Biol. 2020, 199, 105587. [Google Scholar] [CrossRef]
- Judd, S.E.; Tangpricha, V. Vitamin D deficiency and risk for cardiovascular disease. Am. J. Med. Sci. 2009, 338, 40–44. [Google Scholar] [CrossRef]
- Forman, J.P.; Giovannucci, E.; Holmes, M.D.; Bischoff-Ferrari, H.A.; Tworoger, S.S.; Willett, W.C.; Curhan, G.C. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007, 49, 1063–1069. [Google Scholar] [CrossRef]
- Kim, D.H.; Sabour, S.; Sagar, U.N.; Adams, S.; Whellan, D.J. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am. J. Cardiol. 2008, 102, 1540–1544. [Google Scholar] [CrossRef]
- Pilz, S.; Dobnig, H.; Fischer, J.E.; Wellnitz, B.; Seelhorst, U.; Boehm, B.O.; Marz, W. Low vitamin d levels predict stroke in patients referred to coronary angiography. Stroke 2008, 39, 2611–2613. [Google Scholar] [CrossRef]
- Melamed, M.L.; Muntner, P.; Michos, E.D.; Uribarri, J.; Weber, C.; Sharma, J.; Raggi, P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1179–1185. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Iranpanah, H.; Orafai, H.M.; Jamialahmadi, T.; Sahebkar, A. The molecular mechanisms by which vitamin D improve glucose homeostasis: A mechanistic review. Life Sci. 2020, 244, 117305. [Google Scholar] [CrossRef]
- Sypniewska, G.; Pollak, J.; Strozecki, P.; Camil, F.; Kretowicz, M.; Janikowski, G.; Mankowska-Cyl, A.; Pater, A.; Manitius, J. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am. J. Hypertens. 2014, 27, 114–121. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef]
- Tiryaki, O.; Usalan, C.; Sayiner, Z.A. Vitamin D receptor activation with calcitriol for reducing urinary angiotensinogen in patients with type 2 diabetic chronic kidney disease. Ren. Fail. 2016, 38, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; He, Z.; Ding, R.; Wu, F.; Huang, Z.; Wang, J.; Huang, S.; Dai, X.; Zhang, J.; Chen, J.; et al. Reduced Vitamin D Receptor on Circulating Endothelial Progenitor Cells: A New Risk Factor of Coronary Artery Diseases. J. Atheroscler. Thromb. 2018, 25, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Haidar, M.; Placzko, S.; Niendorf, R.; Darashchonak, N.; Hubel, C.A.; von Versen-Hoynck, F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am. J. Physiol. Cell Physiol. 2012, 303, C954–C962. [Google Scholar] [CrossRef] [PubMed]
- Schröder-Heurich, B.; Hardenberg, S.v.; Brodowski, L.; Kipke, B.; Meyer, N.; Borns, K.; Kaisenberg, C.S.V.; Brinkmann, H.; Claus, P.; Versen-Höynck, F.V. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. Faseb J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef]
- Yu, P.; Song, H.; Gao, J.; Li, B.; Liu, Y.; Wang, Y. Vitamin D (1,25-(OH)2D3) regulates the gene expression through competing endogenous RNAs networks in high glucose-treated endothelial progenitor cells. J. Steroid Biochem. Mol. Biol. 2019, 193, 105425. [Google Scholar] [CrossRef]
- Xu, W.; Hu, X.; Qi, X.; Zhu, R.; Li, C.; Zhu, Y.; Yin, S.; Cheng, L.; Zhu, R. Vitamin D Ameliorates Angiotensin II-Induced Human Endothelial Progenitor Cell Injury via the PPAR-gamma/HO-1 Pathway. J. Vasc. Res. 2019, 56, 17–27. [Google Scholar] [CrossRef]
- Hammer, Y.; Soudry, A.; Levi, A.; Talmor-Barkan, Y.; Leshem-Lev, D.; Singer, J.; Kornowski, R.; Lev, E.I. Effect of vitamin D on endothelial progenitor cells function. PloS ONE 2017, 12, e0178057. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Shafiee, A.; Patel, J.; Lee, J.S.; Hutmacher, D.W. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci. Rep. 2017, 7, 13558. [Google Scholar] [CrossRef]
- Kramann, R.; Couson, S.K.; Neuss, S.; Kunter, U.; Bovi, M.; Bornemann, J.; Knuchel, R.; Jahnen-Dechent, W.; Floege, J.; Schneider, R.K. Exposure to uremic serum induces a procalcific phenotype in human mesenchymal stem cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e45–e54. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Li, C.; Pang, L.; Shi, C.; Guo, F.; Chen, A.; Cao, X.; Wan, M. Mesenchymal stem cells recruited by active TGFβ contribute to osteogenic vascular calcification. Stem Cells Dev. 2014, 23, 1392–1404. [Google Scholar] [CrossRef]
