Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade
Abstract
:1. Introduction
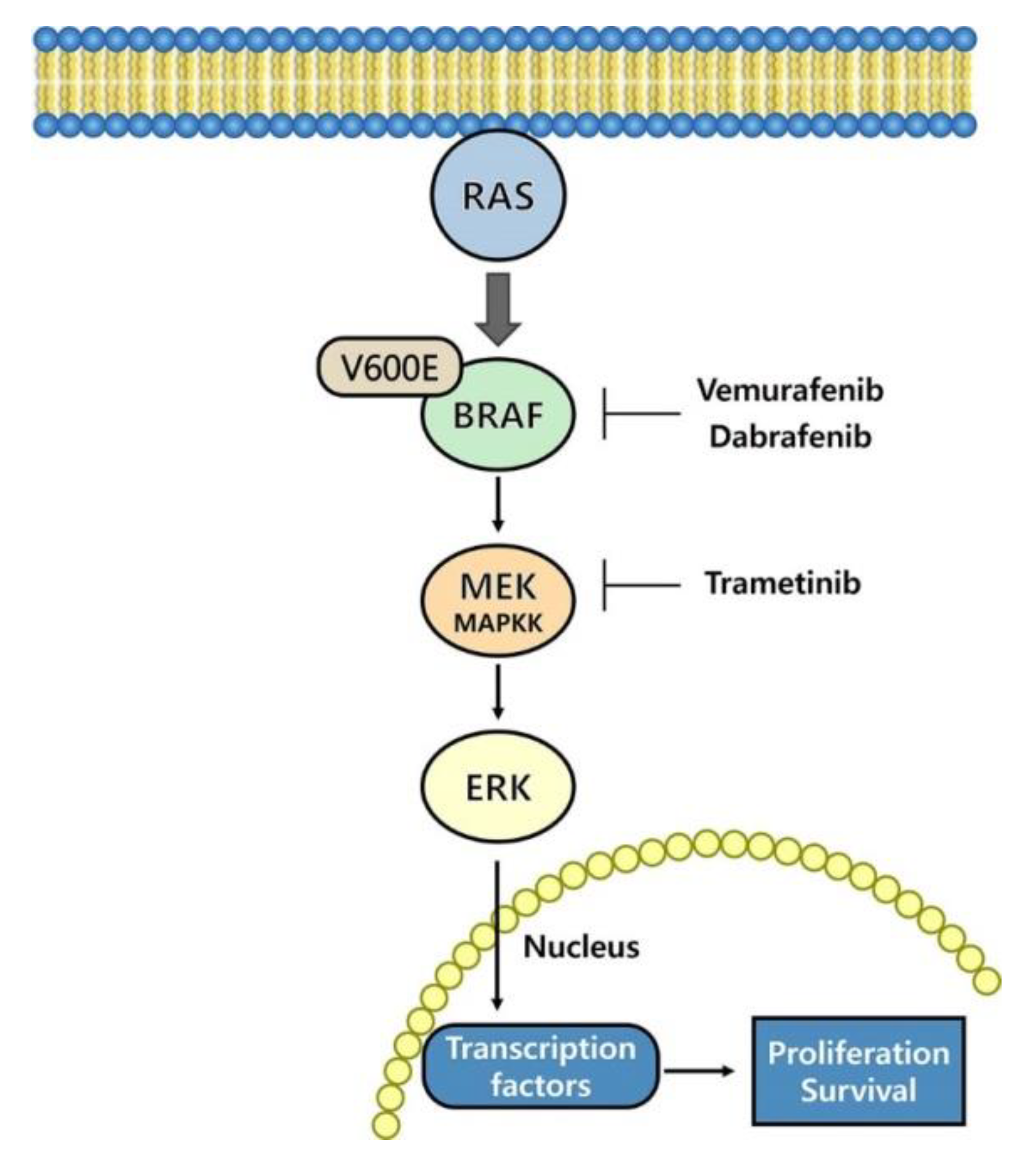
2. Therapies Targeting the MAPK Pathway
3. Immune Checkpoint Blockade
4. Combination of Immunotherapy and Targeted Therapy
4.1. Melanoma
4.2. Non-Small-Cell Lung Cancer (NSCLC)
4.3. Colorectal Cancer (CRC)
4.4. Pancreatic Cancer
4.5. Thyroid Cancer
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MAPK | mitogen-activated protein kinase |
| NSCLC | non-small-cell lung carcinoma |
| MEK | MAPK/ERK kinase |
| PD-1 | programmed death protein 1 |
| PD-L1 | programmed death-ligand 1 |
| PD-L2 | programmed death-ligand 2 |
| CTLA-4 | cytotoxic T cell associated antigen 4 |
| US FDA | United States Food and Drug Administration |
| Tim-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| IL-2 | interleukin 2 |
| PFS | progression-free survival |
| OS | overall survival |
| EGFR | epidermal growth factor receptor |
| ALK | anaplastic lymphoma kinase |
| PI3K | phosphoinositide 3-kinase |
| STAT | signal transducer and activator of transcription |
| CRC | colorectal cancer |
| mCRC | metastatic colorectal cancer |
| MSS | microsatellite stable |
| mAbs | monoclonal antibodies |
| PDAC | pancreatic ductal adenocarcinoma |
| PARP | poly ADP ribose polymerase |
| TME | tumor microenvironment |
| RET/PTC | rearranged in transfusion/papillary thyroid carcinoma |
| RP2D | recommended phase II dose |
| ORR | overall response rate |
| mPFS | median progression-free survival |
| MTD | maximum tolerated dose |
References
- Nussinov, R.; Jang, H.; Tsai, C.J.; Cheng, F. Review: Precision medicine and driver mutations: Computational methods, functional assays and conformational principles for interpreting cancer drivers. PLoS Comput. Biol. 2019, 15, e1006658. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: https://www.cancer.gov/ (accessed on 20 December 2019).
- Yu, C.; Liu, X.; Yang, J.; Zhang, M.; Jin, H.; Ma, X.; Shi, H. Combination of Immunotherapy with Targeted Therapy: Theory and Practice in Metastatic Melanoma. Front. Immunol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghoubi, N.; Soltani, A.; Ghazvini, K.; Hassanian, S.M.; Hashemy, S.I. PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomed. Pharmacother. 2019, 110, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, E.S.; Jones, J.B.; Ashfaq, R.; Adsay, V.; Baker, S.J.; Valentine, V.; Hempen, P.M.; Hilgers, W.; Yeo, C.J.; Hruban, R.H.; et al. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: Potential therapeutic targets. Am. J. Pathol. 2003, 163, 1255–1260. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Boni, A.; Cogdill, A.P.; Dang, P.; Udayakumar, D.; Njauw, C.N.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef] [Green Version]
