Abstract
Nuclear magnetic resonance (NMR) spectroscopy plays important roles in structural biology and drug discovery, as it is a powerful tool to understand protein structures, dynamics, and ligand binding under physiological conditions. The protease of flaviviruses is an attractive target for developing antivirals because it is essential for the maturation of viral proteins. High-resolution structures of the proteases in the absence and presence of ligands/inhibitors were determined using X-ray crystallography, providing structural information for rational drug design. Structural studies suggest that proteases from Dengue virus (DENV), West Nile virus (WNV), and Zika virus (ZIKV) exist in open and closed conformations. Solution NMR studies showed that the closed conformation is predominant in solution and should be utilized in structure-based drug design. Here, we reviewed solution NMR studies of the proteases from these viruses. The accumulated studies demonstrated that NMR spectroscopy provides additional information to understand conformational changes of these proteases in the absence and presence of substrates/inhibitors. In addition, NMR spectroscopy can be used for identifying fragment hits that can be further developed into potent protease inhibitors.
1. Introduction
Flavivirus is a genus of viruses in the family Flaviviridae, and some members such as Dengue virus (DENV), West Nile virus (WNV), Zika virus (ZIKV), tick-borne encephalitis virus, and yellow fever virus are important human pathogens [1,2,3]. Some of these viruses can be transmitted by mosquitoes. Although viral infection usually causes mild symptoms such as fever and joint pain, it can also cause serious diseases such as dengue fever, microcephaly in newborns, and Guillain–Barré syndrome in adults [4,5,6,7]. Chemical therapy and vaccines are needed to prevent viral infection. Developing small-molecule drugs targeting viral proteins or preventing virus–host interactions is a strategy to develop antivirals [8,9,10,11].
The genome of Flaviviridae is a single-stranded RNA which has one open reading frame encoding a single polyprotein [1]. The polyprotein is cleaved into three structural and seven non-structural (NS) proteins by both host and viral proteases [12]. The structural proteins including the capsid protein (C), membrane protein (M), and envelope protein (E) are important for forming viral particles and receptor binding [13]. The seven non-structural proteins include NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 which are important for viral replication [1]. The structures and functions of these proteins are well conserved across the family. Among these non-structural proteins, NS3 and NS5 possess enzymatic activities, making them attractively studied [14,15].
The flavivirus protease is a two-component complex formed by a cytoplasmic region of NS2B and approximately 170 amino acids from the N-terminus of NS3 [16,17,18]. NS2B is a membrane protein with four transmembrane helices and acts as a cofactor for NS3 by regulating its activity and membrane location [18]. The transmembrane domains of NS2B are critical for the membrane location of both NS2B and NS3 [17]. These domains might be also important for the membrane location of replication complex formed by the viral proteins. The protease cofactor region of NS2B comprises approximately 40 amino acids and is critical for folding and protease activity of NS3 by forming a tight complex with the N-terminal region of NS3, with involvement in molecular interactions with substrates [19,20]. The N-terminus of NS3 (NS3pro) contains amino acids critical for the cleavage of substrates. The flavivirus protease is normally referred to as NS2B–NS3 protease (NS2B–NS3pro) due to the presence of two proteins. The viral NS2B–NS3 protease cleaves the joints of NS2A–NS2B, NS2B–NS3, NS3–NS4A, and NS4B–NS5 proteins, which is essential for maturation of these viral proteins [1,2]. Due to the important roles of NS2B–NS3 protease, it is an important antiviral target [21,22].
Structural studies of a target protein and its complex with a ligand/substrate provide useful information for rational drug design [23]. X-ray crystallography is a powerful tool to elucidate structures of proteins and their complexes at a high resolution. Solution NMR spectroscopy, on the other hand, will provide additional information to understand protein structures and dynamics under physiological conditions [24,25,26,27]. In the structural studies of proteases from several members of flaviviruses, these two methods were used together to provide valuable information to understand protease structure, dynamics, and ligand-binding properties [28,29]. The viral protease is an attractive target as it is essential for maturation of non-structural proteins. Therefore, structural information is very useful for designing potent inhibitors. In this review, the roles of solution NMR spectroscopy in understanding the dynamics and structures of proteases from DENV, WNV, and ZIKV are discussed. Accumulated studies showed that NMR, together with X-ray crystallography, is critical in the development of protease inhibitors.
2. X-ray Structures of Flavivirus Proteases
Numerous crystal structures of flavivirus proteases in the absence and presence of inhibitors were determined [30,31,32,33,34,35,36,37,38,39,40]. All these structures use recombinant proteins lacking the transmembrane domains of NS2B, as folding of full-length NS2B requires the presence of membrane systems such as detergent micelles [41,42,43,44]. For DENV and WNV proteases, a construct containing a cofactor region, approximately 40 residues from NS2B and NS3 protease domain (NS3pro) linked via a glycine-rich linker, was used in the structural studies [45]. In this manuscript, NS2B refers to the cofactor region, while full-length NS2B means the entire NS2B protein containing all the transmembrane domains.
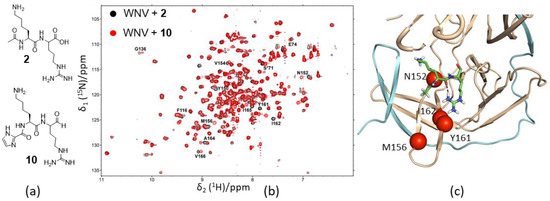
2.1. Structures of Proteases in Complex with Substrates/Inhibitors
In structures of DENV, WNV, and ZIKV NS2B–NS3 proteases, the folds of NS3 in various X-ray structures are almost identical (Figure 1). The N-terminal domain of NS3 is a serine protease containing two β-barrels, and each barrel consists of six β-strands. The catalytic triad formed by His, Asp, and Ser residues is absolutely conserved among these proteases (Figure 1) [30]. The active site is negatively charged, favoring molecular interactions with positively charged residues such as Lys and Arg (Figure 1) [46]. As shown in one of the crystal structures of WNV NS2B–NS3pro in complex with an inhibitor, the S1 pocket of the protease comprises Asp 129, Tyr130, Thr132, Tyr 150, and Gly151. Asp129 stabilizes the positively charged side chain of P1 residue, serving a similar function to Asp189 in trypsin [47]. The S2 pocket is negatively charged by the contribution of electrostatic potential from the backbone carbonyl oxygen atoms of amino acids of NS2B. Asp152 of NS3 and Gly83 of NS2B might form hydrogen bonds with the positively charged group of P2 residue (Figure 1a). The cofactor region of NS2B adopts two different conformations in some crystal structures (see below). In the absence of NS3pro, the free cofactor region of NS2B is unstructured in solution. The N-terminal part of the cofactor region forms a stable complex with NS3 and exists as a β-strand (Figure 1a). This region itself is able to stabilize NS3 protein. In the presence of the C-terminal part of the cofactor, the protease activity was enhanced [47].

Figure 1.
Structures of West Nile virus (WNV) protease in complex with an inhibitor. (a) The crystal structure of WNV NS2B-NS3pro in complex with an inhibitor. The non-structural protein 2B (NS2B) cofactor region and NS3pro are shown in cyan and orange, respectively. The inhibitor is shown in sticks, and residues from NS2B having close contacts with the inhibitor are labeled. Residues from NS2B are labeled with an asterisk. (b) The catalytic triad of WNV protease. The residues forming the catalytic triad are labeled and shown in sticks. (c) Surface representation of WNV protease. Surface charge analysis of the structure is shown. The structure was obtained from the Protein Data Bank (PDB) with access code 2FP7.