- Wang, S.; Tong, M.; Hu, S.; Chen, X. The Bioactive Substance Secreted by MSC Retards Mouse Aortic Vascular Smooth Muscle Cells Calcification. BioMed Res. Int. 2018, 2018, 6053567. [Google Scholar] [CrossRef]
- Wang, S.; Hu, S.; Wang, J.; Liu, Y.; Zhao, R.; Tong, M.; Cui, H.; Wu, N.; Chen, X. Conditioned medium from bone marrow-derived mesenchymal stem cells inhibits vascular calcification through blockade of the BMP2-Smad1/5/8 signaling pathway. Stem Cell Res. Ther. 2018, 9, 160. [Google Scholar] [CrossRef]
- Xie, C.; Ouyang, L.; Chen, J.; Zhang, H.; Luo, P.; Wang, J.; Huang, H. The Emerging Role of Mesenchymal Stem Cells in Vascular Calcification. Stem Cells Int. 2019, 2019, 2875189. [Google Scholar] [CrossRef]
- Lee, K.-M.; Kang, H.-A.; Park, M.; Lee, H.-Y.; Choi, H.-R.; Yun, C.-H.; Oh, J.-W.; Kang, H.-S. Interleukin-24 attenuates β-glycerophosphate-induced calcification of vascular smooth muscle cells by inhibiting apoptosis, the expression of calcification and osteoblastic markers, and the Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2012, 428, 50–55. [Google Scholar] [CrossRef]
- Oma, I.; Andersen, J.K.; Lyberg, T.; Molberg, O.; Whist, J.E.; Fagerland, M.W.; Almdahl, S.M.; Hollan, I. Plasma vitamin D levels and inflammation in the aortic wall of patients with coronary artery disease with and without inflammatory rheumatic disease. Scand. J. Rheumatol. 2017, 46, 198–205. [Google Scholar] [CrossRef]
- Oma, I.; Olstad, O.K.; Andersen, J.K.; Lyberg, T.; Molberg, Ø.; Fostad, I.; Wang Fagerland, M.; Almdahl, S.M.; Rynning, S.E.; Yndestad, A.; et al. Differential expression of vitamin D associated genes in the aorta of coronary artery disease patients with and without rheumatoid arthritis. PLoS ONE 2018, 13, e0202346. [Google Scholar] [CrossRef]
- Wasnik, S.; Rundle, C.H.; Baylink, D.J.; Yazdi, M.S.; Carreon, E.E.; Xu, Y.; Qin, X.; Lau, K.W.; Tang, X. 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. Jci Insight 2018, 3, e98773. [Google Scholar] [CrossRef]
- Zhu, J.; Bing, C.; Wilding, J.P.H. Vitamin D receptor ligands attenuate the inflammatory profile of IL-1beta-stimulated human white preadipocytes via modulating the NF-kappaB and unfolded protein response pathways. Biochem. Biophys. Res. Commun. 2018, 503, 1049–1056. [Google Scholar] [CrossRef]
- Schmidt, N.; Brandsch, C.; Kühne, H.; Thiele, A.; Hirche, F.; Stangl, G.I. Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS ONE 2012, 7, e35316. [Google Scholar] [CrossRef]
- Fu, B.; Wang, H.; Wang, J.; Barouhas, I.; Liu, W.; Shuboy, A.; Bushinsky, D.A.; Zhou, D.; Favus, M.J. Epigenetic regulation of BMP2 by 1,25-dihydroxyvitamin D3 through DNA methylation and histone modification. PLoS ONE 2013, 8, e61423. [Google Scholar] [CrossRef]
- Nguyen-Yamamoto, L.; Tanaka, K.-I.; St-Arnaud, R.; Goltzman, D. Vitamin D-regulated osteocytic sclerostin and BMP2 modulate uremic extraskeletal calcification. Jci Insight 2019, 4, e126467. [Google Scholar] [CrossRef]
- Zhu, M.; Fang, X.; Zhou, S.; Li, W.; Guan, S. Indirect coculture of vascular smooth muscle cells with bone marrow mesenchymal stem cells inhibits vascular calcification and downregulates the Wnt signaling pathways. Mol. Med. Rep. 2016, 13, 5141–5148. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Jia, X.X.; Lin, X.X.; Zhang, W.X. Vitamin D alleviates airway remodeling in asthma by down-regulating the activity of Wnt/beta-catenin signaling pathway. Int. Immunopharmacol. 2019, 68, 88–94. [Google Scholar] [CrossRef]
- Schaub, T.; Gurgen, D.; Maus, D.; Lange, C.; Tarabykin, V.; Dragun, D.; Hegner, B. mTORC1 and mTORC2 Differentially Regulate Cell Fate Programs to Coordinate Osteoblastic Differentiation in Mesenchymal Stromal Cells. Sci. Rep. 2019, 9, 20071. [Google Scholar] [CrossRef]