- Cooper, Z.A.; Juneja, V.R.; Sage, P.T.; Frederick, D.T.; Piris, A.; Mitra, D.; Lo, J.A.; Hodi, F.S.; Freeman, G.J.; Bosenberg, M.W.; et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2014, 2, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Prieto, P.A.; Reuben, A.; Cooper, Z.A.; Wargo, J.A. Targeted Therapies Combined with Immune Checkpoint Therapy. Cancer J. (Sudbury, Mass.) 2016, 22, 138–146. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Schuchter, L.M.; McDermott, D.F.; Kramer, A.; Giles, L.; Gramlich, K.; Carberry, M.; Troxel, A.B.; Letrero, R.; Nathanson, K.L.; et al. Phase II Trial of Temozolomide and Sorafenib in Advanced Melanoma Patients with or without Brain Metastases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7711–7718. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [Green Version]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet (Lond. Engl.) 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karachaliou, N.; Gonzalez-Cao, M.; Sosa, A.; Berenguer, J.; Bracht, J.W.P.; Ito, M.; Rosell, R. The combination of checkpoint immunotherapy and targeted therapy in cancer. Ann. Transl. Med. 2017, 5, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, P.E.; Caenepeel, S.; Wu, L.C. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016, 37, 462–476. [Google Scholar] [CrossRef]
- Frederick, D.T.; Piris, A.; Cogdill, A.P.; Cooper, Z.A.; Lezcano, C.; Ferrone, C.R.; Mitra, D.; Boni, A.; Newton, L.P.; Liu, C.; et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra137. [Google Scholar] [CrossRef] [Green Version]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.N.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef] [Green Version]
- Vella, L.J.; Pasam, A.; Dimopoulos, N.; Andrews, M.; Knights, A.; Puaux, A.L.; Louahed, J.; Chen, W.; Woods, K.; Cebon, J.S. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol. Res. 2014, 2, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Comin-Anduix, B.; Chodon, T.; Sazegar, H.; Matsunaga, D.; Mock, S.; Jalil, J.; Escuin-Ordinas, H.; Chmielowski, B.; Koya, R.C.; Ribas, A. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 6040–6048. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. (Baltimore, Md. 1950) 2014, 192, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Reuben, A.; Cooper, Z.A.; Wargo, J.A. Update on use of aldesleukin for treatment of high-risk metastatic melanoma. Immuno. Targets Ther. 2015, 4, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.-J.; Lee, K.-M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e17. [Google Scholar]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science (New York, N.Y.) 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Jaffee, E.M.; Zaidi, N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Investig. 2018, 128, 3209–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khair, D.O.; Bax, H.J.; Mele, S.; Crescioli, S.; Pellizzari, G.; Khiabany, A.; Nakamura, M.; Harris, R.J.; French, E.; Hoffmann, R.M.; et al. Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Front. Immunol. 2019, 10, 453. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Atkins, M.B.; Larkin, J. Immunotherapy Combined or Sequenced With Targeted Therapy in the Treatment of Solid Tumors: Current Perspectives. J. Natl. Cancer Inst. 2016, 108, djv414. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 20 December 2019).