2.2. Open and Closed Conformations of the Protease
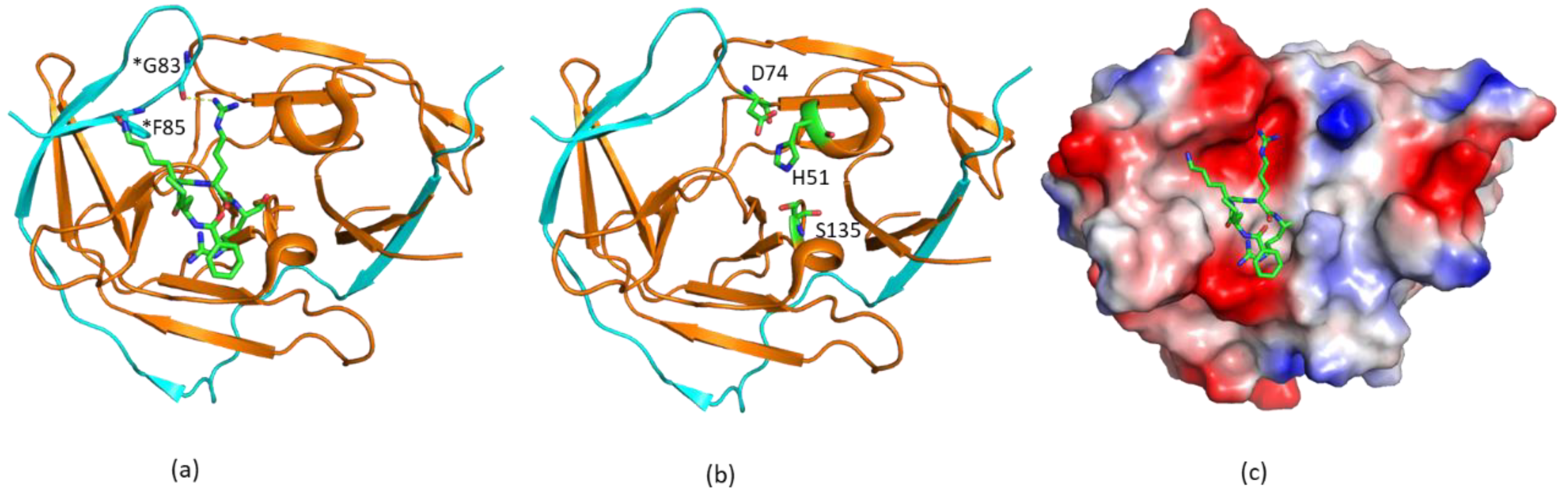
The structures of free WNV and DENV NS2B–NS3pro were first determined, and they suggested that the free protease exists in an open conformation which lacks protease activity [37,38,47]. Although the folding of NS3pro is almost the same as those in complexes with inhibitors, the C-terminal region of the NS2B cofactor positions away from the active site (Figure 2). This conformation is, therefore, indicated as the open conformation. This open conformation is inactive as the protease activity requires the presence of amino acids from the C-terminal section of NS2B cofactor region. These residues form a β-hairpin structure through molecular interactions with the substrate [48,49]. Therefore, several strategies were applied to develop protease inhibitors based on the available structures, namely, inhibitors derived from substrate sequences, small-molecule compounds binding to the active site, allosteric inhibitors to induce the open conformation, and inhibitors to stabilize the open conformation [50]. A structural study indicated that free ZIKV NS2B–NS3 protease exists in the closed conformation [33], and accumulated NMR studies demonstrated that the closed conformation of the protease should be used in structure-based drug design [51]. The open conformation observed in the X-ray studies might be due to crystal packing, while exchanges are indeed present at the binding interface of NS3pro and the C-terminal region of NS2B cofactor.
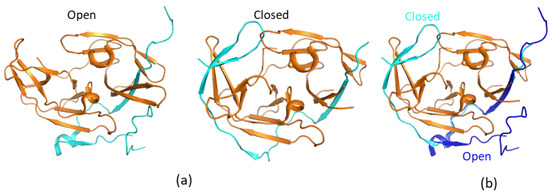
Figure 2.
Open and closed conformations of the proteases. (a) Open and closed conformations of WNV protease. The non-structural protein 2B (NS2B) and NS3pro are shown in cyan and orange, respectively. (b) Structural comparison of open and closed proteases. The structures of open (PDB identifier (ID) 2FOM) and closed (PDB ID 2FP7) proteases are shown. The NS2B C-terminal portion from the cofactor is away from the active site to form an open conformation. NS2B in the open and closed conformations is shown in blue and cyan, respectively. To make it clear, only NS3 from the closed conformation is shown.
3. Solution NMR Studies on Flavivirus Proteases
Although X-ray structures of flavivirus proteases provide detailed information to understand the interactions between protease and inhibitors, solution NMR spectroscopy provides complimentary information to better understand the structural information obtained using X-ray crystallography. Solution NMR spectroscopy was used to probe protease and inhibitor interactions. In addition, NMR studies suggested that the closed conformation should be used in structure-based drug design.
3.1. Ligand-Bound Proteases Form Closed Conformation
Solution NMR studies on WNV NS2B–NS3pro protease to understand its structures were first carried out by Otting’s group. In one study, a modified protocol was developed to produce an isotopically labeled WNV protease for NMR studies [52]. The solution NMR spectra of WNV NS2B–NS3pro in complex with a small-molecule inhibitor were collected, and resonance assignment was obtained to analyze the solution structures of WNV protease. The study demonstrated that WNV protease adopted the closed conformation when it was bound to a small-molecule compound. This compound was proven to bind to the active site of the protease as nuclear Overhauser effects (NOEs) between protease and the compound were identified [52]. In a follow-up study, the 1H–15N-heteronuclear single quantum coherence (HSQC) spectra of WNV protease in the absence and presence of an inhibitor were collected and compared. Significant changes in the numbers of the detectable cross-peaks in the 1H–15N-HSQC spectra were observed when the protease formed a complex with an inhibitor [53].