- Lisse, T.S.; Hewison, M. Vitamin D: A new player in the world of mTOR signaling. Cell Cycle 2011, 10, 1888–1889. [Google Scholar] [CrossRef]
- Valle, Y.L.; Almalki, S.G.; Agrawal, D.K. Vitamin D machinery and metabolism in porcine adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 118. [Google Scholar] [CrossRef]
- Pesarini, J.R.; Oliveira, R.J.; Pessatto, L.R.; Antoniolli-Silva, A.; Felicidade, I.; Nardi, N.B.; Camassola, M.; Mantovani, M.S.; Ribeiro, L.R. Vitamin D: Correlation with biochemical and body composition changes in a southern Brazilian population and induction of cytotoxicity in mesenchymal stem cells derived from human adipose tissue. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 91, 861–871. [Google Scholar] [CrossRef]
- Song, I.; Kim, B.S.; Kim, C.S.; Im, G.I. Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem. Biophys. Res. Commun. 2011, 408, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Darmon, P.; Landrier, J.-F. Vitamin D Limits Chemokine Expression in Adipocytes and Macrophage Migration In Vitro and in Male Mice. Endocrinology 2015, 156, 1782–1793. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. CMLS 2009, 66, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Shyu, J.-F.; Wu, C.-C.; Hung, C.-F.; Liao, M.-T.; Liu, W.-C.; Zheng, C.-M.; Hou, Y.-C.; Lin, Y.-F.; Lu, K.-C. Association of Anabolic Effect of Calcitriol with Osteoclast-Derived Wnt 10b Secretion. Nutrients 2018, 10, 1164. [Google Scholar] [CrossRef] [PubMed]

| Performance of EPCs | Characteristics | Surface Marker |
|---|---|---|
| Vitamin D receptors on EPCs | Decrease in coronary artery disease (CAD) [124] | CD45dim, CD34+, and KDR+ |
| EPCs migration and differentiation | Accelerated [125] | CD34+, CD31+, CD45−, and CD133− |
| Endothelial colony-forming cells expressed mRNA of VEGF and pro–matrix metalloproteinase (pro-MMP) activity | Increased [125] | CD34+, CD31+, CD45−, and CD133− |
| Endothelial progenitor adhesion | Increased [126] | CD31+, CD45+, and CD133+ |
| Migration of the EPCs from the bone marrow | Increased [127] | 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated low density lipoprotein and fluorescein isothiocyanate -Ulex europaeus agglutinin-1 |
| Formation of VE-cadherin adhesion junctions on the EPCs | Increased [126] | CD31+, CD45+, and CD133+ |
| EPC injury by Ang II through modulating the PPAR-γ/HO-1 pathway | Decreased [128] | VEGF-2+ and CD13+ |
| EPC viability | Improved [129] | CD34+ and KDR+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.-C.; Lu, C.-L.; Zheng, C.-M.; Liu, W.-C.; Yen, T.-H.; Chen, R.-M.; Lin, Y.-F.; Chao, C.-T.; Lu, K.-C. The Role of Vitamin D in Modulating Mesenchymal Stem Cells and Endothelial Progenitor Cells for Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 2466. https://doi.org/10.3390/ijms21072466
Hou Y-C, Lu C-L, Zheng C-M, Liu W-C, Yen T-H, Chen R-M, Lin Y-F, Chao C-T, Lu K-C. The Role of Vitamin D in Modulating Mesenchymal Stem Cells and Endothelial Progenitor Cells for Vascular Calcification. International Journal of Molecular Sciences. 2020; 21(7):2466. https://doi.org/10.3390/ijms21072466
Chicago/Turabian StyleHou, Yi-Chou, Chien-Lin Lu, Cai-Mei Zheng, Wen-Chih Liu, Tzung-Hai Yen, Ruei-Ming Chen, Yuh-Feng Lin, Chia-Ter Chao, and Kuo-Cheng Lu. 2020. "The Role of Vitamin D in Modulating Mesenchymal Stem Cells and Endothelial Progenitor Cells for Vascular Calcification" International Journal of Molecular Sciences 21, no. 7: 2466. https://doi.org/10.3390/ijms21072466
APA StyleHou, Y.-C., Lu, C.-L., Zheng, C.-M., Liu, W.-C., Yen, T.-H., Chen, R.-M., Lin, Y.-F., Chao, C.-T., & Lu, K.-C. (2020). The Role of Vitamin D in Modulating Mesenchymal Stem Cells and Endothelial Progenitor Cells for Vascular Calcification. International Journal of Molecular Sciences, 21(7), 2466. https://doi.org/10.3390/ijms21072466