- Wang, M.; Liu, Y.; Cheng, Y.; Wei, Y.; Wei, X. Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment. Biochimica Et Biophys. Acta Rev. Cancer 2019, 1871, 199–224. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Cerezo, M.; Lehraiki, A.; Millet, A.; Rouaud, F.; Plaisant, M.; Jaune, E.; Botton, T.; Ronco, C.; Abbe, P.; Amdouni, H.; et al. Compounds Triggering ER Stress Exert Anti-Melanoma Effects and Overcome BRAF Inhibitor Resistance. Cancer Cell 2016, 29, 805–819. [Google Scholar] [CrossRef] [Green Version]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Menzies, A.M.; Long, G.V.; Murali, R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des. Dev. Ther. 2012, 6, 391–405. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Vis, D.J.; Bruna, A.; Sustic, T.; van Wageningen, S.; Batra, A.S.; Rueda, O.M.; Bosdriesz, E.; Caldas, C.; Wessels, L.F.A.; et al. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018, 28, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Z.; Ribas, A. Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brighton, H.E.; Angus, S.P.; Bo, T.; Roques, J.; Tagliatela, A.C.; Darr, D.B.; Karagoz, K.; Sciaky, N.; Gatza, M.L.; Sharpless, N.E.; et al. New Mechanisms of Resistance to MEK Inhibitors in Melanoma Revealed by Intravital Imaging. Cancer Res. 2018, 78, 542–557. [Google Scholar] [CrossRef] [Green Version]
- Shirley, M. Encorafenib and Binimetinib: First Global Approvals. Drugs 2018, 78, 1277–1284. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet (Lond. Engl.) 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Park, B.J.; Zakowski, M.F.; Ladanyi, M.; Pao, W.; D’Angelo, S.P.; Kris, M.G.; Shen, R.; Zheng, J.; Azzoli, C.G. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Shen, Y.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Xu, L.; et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet. Oncol. 2018, 19, 139–148. [Google Scholar] [CrossRef]
- Lim, S.M.; Hong, M.H.; Kim, H.R. Immunotherapy for Non-small Cell Lung Cancer: Current Landscape and Future Perspectives. Immune Netw. 2020, 20, e10. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annual Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Sun, W.; Zhou, Y.; Li, P.; Chen, F.; Chen, H.; Xia, D.; Xu, E.; Lai, M.; Wu, Y.; et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018, 37, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Le, D.T. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 373, 1979. [Google Scholar] [CrossRef] [Green Version]
- Delord, J.P.; Robert, C.; Nyakas, M.; McArthur, G.A.; Kudchakar, R.; Mahipal, A.; Yamada, Y.; Sullivan, R.; Arance, A.; Kefford, R.F.; et al. Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5339–5348. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.C.; Javle, M.; Bekaii-Saab, T.S.; Finn, R.S.; Wainberg, Z.A.; Laheru, D.A.; Weekes, C.D.; Tan, B.R.; Khan, G.N.; Zalupski, M.M.; et al. A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br. J. Cancer 2017, 116, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Korc, M. Driver mutations: A roadmap for getting close and personal in pancreatic cancer. Cancer Biol. Ther. 2010, 10, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.H.; Bekaii-Saab, T.; Van Ziffle, J.; Mirzoeva, O.M.; Joseph, N.M.; Talasaz, A.; Kuhn, P.; Tempero, M.A.; Collisson, E.A.; Kelley, R.K.; et al. A Multicenter, Open-Label Phase II Clinical Trial of Combined MEK plus EGFR Inhibition for Chemotherapy-Refractory Advanced Pancreatic Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, P.L.; Tabernero, J.; Janku, F.; Wainberg, Z.A.; Paz-Ares, L.; Vansteenkiste, J.; Van Cutsem, E.; Perez-Garcia, J.; Stathis, A.; Britten, C.D.; et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolcher, A.W.; Bendell, J.C.; Papadopoulos, K.P.; Burris, H.A., 3rd; Patnaik, A.; Jones, S.F.; Rasco, D.; Cox, D.S.; Durante, M.; Bellew, K.M.; et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Groenendijk, F.H.; Bernards, R. Drug resistance to targeted therapies: Deja vu all over again. Mol. Oncol. 2014, 8, 1067–1083. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar] [CrossRef]
- Sideras, K.; Braat, H.; Kwekkeboom, J.; van Eijck, C.H.; Peppelenbosch, M.P.; Sleijfer, S.; Bruno, M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat. Rev. 2014, 40, 513–522. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet. Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Mazieres, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet. Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Pacini, F.; Tuttle, R.M.; Schlumberger, M. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol. 2016, 4, 64–71. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Zafereo, M.; Gunn, G.B.; Ferrarotto, R. Anaplastic Thyroid Carcinoma: Treatment in the Age of Molecular Targeted Therapy. J. Oncol. Pract. 2016, 12, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Tavangar, S.M. A Review of Driver Genetic Alterations in Thyroid Cancers. Iran. J. Pathol. 2018, 13, 125–135. [Google Scholar] [CrossRef]
- Bikas, A.; Vachhani, S.; Jensen, K.; Vasko, V.; Burman, K.D. Targeted therapies in thyroid cancer: An extensive review of the literature. Expert Rev. Clin. Pharmacol. 2016, 9, 1299–1313. [Google Scholar] [CrossRef]

| Type of Cancer | Mutations |
|---|---|
| Melanoma | IDH1, RB1, DDX3X, NF1, BRAF, RAS, |
| Non-small-cell lung cancer | PI3K, FGFR, DDR2, PTEN, KRAS, EGFR, BRAF, ALK |
| Colorectal cancer | APC, KRAS, TP53, SMAD4, FBXW7, BRAF, PI3K |
| Pancreatic cancer | KRAS,BRAF,TP53, CDKN2A, SMAD4, MLL3, TGFBR2, ARID1A, SF3B1 |
| Thyroid cancer | RAS, BRAF, TP53, PI3K, RET/PTC |
| Name of Drug | Company | Target | Conditions |
|---|---|---|---|
| Everolimus (Afinitor) | Novartis | mTOR |
|
| Tamoxifen (Nolvadex) | AstraZeneca | Estrogen receptor (ER)-positive breast cancer |
|
| Lapatinib (Tykerb) | GlaxoSmithKline | HER2 (ERBB2/neu), EGFR (HER1/ERBB1) |
|
| Neratinib (Nerlynx) | Puma Biotech | HER2 (ERBB2/neu) |
|
| Palbociclib (Ibrance) | Pfizer | CDK4, CDK6 |
|
| Ribociclib (Kisqali) | Novartis | CDK4, CDK6 |
|
| Ado-trastuzumab emtansine (Kadcyla) | Genentech | HER2 (ERBB2/neu) |
|
| Trastuzumab (Herceptin) | Genentech | HER2 (ERBB2/neu) |
|
| Erdafitinib (Balversa™) | Astex Pharmaceuticals and Janssen Pharmaceutical | FGFR |
|
| Axitinib (Inlyta) | Chiron | KIT, PDGFRβ, VEGFR1/2/3 |