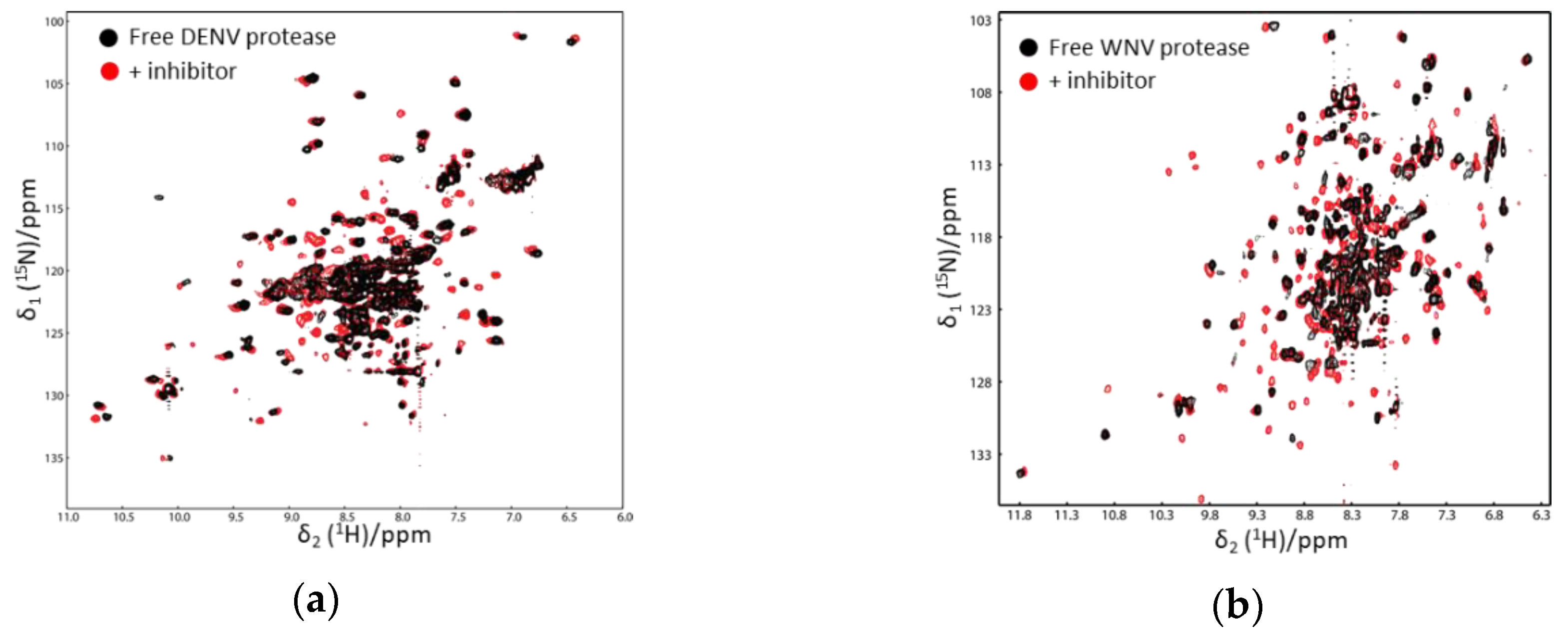
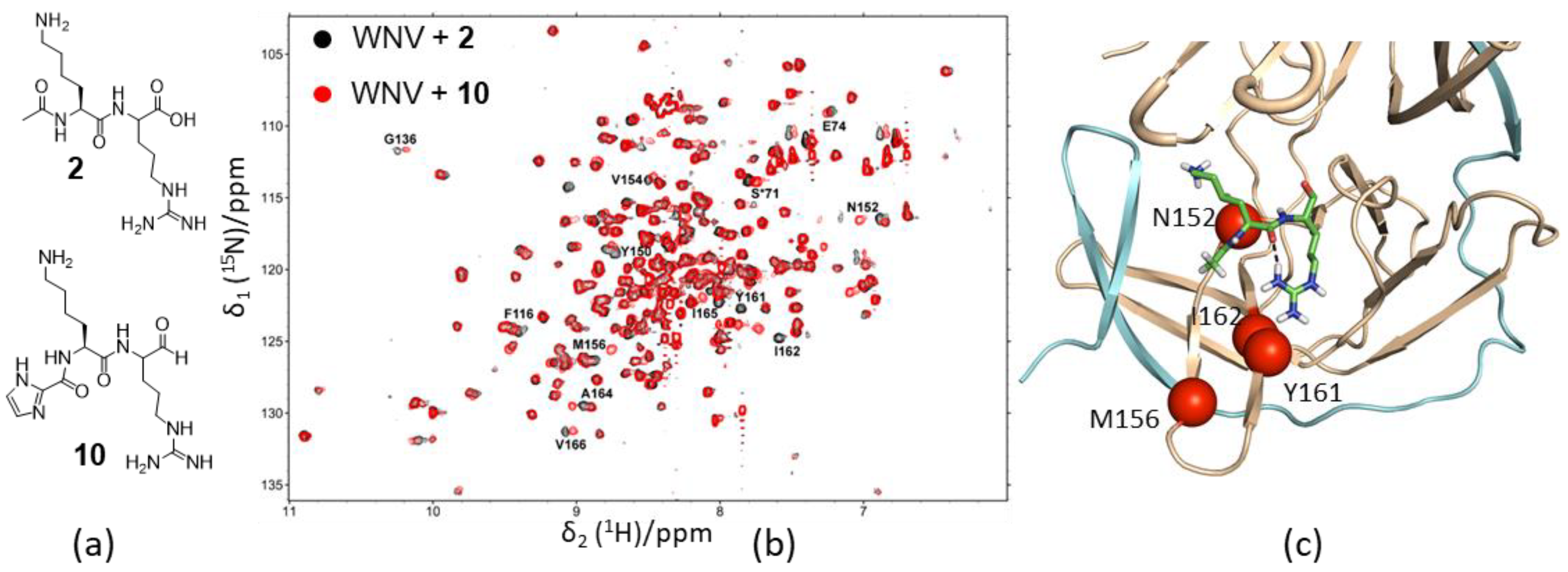
In the 1H–15N-HSQC spectrum of free WNV protease, the cross-peaks corresponding to several residues at the active sites and from the C-terminus of the NS2B cofactor were not observed, which might be caused by the conformational exchanges of the C-terminal part of the cofactor region [53]. On the other hand, signals of residues from the N-terminal portion of the cofactor were observed in the 1H–15N-HSQC spectra of both free and ligand-bound proteases [53,54]. Such results demonstrate that the N-terminal portion of the factor forms a stable complex with NS3. The cross-peaks corresponding to the residues from the C-terminal part of the cofactor region and close to the active site of NS3pro were observed when the protease formed a complex with inhibitors. Similar results were found for DENV and ZIKV proteases [55,56,57]. The protease in complex with inhibitors/substrates exhibited well-dispersed cross-peaks in the 1H–15N-HSQC spectra [35,51,54,58,59,60] (Figure 3). Further chemical shift analysis demonstrated that the C-terminal region of NS2B cofactor region formed ordered structures in solution, and the protease was in the closed conformation [35,51,55,56,57]. Pseudocontact shift (PCS) was used to analyze a DENV protease construct in which a protease cleavage site was introduced. The result indicated that the protease adopted the closed conformation in solution [61]. A later study also showed that adding a protease cleavage site in the ZIKV protease construct resulted in a protease forming the closed conformation, in which the residues at the cleavage site had interactions with both NS3 and the C-terminus of NS2B cofactor [35].
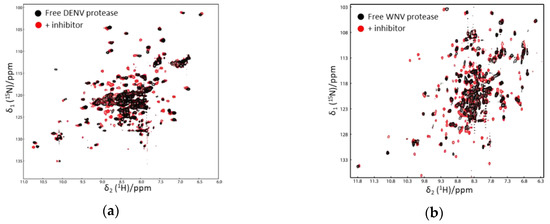
Figure 3.
1H–15N-heteronuclear single quantum coherence (HSQC) spectra of Dengue virus (DENV) (a) and WNV protease (b) in the absence and presence of an inhibitor. The spectra of free and ligand-bound proteases are shown in black and red, respectively. More cross-peaks were observed when the protease formed a complex with an inhibitor. The spectra were obtained from Reference [58] with slight modifications. Similar results were also obtained in other studies [52,53].
3.2. Free NS2B–NS3pro Forms Closed Conformation in Solution
To further probe the conformations of free protease in solution, a protease construct was labeled with paramagnetic tags. A paramagnetic relaxation enhancement (PRE) experiment and relaxation analysis demonstrated that free WNV protease was predominant in the closed conformation [61]. The C-terminal region of NS2B cofactor might exist in multiple conformations or dissociate from NS3, which contributes to signal broadening in the 1H–15N-HSQC spectrum of free protease. In the absence and presence of an inhibitor, the artificial linker used in the study is dynamic in solution. This linker was used for improving protease yield and purity without affecting enzymatic activities [19]. The DENV protease containing the same glycine-rich linker exhibited poorly dispersed cross-peaks in the 1H–15N-HSQC, making backbone resonance assignment challenging [62]. By tagging DENV protease with paramagnetic lanthanides, the protease was confirmed to contain the open conformation in its free form. The closed conformation can be readily induced by adding positively changed small-molecule inhibitors [62]. The conformational exchanges of DENV protease were clearly investigated using PRE experiments with different lanthanides. Using an 19F-NMR study, the free DENV protease with a glycine-rich linker was shown to contain both open and closed conformations in solution. Addition of a trypsin inhibitor (bovine pancreatic trypsin inhibitor (BPTI)) enabled reducing the population of the open conformation [63]. The conformation of DENV protease in solution was explored by NMR using an NS2B–NS3pro complex without any artificial linker. The protease complex in solution exhibited nicely dispersed cross-peaks in the 1H–15N-HSQC spectrum. Backbone assignment and a PRE experiment demonstrated that the free DENV protease existed in the closed conformation [20]. The artificial linker might affect the dynamics of NS2B to generate a spectrum with broadened signals. Indeed, comparison of the 1H–15N-HSQC spectra of ZIKV protease in the absence and presence of the glycine-rich linker revealed obvious chemical shift changes on a few residues, demonstrating that the linker is able to affect the chemical environment of some residues [57].
3.3. Free NS2B Cofactor Region and NS3pro Are Unstructured
Free DENV NS3pro was not soluble and present in the inclusion bodies when it was expressed in Escherichia coli. It was able to be purified in the denatured form in the presence of 8 M urea. Purified NS3pro was able to be refolded into a buffer while the far-ultraviolet (UV) circular dichroism (CD) spectrum suggested that NS3pro alone contained approximately 29% extended strand and 4% helix. The narrow dispersion of the cross-peaks in the 1H–15N-HSQC spectrum suggests the lack of tertiary packing [64]. Backbone resonance assignment for the refolded NS3pro was achieved using heteronuclear NMR experiments, and chemical shift analysis of the Cα and Cβ atoms demonstrated that free NS3pro was not structured in solution [64]. In the presence of the cofactor region, NS3pro formed a well-folded structure which is similar to that derived from X-ray crystallography [20]. The structure of the free full-length NS2B in detergent micelles was investigated using solution NMR study [18]. It was observed that the transmembrane regions of full-length NS2B formed helical structures in solution, while the cofactor region was unstructured in the absence of NS3pro [18]. This cofactor region alone could be over-expressed and purified in E. coli. It was also soluble in water, and CD analysis suggested that it may contain some helical structures [64]. For the cofactor region from ZIKV NS2B, the free form was unstructured in solution [33]. Although the NMR studies on free cofactor region, full-length NS2B, and NS3pro did not provide atomic structures, these studies provided direct evidence to show the importance of the complex in regulating the function of viral protease.
3.4. NMR in Guiding Protease Construct Design
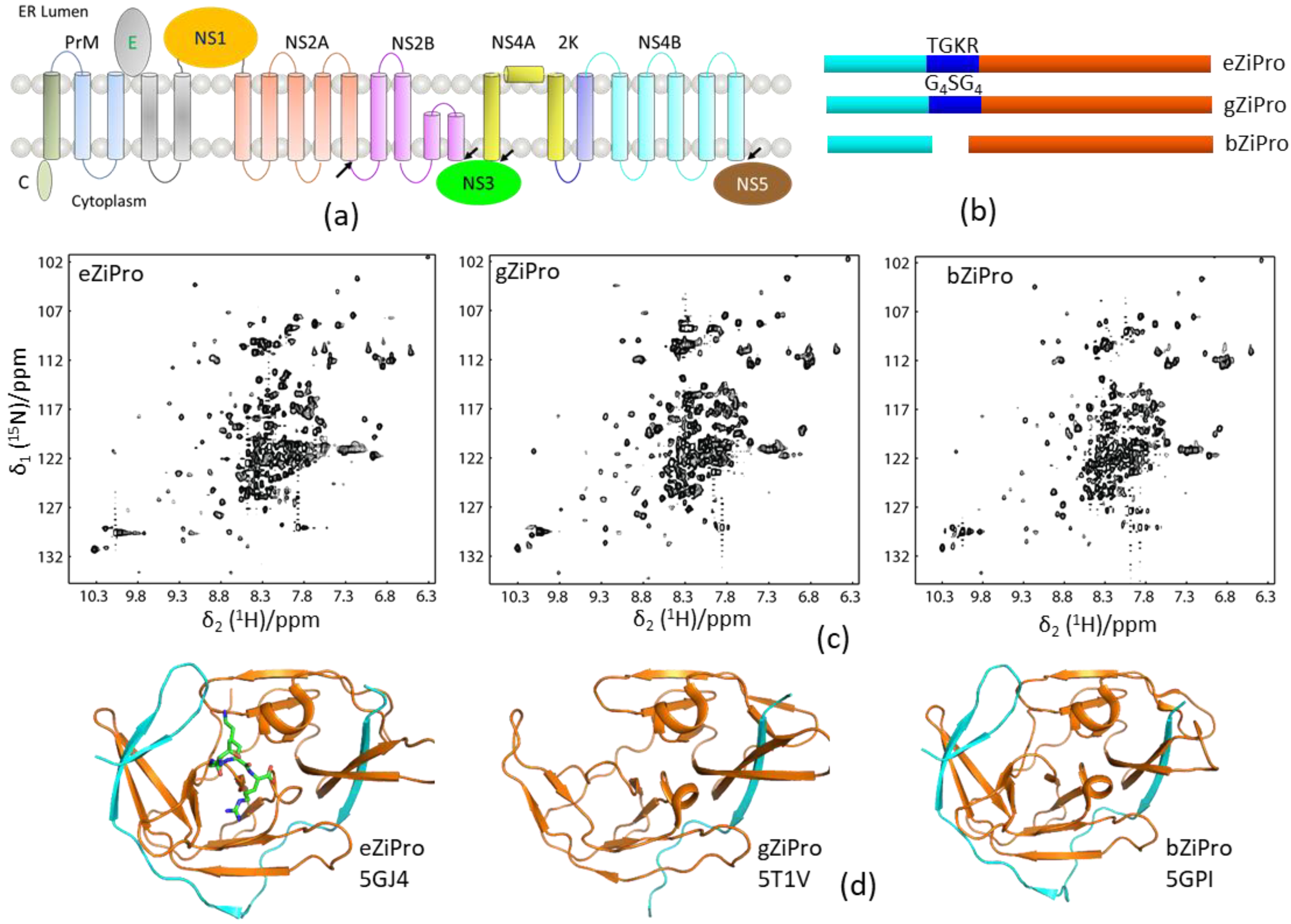
One-dimensional (1D) proton NMR can be used to monitor the folding of a purified protein. Constructs with high-quality X-ray structures usually generate high-quality NMR spectra [65]. NMR spectroscopy is, therefore, useful for evaluating the folding of the protease constructs which can be used for X-ray studies. As aforementioned, an unlinked DENV protease construct exhibited a better resolved 1H–15N-HSQC spectrum than the conventional DENV protease that contains a glycine-rich linker (G4SG4). In the structural studies of ZIKV protease, three constructs bZiPro, eZiPro, and gZiPro were designed (Figure 4). These constructs contained NS2B cofactor region and NS3pro without a linker, an enzyme cleavage site (TGKR), and a glycine-rich linker (G4SG4). All these constructs were able to be crystallized, and the studies showed that the C-terminal region of NS2B cofactor was structured in bZiPro and eZiPro and unstructured in gZiPro (Figure 4). NMR studies indicated that exchanges exist in bZiPro and gZiPro, while addition of substrate or inhibitors can suppress the exchanges to give rise to spectra in which the signals from residues at the NS2B–NS3 interface are detectable (Figure 4). The bZiPro construct was utilized in providing protein–ligand interactions because several crystal structures of protease complexes were solved [33,55,56,66]. Although all different constructs exhibited similar enzymatic activities, the unlinked protease complex without any artificial linker should be used in drug discovery.