|
| Lenvatinib (Lenvima) | Eisai | VEGFR2 |
|
| Sorafenib (Nexavar) | Bayer | VEGFR, PDGFR, KIT, RAF |
|
| Temsirolimus (Torisel) | Pfizer | mTOR |
|
| Pazopanib (Votrient) | GlaxoSmithKline | VEGFR, PDGFR, KIT |
|
| Cabozantinib (Cabometyx (tablet), Cometriq (capsule])) | Exelixis | FLT3, KIT, MET, RET, VEGFR2 |
|
| Afatinib (Gilotrif) | Boehringer Ingelheim | EGFR (HER1/ERBB1), HER2 (ERBB2/neu) |
|
| Alectinib (Alecensa) | Genentech | ALK |
|
| Brigatinib (Alunbrig) | Takeda Pharmaceutical | ALK |
|
| Ceritinib (Zykadia) | Novartis | ALK |
|
| Crizotinib (Xalkori) | Pfizer | ALK, MET, ROS1 |
|
| Erlotinib (Tarceva) | Roche | EGFR (HER1/ERBB1) |
|
| Gefitinib (Iressa) | AstraZeneca | EGFR (HER1/ERBB1) |
|
| Osimertinib (Tagrisso) | AstraZeneca | EGFR |
|
| Cobimetinib (Cotellic) | Genentech | MEK |
|
| Dabrafenib (Tafinlar) | GlaxoSmithKline | BRAF |
|
| Necitumumab (Portrazza) | Eli Lilly | EGFR (HER1/ERBB1) |
|
| Bortezomib (Velcade) | Takeda | Proteasome |
|
| Bosutinib (Bosulif) | Pfizer | ABL |
|
| Carfilzomib (Kyprolis) | Onyx | Proteasome |
|
| Dasatinib (Sprycel) | Bristol-Myers Squibb | ABL |
|
| Enasidenib (Idhifa) | Agios Pharmaceuticals/Celgene | IDH2 |
|
| Venetoclax (Venclexta) | AbbVie and Roche | BCL2 |
|
| Ibrutinib (Imbruvica) | Johnson &Johnson | BTK |
|
| Idelalisib (Zydelig) | Gilead | PI3Kδ |
|
| Ixazomib (Ninlaro) | Takeda Pharmaceutical | Proteasome |
|
| Midostaurin (Rydapt) | Novartis | FLT3 |
|
| Nilotinib (Tasigna) | Novartis | ABL |
|
| Ponatinib (Iclusig) | ARIAD | ABL, FGFR1–3, FLT3, VEGFR2 |
|
| Trametinib (Mekinist) | GlaxoSmithKline | MEK |
|
| Vemurafenib (Zelboraf) | Genentech | BRAF |
|
| Cetuximab (Erbitux) | Eli Lilly | EGFR (HER1/ERBB1) |
|
| Ziv-aflibercept (Zaltrap) | Sanofi-Aventis | PIGF, VEGFA/B |
|
| Panitumumab (Vectibix) | Amgen | EGFR (HER1/ERBB1) |
|
| Ramucirumab (Cyramza) | Eli Lilly | VEGFR2 |
|
| Regorafenib (Stivarga) | Bayer | KIT, PDGFRβ, RAF, RET, VEGFR1–3 |
|
| Rucaparib (Rubraca) | Clovis Oncology | PARP |
|
| Niraparib (Zejula) | Tesaro | PARP |
|
| Olaparib (Lynparza) | AstraZeneca | PARP |
|
| Denosumab (Xgeva) | Amgen | RANKL |
|
| Dinutuximab (Unituxin) | United Therapeutics | B4GALNT1 (GD2) |
|
| Imatinib (Gleevec) | Novartis | KIT, PDGFR, ABL |
|
| Sonidegib (Odomzo) | Novartis | Smoothened |
|
| Vismodegib (Erivedge) | Roche | PTCH, Smoothened |
|
| Olaratumab (Lartruvo) | Eli Lilly | PDGFRα |
|
| Ruxolitinib (Jakafi) | Incyte | JAK1/2 |
|
| Tofacitinib (Xeljanz) | Pfizer | JAK3 |
|
| Vandetanib (Caprelsa) | AstraZeneca | EGFR (HER1/ERBB1), RET, VEGFR2 |
|
| Name of Drug | Company | Target | Conditions |
|---|---|---|---|
| Alemtuzumab (Campath) | Sanofi | CD52 |
|
| Atezolizumab (Tecentriq) | Genentech | PD-L1 |
|
| Avelumab (Bavencio) | Merck KGaA and Pfizer | PD-L1 |
|
| Blinatumomab (Blincyto) | Amgen | CD19/CD3 |
|
| Brentuximab vedotin (Adcetris) | Takeda Pharmaceutical | CD30 |
|
| Canakinumab (Ilaris) | Novartis | IL-1β |
|
| Daratumumab (Darzalex) | Janssen Pharmaceutical | CD38 |
|
| Durvalumab (Imfinzi) | MedImmune/AstraZeneca | PD-L1 |
|
| Elotuzumab (Empliciti) | Bristol-Myers Squibb | SLAMF7 (CS1/CD319/CRACC) |
|