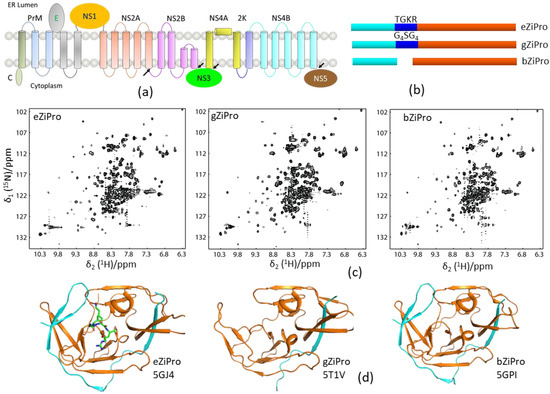
Figure 4.
Solution NMR spectra of different protease constructs. (a) Diagram of the viral polypeptide. The arrows indicate the viral protease cleavage sites. (b) Current constructs used for ZIKV protease. The NS2B cofactor, linker, and NS3pro are shown in cyan, blue, and orange, respectively. (c) 1H–15N-HSQC spectra of the three protease constructs. (d) X-ray structures of these three constructs. The PDB access codes of structures are shown. The NS2B and NS3 are shown in cyan and orange, respectively. The enzyme cleavage site (TGKR) peptide in eZiPro is shown in sticks.
3.5. Other Applications
As NS2B is a membrane protein, NS3 might also have molecular interactions with cell membranes. A hydrophobic loop from DENV NS3 conserved among flaviviruses was suggested to bind to the cell membrane [67]. NMR was then used to investigate molecular interactions between WNV protease and membranes. Using a titration experiment, several residues were identified to bind to detergent micelles which were used as a mimic of the cell membrane [68]. Such a study provided direct evidence to demonstrate the specific interactions between NS3 and the cell membrane.
In addition, flaviviral proteases were used as a model system for developing novel NMR methods for structure determination and ligand binding studies [69,70,71]. Distance restraints for structural determination are usually obtained from NEOSY spectra, which is challenging for some proteins due to signal overlaps or conformational exchanges. PCS data from DENV protease were generated by tagging lanthanide to the target protein [72]. The resulting data can be used as distance restraints or used as an input for generating computational models. The structures of NS2B can be obtained using a combination of PCS and computational model using Rosetta [70]. Such a strategy is an efficient and reliable way for determining structures of proteins [73].
Using ZIKV protease as one of the model proteins, 1H-NMR was shown to be able to probe protein–ligand interactions. Although 1H-NMR is very sensitive, the overlaps between protein and ligand signals hinders its application in probing protein and ligand interactions. In a recent study, a trimethylsilyl (TMS) tag was developed to solve this problem, as TMS exhibits a chemical shift near 0 ppm in 1H-NMR spectra. A cysteine residue is required in a protein for the tagging reaction. As ZIKV protease harbors enzymatic activity, the impact of tag modification on the activity of the target protein can be analyzed. It was shown that the TMS tags were able to react with a cysteine residue introduced into ZIKV protease without affecting enzymatic activity. The resulting protease was able to be utilized for probing protein–ligand interactions. In addition, 1H-NMR can also be used to estimate the dissociation constant for the interactions in the slow exchange regime [71].
3.6. Summary
NMR studies provide information to understand structures, conformational exchanges, and dynamics of these proteases. Introducing a linker in the protease is a great strategy in recombinant protein production, while chemical environment of some residues could be affected even though the linker is unstructured. Together with X-ray crystallography and computational methods, NMR provides a meaningful view of protein structures.
5. Conclusions
Numerous NMR studies on proteases from DENV, WNV, and ZIKV were carried out, which provide insights into conformational exchanges and dynamics. NMR spectroscopy was able to test the folding of different protease constructs which could be used in evaluating different types of inhibitors. This technique can play important roles in hit identification and confirmation steps, especially in FBDD. Together with X-ray crystallography, the mechanism of action for an inhibitor can be well understood. It should also be noted that NMR plays important roles in structural studies of viral proteins, as several viral proteins are challenging to be crystallized.
Author Contributions
Conceptualization, Q.L. and C.K.; All authors read and agreed to the published version of the manuscript.
Funding
This research was supported by funding from the “Hundred-Talent Program” (Grant Numbers: 2020GDASYL-20200102010 and 2020GDASYL-20200102009), Guangdong Academy of Sciences, China. This research was also supported by Singapore Ministry of Health’s National Medical Research Council under its open fund individual research grant (OFIRG17may050, NMRC/OFIRG/0051/2017).
Acknowledgments
Q.L. appreciates the support from Guangdong Bioengineering Institute (Guangzhou Sugarcane Industry Research Institute), Guangdong Academy of Sciences, Guangdong, China. C.K. thanks the support from BMRC, A*STAR, Singapore.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| NMR | Nuclear magnetic resonance |
| HSQC | Heteronuclear single quantum coherence |
| DENV | Dengue virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
| BPTI | Bovine pancreatic trypsin inhibitor |
| PRE | Paramagnetic relaxation enhancement |
| NOE | Nuclear Overhauser effect |
| PCS | Pseudocontact shift |
| UV | Ultraviolet |
| CD | Circular dichroism |
| 1D | One-dimensional |
| STD | Saturation transfer difference |
| HTS | High-throughput screening |
| MTSL | 1-Oxyl-2,2,5,5-tetramethyl-Δ3-pyrroline-3-methylmethanethiosulfonate |
| FBDD | Fragment-based drug design |
References
- Pierson, T.C.; Keilian, M. Flaviviruses: Braking the entering. Curr. Opin. Virol. 2013, 3, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Thiel, H.J.; Rice, C.M. Flaviviridae: The Viruses and Their Replication. In Fields Virology; Lippincott-Raven Publishers: Philadelphia, PA, USA, 2013; pp. 712–746. [Google Scholar]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Broutet, N.; Krauer, F.; Riesen, M.; Khalakdina, A.; Almiron, M.; Aldighieri, S.; Espinal, M.; Low, N.; Dye, C. Zika Virus as a Cause of Neurologic Disorders. N. Engl. J. Med. 2016, 374, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugieres, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Mecharles, S.; Herrmann, C.; Poullain, P.; Tran, T.H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef]
- Lim, S.P.; Wang, Q.Y.; Noble, C.G.; Chen, Y.L.; Dong, H.; Zou, B.; Yokokawa, F.; Nilar, S.; Smith, P.; Beer, D.; et al. Ten years of dengue drug discovery: progress and prospects. Antiviral Res. 2013, 100, 500–519. [Google Scholar] [CrossRef]
- Lim, S.P.; Shi, P.Y. West Nile virus drug discovery. Viruses 2013, 5, 2977–3006. [Google Scholar] [CrossRef]
- Xie, X.; Zou, J.; Wang, Q.Y.; Shi, P.Y. Targeting dengue virus NS4B protein for drug discovery. Antiviral Res. 2015, 118, 39–45. [Google Scholar] [CrossRef]
- Lim, S.P.; Noble, C.G.; Shi, P.Y. The dengue virus NS5 protein as a target for drug discovery. Antiviral Res. 2015, 119, 57–67. [Google Scholar] [CrossRef]
- Shiryaev, S.A.; Ratnikov, B.I.; Aleshin, A.E.; Kozlov, I.A.; Nelson, N.A.; Lebl, M.; Smith, J.W.; Liddington, R.C.; Strongin, A.Y. Switching the substrate specificity of the two-component NS2B-NS3 flavivirus proteinase by structure-based mutagenesis. J. Virol. 2007, 81, 4501–4509. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Noble, C.G.; Shi, P.Y. Structural biology of dengue virus enzymes: Towards rational design of therapeutics. Antiviral Res. 2012, 96, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P. Dengue drug discovery: Progress, challenges and outlook. Antiviral Res 2019, 163, 156–178. [Google Scholar] [CrossRef]
- Baronti, C.; Piorkowski, G.; Charrel, R.N.; Boubis, L.; Leparc-Goffart, I.; de Lamballerie, X. Complete Coding Sequence of Zika Virus from a French Polynesia Outbreak in 2013. Genome Announc. 2014, 2, e00500-14. [Google Scholar] [CrossRef]
- Clum, S.; Ebner, K.E.; Padmanabhan, R. Cotranslational membrane insertion of the serine proteinase precursor NS2B-NS3(Pro) of dengue virus type 2 is required for efficient in vitro processing and is mediated through the hydrophobic regions of NS2B. J. Biol. Chem. 1997, 272, 30715–30723. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wong, Y.L.; Liew, L.S.; Kang, C. Membrane topology of NS2B of dengue virus revealed by NMR spectroscopy. Biochim. Biophys. Acta 2015, 1848, 2244–2252. [Google Scholar] [CrossRef]
- Leung, D.; Schroder, K.; White, H.; Fang, N.X.; Stoermer, M.J.; Abbenante, G.; Martin, J.L.; Young, P.R.; Fairlie, D.P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001, 276, 45762–45771. [Google Scholar] [CrossRef]
- Kim, Y.M.; Gayen, S.; Kang, C.; Joy, J.; Huang, Q.; Chen, A.S.; Wee, J.L.; Ang, M.J.; Lim, H.A.; Hung, A.W.; et al. NMR Analysis of a Novel Enzymatically Active Unlinked Dengue NS2B-NS3 Protease Complex. J. Biol. Chem. 2013, 288, 12891–12900. [Google Scholar] [CrossRef]
- Kang, C.; Keller, T.H.; Luo, D. Zika Virus Protease: An Antiviral Drug Target. Trends Microbiol. 2017, 25, 797–808. [Google Scholar] [CrossRef]
- Nitsche, C. Proteases from dengue, West Nile and Zika viruses as drug targets. Biophys. Rev. 2019, 11, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Ahmad, B.; Choi, S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019, 20, 2783. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Baskaran, K.; Arthanari, H. Structure determination using solution NMR: Is it worth the effort? J. Magn. Reson. 2019, 306, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.B. 19F-NMR in target-based drug discovery. Curr. Med. Chem. 2019, 26, 4964–4983. [Google Scholar] [CrossRef]
- Kang, C. Applications of In-Cell NMR in Structural Biology and Drug Discovery. Int. J. Mol. Sci. 2019, 20, 139. [Google Scholar] [CrossRef]
- Sugiki, T.; Furuita, K.; Fujiwara, T.; Kojima, C. Current NMR Techniques for Structure-Based Drug Discovery. Molecules 2018, 23, 148. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, G.F. Structural Biology of the Zika Virus. Trends Biochem. Sci. 2017, 42, 443–456. [Google Scholar] [CrossRef]
- Brecher, M.; Zhang, J.; Li, H. The flavivirus protease as a target for drug discovery. Virol. Sin. 2013, 28, 326–336. [Google Scholar] [CrossRef]
- Phoo, W.W.; Zhang, Z.; Wirawan, M.; Chew, E.J.C.; Chew, A.B.L.; Kouretova, J.; Steinmetzer, T.; Luo, D. Structures of Zika virus NS2B-NS3 protease in complex with peptidomimetic inhibitors. Antivir. Res. 2018, 160, 17–24. [Google Scholar] [CrossRef]
- Shiryaev, S.A.; Farhy, C.; Pinto, A.; Huang, C.-T.; Simonetti, N.; Ngono, A.E.; Dewing, A.; Shresta, S.; Pinkerton, A.B.; Cieplak, P.; et al. Characterization of the Zika virus two-component NS2B-NS3 protease and structure-assisted identification of allosteric small-molecule antagonists. Antivir. Res. 2017, 143, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Loh, Y.R.; Phoo, W.W.; Hung, A.W.; Kang, C.; Luo, D. Crystal structure of unlinked NS2B-NS3 protease from Zika virus. Science 2016, 354, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ji, X.; Yang, X.; Xie, W.; Yang, K.; Chen, C.; Wu, C.; Chi, H.; Mu, Z.; Wang, Z.; et al. The crystal structure of Zika virus helicase: basis for antiviral drug design. Protein Cell 2016, 7, 450–454. [Google Scholar] [CrossRef]