| Ibritumomab tiuxetan (Zevalin) | Biogen IDEC | CD20 |
|
| Ipilimumab (Yervoy) | Bristol-Myers Squibb | CTLA-4 |
|
| Nivolumab (Opdivo) | Bristol-Myers Squibb | PD-1 |
|
| Obinutuzumab (Gazyva) | Roche | CD20 |
|
| Ofatumumab (Arzerra, HuMax-CD20) | Roche | CD20 |
|
| Pembrolizumab (Keytruda) | Merck &Co | PD-1 |
|
| Rituximab (Rituxan, Mabthera) | Roche | CD20 |
|
| Rituximab/hyaluronidase human (Rituxan Hycela) | Roche | CD20 |
|
| Siltuximab (Sylvant) | Janssen Pharmaceutical | IL-6 |
|
| Tocilizumab (Actemra) | Genentech | IL-6R |
|
| Tositumomab (Bexxar) | Corixa | CD20 |
|
| Name of Drug | Company | Target | Indications |
|---|---|---|---|
| Ipilimumab | Bristol-Myers Squibb | CTLA-4 |
|
| Tremelimumab | AstraZeneca | CTLA-4 |
|
| Nivolumab | Bristol-Myers | PD-1 |
|
| Pembrolizumab | Merck | PD-1 |
|
| Atezolizumab | Roche | PD-L1 |
|
| Avelumab | Merck Pfizer | PD-L1 |
|
| Durvalumab | MedImmune/AstraZeneca | PD-L1 |
|
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 01400451 | Ph I Ipilimumab Vemurafenib Combo in Patients with v-Raf Murine Sarcoma Viral Oncogene Homolog B1 (BRAF) | Terminated (unexpected grade 2/3 hepatotoxicity) | •Melanoma | •Drug: Ipilimumab (BMS-734016) •Drug: Vemurafenib | Phase 1 | November 2011 |
| NCT 01673854 | Phase II Safety Study of Vemurafenib Followed by Ipilimumab in Subjects with V600 BRAF Mutated Advanced Melanoma | Completed (no severe hepatotoxicity, but reported a grade 3/4 skin adverse event) | •Melanoma | •Drug: Ipilimumab •Biological: Vemurafenib | Phase 2 | 13 September 2012 |
| NCT 03554083 | Neoadjuvant Combination Targeted and Immunotherapy for Patients with High-Risk Stage III Melanoma | Recruiting | •Clinical stage iii cutaneous melanoma ajcc v8 •Pathologic Stage III Cutaneous Melanoma AJCC v8 •Pathologic Stage IIIA Cutaneous Melanoma AJCC v8 •Pathologic Stage IIIB Cutaneous Melanoma AJCC v8 •Pathologic Stage IIIC Cutaneous Melanoma AJCC v8 •Pathologic Stage IIID Cutaneous Melanoma AJCC v8 | •Drug: Atezolizumab •Drug: Cobimetinib •Drug: Vemurafenib | Phase 2 | 22 June 2018 |
| NCT 03235245 | Immunotherapy with Ipilimumab and Nivolumab Preceded or Not by a Targeted Therapy with Encorafenib and Binimetinib | Recruiting | •Unresectable Stage III Melanoma •Stage IV Melanoma | •Drug: Nivolumab + Ipilimumab •Drug: Encorafenib+ Binimetinib | Phase 2 | 30 October 2018 |
| NCT 02967692 | A Study of the Anti-PD1 Antibody PDR001, in Combination with Dabrafenib and Trametinib in Advanced Melanoma | Recruiting | •Melanoma | •Biological: Spartalizumab (PDR001) •Other: Placebo •Drug: Dabrafenib •Drug: Trametinib | Phase 3 | 17 February 2017 |
| NCT 02902042 | Encorafenib + Binimetinib + Pembrolizumab in Patients with Unresectable or Metastatic BRAF V600 Mutant Melanoma | Recruiting | •Malignant Melanoma | •Drug: Encorafenib •Drug: Binimetinib •Drug: Pembrolizumab •Drug: Pembrolizumabalone | •Phase 1 •Phase 2 | 24 April 2018 |
| NCT 02858921 | Neoadjuvant Dabrafenib, Trametinib and/or Pembrolizumab in BRAF Mutant Resectable Stage III Melanoma | Recruiting | •Melanoma | •Melanoma •Drug: Dabrafenib •Drug: Trametinib •Drug: Pembrolizumab | Phase 2 | 8 November 2017 |