- Phoo, W.W.; Li, Y.; Zhang, Z.; Lee, M.Y.; Loh, Y.R.; Tan, Y.B.; Ng, E.Y.; Lescar, J.; Kang, C.; Luo, D. Structure of the NS2B-NS3 protease from Zika virus after self-cleavage. Nat. Commun. 2016, 7, 13410. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Hansen, G.; Nitsche, C.; Klein, C.D.; Zhang, L.; Hilgenfeld, R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science 2016, 353, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, A.E.; Shiryaev, S.A.; Strongin, A.Y.; Liddington, R.C. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 2007, 16, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, S.; Joseph, J.S.; Daudenarde, S.; Gatchalian, J.; Cornillez-Ty, C.; Kuhn, P. Serotype-specific structural differences in the protease-cofactor complexes of the dengue virus family. J. Virol. 2010, 84, 3059–3067. [Google Scholar] [CrossRef]
- Robin, G.; Chappell, K.; Stoermer, M.J.; Hu, S.H.; Young, P.R.; Fairlie, D.P.; Martin, J.L. Structure of West Nile virus NS3 protease: ligand stabilization of the catalytic conformation. J. Mol. Biol. 2009, 385, 1568–1577. [Google Scholar] [CrossRef]
- Hammamy, M.Z.; Haase, C.; Hammami, M.; Hilgenfeld, R.; Steinmetzer, T. Development and characterization of new peptidomimetic inhibitors of the West Nile virus NS2B-NS3 protease. Chem. Med. Chem. 2013, 8, 231–241. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, A.S.; Li, Q.; Kang, C. Expression, purification, and initial structural characterization of nonstructural protein 2B, an integral membrane protein of Dengue-2 virus, in detergent micelles. Protein Expr. Purif. 2011, 80, 169–175. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Joy, J.; Chen, A.S.; Ruiz-Carrillo, D.; Hill, J.; Lescar, J.; Kang, C. Lyso-myristoyl phosphatidylcholine micelles sustain the activity of Dengue non-structural (NS) protein 3 protease domain fused with the full-length NS2B. Protein Expr. Purif. 2013, 92, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.S.; Lee, M.Y.; Wong, Y.L.; Cheng, J.; Li, Q.; Kang, C. Selection of suitable detergents for obtaining an active dengue protease in its natural form from E. coli. Protein Expr. Purif. 2016, 121, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.; Loh, Y.R.; Li, Y.; Li, Q.; Kang, C. Expression, purification of Zika virus membrane protein-NS2B in detergent micelles for NMR studies. Protein Expr. Purif. 2019, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.E.; Chappell, K.J.; Stoermer, M.J.; Chow, S.Y.; Kok, W.M.; Fairlie, D.P.; Young, P.R. Simultaneous uncoupled expression and purification of the Dengue virus NS3 protease and NS2B co-factor domain. Protein Expr. Purif. 2015, 119, 124–129. [Google Scholar] [CrossRef]
- Poulsen, A.; Kang, C.; Keller, T.H. Drug design for flavivirus proteases: what are we missing? Curr. Pharm. Des. 2014, 20, 3422–3427. [Google Scholar] [CrossRef]
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef]
- Radichev, I.; Shiryaev, S.A.; Aleshin, A.E.; Ratnikov, B.I.; Smith, J.W.; Liddington, R.C.; Strongin, A.Y. Structure-based mutagenesis identifies important novel determinants of the NS2B cofactor of the West Nile virus two-component NS2B-NS3 proteinase. J. Gen. Virol. 2008, 89, 636–641. [Google Scholar] [CrossRef]
- Chappell, K.J.; Stoermer, M.J.; Fairlie, D.P.; Young, P.R. Mutagenesis of the West Nile virus NS2B cofactor domain reveals two regions essential for protease activity. J. Gen. Virol. 2008, 89, 1010–1014. [Google Scholar] [CrossRef]
- Yildiz, M.; Ghosh, S.; Bell, J.A.; Sherman, W.; Hardy, J.A. Allosteric Inhibition of the NS2B-NS3 Protease from Dengue Virus. ACS Chem. Biol. 2013, 17, 76–80. [Google Scholar] [CrossRef]
- Chen, W.N.; Loscha, K.V.; Nitsche, C.; Graham, B.; Otting, G. The dengue virus NS2B-NS3 protease retains the closed conformation in the complex with BPTI. FEBS Lett. 2014, 588, 2206–2211. [Google Scholar] [CrossRef]
- Su, X.C.; Ozawa, K.; Yagi, H.; Lim, S.P.; Wen, D.; Ekonomiuk, D.; Huang, D.; Keller, T.H.; Sonntag, S.; Caflisch, A.; et al. NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease. FEBS J. 2009, 276, 4244–4255. [Google Scholar] [CrossRef] [PubMed]
- Su, X.C.; Ozawa, K.; Qi, R.; Vasudevan, S.G.; Lim, S.P.; Otting, G. NMR analysis of the dynamic exchange of the NS2B cofactor between open and closed conformations of the West Nile virus NS2B-NS3 protease. PLoS Negl. Trop. Dis. 2009, 3, e561. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Gayen, S.; Wang, W.; Severin, R.; Chen, A.S.; Lim, H.A.; Chia, C.S.; Schuller, A.; Doan, D.N.; Poulsen, A.; et al. Exploring the binding of peptidic West Nile virus NS2B-NS3 protease inhibitors by NMR. Antivir. Res. 2013, 97, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Li, R.; Yang, H.Y.; Jansson, A.E.; Hill, J.; Keller, T.H.; Nacro, K.; et al. Structural Insights into the Inhibition of Zika Virus NS2B-NS3 Protease by a Small-Molecule Inhibitor. Structure 2018, 26, 555–564.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Phoo, W.W.; Loh, Y.R.; Wang, W.; Liu, S.; Chen, M.W.; Hung, A.W.; Keller, T.H.; Luo, D.; et al. Structural Dynamics of Zika Virus NS2B-NS3 Protease Binding to Dipeptide Inhibitors. Structure 2017, 25, 1242–1250.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Phoo, W.W.; Loh, Y.R.; Zhang, Z.; Ng, E.Y.; Wang, W.; Keller, T.H.; Luo, D.; Kang, C. Structural characterization of the linked NS2B-NS3 protease of Zika virus. FEBS Lett. 2017, 591, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Koh-Stenta, X.; Joy, J.; Wang, S.F.; Kwek, P.Z.; Wee, J.L.; Wan, K.F.; Gayen, S.; Chen, A.S.; Kang, C.; Lee, M.A.; et al. Identification of covalent active site inhibitors of dengue virus protease. Drug Des. Devel. Ther. 2015, 9, 6389–6399. [Google Scholar] [CrossRef]
- Bi, Y.; Zhu, L.; Li, H.; Wu, B.; Liu, J.; Wang, J. Backbone (1)H, (13)C and (15)N resonance assignments of dengue virus NS2B-NS3p in complex with aprotinin. Biomol. NMR Assign. 2012, 7, 137–139. [Google Scholar] [CrossRef]
- Bodenreider, C.; Beer, D.; Keller, T.H.; Sonntag, S.; Wen, D.; Yap, L.; Yau, Y.H.; Shochat, S.G.; Huang, D.; Zhou, T.; et al. A fluorescence quenching assay to discriminate between specific and nonspecific inhibitors of dengue virus protease. Anal. Biochem. 2009, 395, 195–204. [Google Scholar] [CrossRef]