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 03991819 | Study of Binimetinib in Combination with Pembrolizumab in Advanced Non-Small-Cell Lung Cancer | Recruiting | •Non-Small-Cell Carcinoma | •Drug: Binimetinib •Drug: Pembrolizumab | •Phase 1 | 20 September 2019 |
| NCT 03600701 | Atezolizumab and Cobimetinib in Treating Patients with Metastatic, Recurrent, or Refractory Non-Small-Cell Lung Cancer | Recruiting | •Recurrent Lung Non-Small-Cell Carcinoma •Refractory Lung Non-Small-Cell Carcinoma •Stage IV Lung Non- Small-Cell Cancer AJCC v7 | •Drug: Atezolizumab •Drug: Cobimetinib | Phase 2 | 20 July 2018 |
| NCT 03581487 | Durvalumab, Tremelimumab, and Selumetinib in Treating Participants with Recurrent or Stage IV Non-Small-Cell Lung Cancer | Recruiting | •Recurrent Lung Non-Small-Cell Carcinoma •Stage IV Lung Cancer AJCC v8 •Stage IVA Lung Cancer AJCC v8 •Stage IVB Lung Cancer AJCC v8 | •Biological: Durvalumab •Drug: Selumetinib •Biological:Tremelimumab | •Phase 1 •Phase 2 | 1 April 2019 |
| NCT 03299088 | Pembrolizumab and Trametinib in Treating Patients with Stage IV Non-Small-Cell Lung Cancer and KRAS Gene Mutations | Recruiting | •KRAS Gene Mutation •Metastatic Non- Squamous Non- Small Cell Lung Carcinoma •Recurrent Non- Squamous Non- Small Cell Lung Carcinoma •Stage IV Non-Small-Cell Lung Cancer AJCC v7 | •Biological: Pembrolizumab •Drug: Trametinib | Phase 1 | 26 June 2018 |
| NCT 03225664 | Trametinib and Pembrolizumab in Treating Patients with Recurrent Non-Small-Cell Lung Cancer That Is Metastatic, Unresectable, or Locally Advanced | Recruiting | •Metastatic Lung Non-Small-Cell Carcinoma •Recurrent Lung Non-Small-Cell Carcinoma •Stage III Lung Cancer AJCC v8 •Stage IIIA Lung Cancer AJCC v8 •Stage IIIB Lung Cancer AJCC v8 •Stage IIIC Lung Cancer AJCC v8 •Stage IV Lung Cancer AJCC v8 •Stage IVA Lung Cancer AJCC v8 •Stage IVB Lung Cancer AJCC v8 •Unresectable Lung Non-Small-Cell Carcinoma | •Biological: Pembrolizumab •Other: Pharmacokinetic Study •Drug: Trametinib | •Phase 1 •Phase 2 | 3 February 2018 |
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 01436656 | A Phase I Study of Oral LGX818 in Adult Patients with Advanced or Metastatic BRAF Mutant Melanoma | Active, not recruiting | •Melanoma and Metastatic Colorectal Cancer | •Drug: LGX818 | •Phase 1 | September 2011 |
| NCT 00959127 | A Study of ARRY-438162 (MEK162) in Patients with Advanced Cancer | Completed | •Advanced Solid Tumors •Advanced or Metastatic Biliary Cancer •Metastatic Colorectal Cancer | •Drug: ARRY-438162 (MEK162), MEK inhibitor; oral | Phase 1 | August 2009 |
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 04044430 | Encorafenib, Binimetinib, and Nivolumab in Treating Patients with Microsatellite Stable BRAFV600E Metastatic Colorectal Cancer | Not yet recruiting | •Metastatic Colon Adenocarcinoma •Metastatic Colorectal Adenocarcinoma •Metastatic Microsatellite Stable Colorectal Carcinoma •Metastatic Rectal Adenocarcinoma •Stage III Colon Cancer •Stage III Colorectal Cancer •Stage III Rectal Cancer •Stage IIIA Colon Cancer •Stage IIIA Colorectal Cancer •Stage IIIA Rectal Cancer •and 18 more | •Drug: Binimetinib •Drug: Encorafenib •Biological: Nivolumab •Other: Questionnaire Administration | •Phase 1 •Phase 2 | 1 December 2019 |
| NCT 03428126 | Study of Durvalumab (MEDI4736) (Anti-PD-L1) and Trametinib (MEKi) in MSS Metastatic Colon Cancer | Enrolling by invitation | •Malignant Neoplasms of Digestive Organs •Colorectal Cancer •Colon Cancer | •Drug: Durvalumab •Drug: Trametinib | Phase 2 | 21 March 2018 |
| NCT 03374254 | Safety and Efficacy of Pembrolizumab (MK-3475) Plus Binimetinib Alone or Pembrolizumab Plus Chemotherapy with or without Binimetinib in Metastatic Colorectal Cancer (mCRC) Participants (MK-3475-651) | Recruiting | •Metastatic Colorectal Cancer | •Biological: Pembrolizumab •Drug: Binimetinib •Drug: Oxaliplatin •Drug: Leucovorin •Drug: 5-Fluorouracil [5-FU] •Drug: Irinotecan | Phase 1 | 16 February 2018 |
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 03193190 | A Study of Multiple Immunotherapy-Based Treatment Combinations in Participants with Metastatic Pancreatic Ductal Adenocarcinoma (Morpheus Pancreatic Cancer) | Recruiting | •Pancreatic Adenocarcinoma | •Drug: NabPaclitaxel •Drug: Gemcitabine •Drug: Oxaliplatin •Drug: Leucovorin •Drug: Fluorouracil •Drug: Atezolizumab •Drug: Cobimetinib •Drug: PEGPH20 •Drug: BL-8040 •Drug: Selicrelumab •and 3 more | •Phase 1 •Phase 2 | 5 July 2017 |
| NCT 03637491 | A Study of Avelumab, Binimetinib and Talazoparib in Patients with Locally Advanced or Metastatic RAS-mutant Solid Tumors | Recruiting | •Pancreatic Cancer | •Drug: Avelumab •Drug: Binimetinib •Drug: Talazoparib | Phase 2 | 15 August 2018 |
| National Clinical Trial (NCT) Number | Title | Status | Conditions | Interventions | Phase | Start Date |
|---|---|---|---|---|---|---|
| NCT 04061980 | Encorafenib and Binimetinib with or without Nivolumab in Treating Patients with Metastatic Radioiodine Refractory BRAF V600 Mutant Thyroid Cancer | Not yet recruiting | •BRAF NP_004324.2:p.V600M •BRAF V600E Mutation Present •Metastatic Thyroid Gland Carcinoma •Refractory Thyroid Gland Carcinoma •Stage IV Differentiated Thyroid Gland Carcinoma AJCC v8 •Stage IVA Differentiated Thyroid Gland Carcinoma AJCC v8 •Stage IVB Differentiated Thyroid Gland Carcinoma AJCC v8 | •Drug: Binimetinib •Drug: Encorafenib •Biological: Nivolumab | Phase 2 | 30 August 2019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Lee, K.-M. Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade. Int. J. Mol. Sci. 2020, 21, 2531. https://doi.org/10.3390/ijms21072531
Shin MH, Kim J, Lim SA, Kim J, Lee K-M. Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade. International Journal of Molecular Sciences. 2020; 21(7):2531. https://doi.org/10.3390/ijms21072531
Chicago/Turabian StyleShin, Min Hwa, Jiyoung Kim, Siyoung A. Lim, Jeongsoo Kim, and Kyung-Mi Lee. 2020. "Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade" International Journal of Molecular Sciences 21, no. 7: 2531. https://doi.org/10.3390/ijms21072531
APA StyleShin, M. H., Kim, J., Lim, S. A., Kim, J., & Lee, K.-M. (2020). Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade. International Journal of Molecular Sciences, 21(7), 2531. https://doi.org/10.3390/ijms21072531