- de la Cruz, L.; Chen, W.N.; Graham, B.; Otting, G. Binding mode of the activity-modulating C-terminal segment of NS2B to NS3 in the dengue virus NS2B-NS3 protease. FEBS J. 2014, 281, 1517–1533. [Google Scholar] [CrossRef]
- de la Cruz, L.; Nguyen, T.H.; Ozawa, K.; Shin, J.; Graham, B.; Huber, T.; Otting, G. Binding of low molecular weight inhibitors promotes large conformational changes in the dengue virus NS2B-NS3 protease: fold analysis by pseudocontact shifts. J. Am. Chem. Soc. 2011, 133, 19205–19215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, J.; Li, H.; Sun, H.; Liu, J.; Wang, J. Conformational change study of dengue virus NS2B-NS3 protease using 19F NMR spectroscopy. Biochem. Biophys. Res. Commun. 2015, 461, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Lim, L.; Song, J. NMR and MD Studies Reveal That the Isolated Dengue NS3 Protease Is an Intrinsically Disordered Chymotrypsin Fold Which Absolutely Requests NS2B for Correct Folding and Functional Dynamics. PLoS ONE 2015, 10, e0134823. [Google Scholar] [CrossRef]
- Page, R.; Peti, W.; Wilson, I.A.; Stevens, R.C.; Wuthrich, K. NMR screening and crystal quality of bacterially expressed prokaryotic and eukaryotic proteins in a structural genomics pipeline. Proc. Natl. Acad. Sci. USA 2005, 102, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.P.; Liu, S.; Zhang, Z.; Li, Y.; Ng, E.Y.; Loh, Y.R.; Hung, A.W.; Luo, D.; Kang, C. Identification and structural characterization of small molecule fragments targeting Zika virus NS2B-NS3 protease. Antivir. Res. 2020, 175, 104707. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wei, N.; Doan, D.N.; Paradkar, P.N.; Chong, Y.; Davidson, A.D.; Kotaka, M.; Lescar, J.; Vasudevan, S.G. Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J. Biol. Chem. 2010, 285, 18817–18827. [Google Scholar] [CrossRef] [PubMed]
- Gayen, S.; Chen, A.S.; Huang, Q.; Kang, C. West Nile Virus (WNV) protease and membrane interactions revealed by NMR spectroscopy. Biochem. Biophys. Res. Commun. 2012, 423, 799–804. [Google Scholar] [CrossRef]
- Nitsche, C.; Mahawaththa, M.C.; Becker, W.; Huber, T.; Otting, G. Site-selective tagging of proteins by pnictogen-mediated self-assembly. Chem. Commun. 2017, 53, 10894–10897. [Google Scholar] [CrossRef]
- Pilla, K.B.; Leman, J.K.; Otting, G.; Huber, T. Capturing conformational States in proteins using sparse paramagnetic NMR data. PLoS ONE 2015, 10, e0127053. [Google Scholar] [CrossRef]
- Becker, W.; Adams, L.A.; Graham, B.; Wagner, G.E.; Zangger, K.; Otting, G.; Nitsche, C. Trimethylsilyl tag for probing protein-ligand interactions by NMR. J. Biomol. NMR 2018, 70, 211–218. [Google Scholar] [CrossRef]
- Graham, B.; Loh, C.T.; Swarbrick, J.D.; Ung, P.; Shin, J.; Yagi, H.; Jia, X.; Chhabra, S.; Barlow, N.; Pintacuda, G.; et al. DOTA-amide lanthanide tag for reliable generation of pseudocontact shifts in protein NMR spectra. Bioconjugate Chem. 2011, 22, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Vernon, R.; Otting, G.; Baker, D.; Huber, T. Protein structure determination from pseudocontact shifts using ROSETTA. J. Mol. Biol. 2012, 416, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Ekonomiuk, D.; Su, X.C.; Ozawa, K.; Bodenreider, C.; Lim, S.P.; Yin, Z.; Keller, T.H.; Beer, D.; Patel, V.; Otting, G.; et al. Discovery of a non-peptidic inhibitor of west nile virus NS3 protease by high-throughput docking. PLoS Negl. Trop. Dis. 2009, 3, e356. [Google Scholar] [CrossRef] [PubMed]
- Ekonomiuk, D.; Su, X.C.; Ozawa, K.; Bodenreider, C.; Lim, S.P.; Otting, G.; Huang, D.; Caflisch, A. Flaviviral protease inhibitors identified by fragment-based library docking into a structure generated by molecular dynamics. J. Med. Chem. 2009, 52, 4860–4868. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Loh, Y.R.; Hung, A.W.; Kang, C. Characterization of molecular interactions between Zika virus protease and peptides derived from the C-terminus of NS2B. Biochem. Biophys. Res. Commun. 2018, 503, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.D.; Loh, C.T.; Shin, J.; Chhabra, S.; Dennis, M.L.; Otting, G.; Swarbrick, J.D.; Graham, B. Compact, hydrophilic, lanthanide-binding tags for paramagnetic NMR spectroscopy. Chem. Sci. 2015, 6, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Erlanson, D.A.; Fesik, S.W.; Hubbard, R.E.; Jahnke, W.; Jhoti, H. Twenty years on: the impact of fragments on drug discovery. Nat. Rev. Drug Discov. 2016, 15, 605–619. [Google Scholar] [CrossRef]
- Kessler, D.; Gmachl, M.; Mantoulidis, A.; Martin, L.J.; Zoephel, A.; Mayer, M.; Gollner, A.; Covini, D.; Fischer, S.; Gerstberger, T.; et al. Drugging an undruggable pocket on KRAS. Proc. Natl. Acad. Sci. 2019, 116, 15823–15829. [Google Scholar] [CrossRef]
- Stamford, A.; Strickland, C. Inhibitors of BACE for treating Alzheimer’s disease: a fragment-based drug discovery story. Curr. Opin. Chem. Biol. 2013, 17, 320–328. [Google Scholar] [CrossRef]
- Schöne, T.; Grimm, L.L.; Sakai, N.; Zhang, L.; Hilgenfeld, R.; Peters, T. STD-NMR experiments identify a structural motif with novel second-site activity against West Nile virus NS2B-NS3 protease. Antivir. Res. 2017, 146, 174–183. [Google Scholar] [CrossRef]
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-Bound Structures of the Dengue Virus Protease Reveal the Active Conformation. J. Virol. 2012, 86, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Passioura, T.; Varava, P.; Mahawaththa, M.C.; Leuthold, M.M.; Klein, C.D.; Suga, H.; Otting, G. De Novo Discovery of Nonstandard Macrocyclic Peptides as Noncompetitive Inhibitors of the Zika Virus NS2B-NS3 Protease. ACS Med. Chem. Lett. 2019, 10, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huo, T.; Lin, Y.L.; Nie, S.; Wu, F.; Hua, Y.; Wu, J.; Kneubehl, A.R.; Vogt, M.B.; Rico-Hesse, R.; et al. Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease. J. Am. Chem. Soc. 2019, 141, 6832–6836. [Google Scholar] [CrossRef] [PubMed]
- Mahawaththa, M.C.; Pearce, B.J.; Szabo, M.; Graham, B.; Klein, C.D.; Nitsche, C.; Otting, G. Solution conformations of a linked construct of the Zika virus NS2B-NS3 protease. Antivir. Res. 2017, 142, 141–147. [Google Scholar] [CrossRef]
- Norton, R.S.; Leung, E.W.W.; Chandrashekaran, I.R.; MacRaild, C.A. Applications of 19F-NMR in Fragment-Based Drug Discovery. Molecules 2016, 21, 860. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).