Histopathologic Features of Lymphedema: A Molecular Review
Abstract
1. Introduction
2. Clinical Presentation and Staging
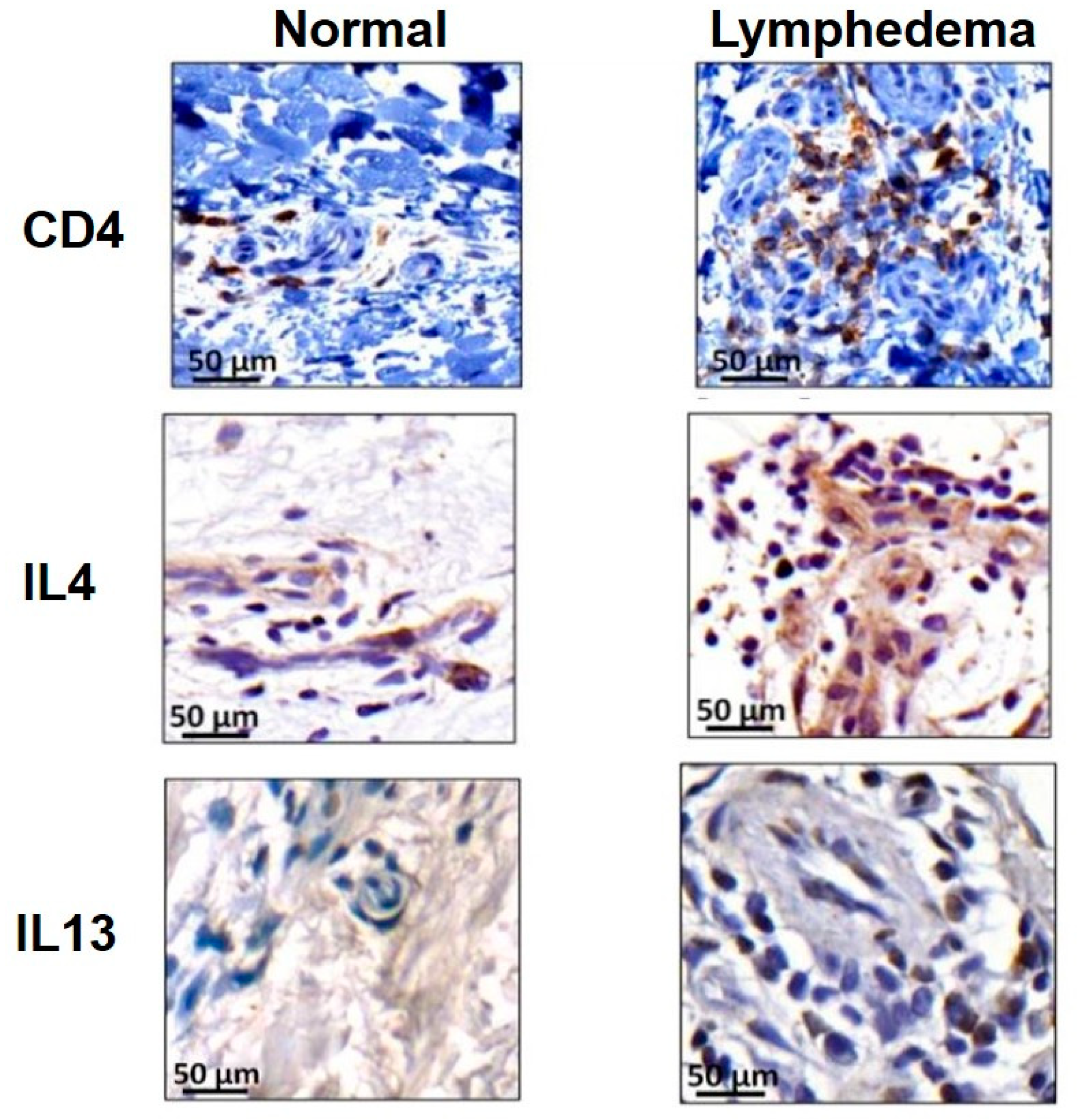
3. Role of Inflammation in Development of Lymphedema
4. Lymphedema as Fibrotic End-Organ Failure of Lymphatic System
5. Lymphedema Results in Adipose Deposition
6. Lymphedema Is Associated with Recurrent Infections
7. Future Directions and Conclusion
Funding
Conflicts of Interest
Abbreviations
| CDT | complete decongestive therapy |
| ISL | International Society of Lymphology |
| PLND | popliteal lymph node dissection |
| WT | wild-type |
| VEGF-C | vascular endothelial growth factor C |
| DC | dendritic cell |
| iNOS | inducible nitric oxide synthase |
| LEC | lymphatic endothelial cell |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| TGF-β1 | transforming growth factor-β1 |
| BMI | body mass index |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| LMC | lymphatic muscle cell |
| Treg | T-regulatory cell |
| LTB4 | leukotriene B4 |
References
- Szuba, A.; Rockson, S.G. Lymphedema: Anatomy, physiology and pathogenesis. Vasc. Med. 1997, 2, 321–326. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Giron, G.L.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Riedel, E.R.; Van Zee, K.J. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.M.; Fu, M.R.; Wainstock, J.M.; Zagar, E.; Jacobs, L.K. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology 2004, 37, 73–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwan, M.L.; Darbinian, J.; Schmitz, K.H.; Citron, R.; Partee, P.; Kutner, S.E.; Kushi, L.H. Risk factors for lymphedema in a prospective breast cancer survivorship study: The pathways study. Arch. Surg. 2010, 145, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Yamamoto, R.; Minobe, S.; Suzuki, Y.; Takeshi, U.; Nakatani, M.; Aoyagi, Y.; Ohba, Y.; Okamoto, K.; Kato, H. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol. Oncol. 2010, 119, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.A.; Localio, A.R.; Kallan, M.J.; Weber, A.L.; Torpey, H.A.; Potashnik, S.L.; Miller, L.T.; Fox, K.R.; DeMichele, A.; Solin, L.J. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Starritt, E.C.; Joseph, D.; McKinnon, J.G.; Lo, S.K.; De Wilt, J.H.; Thompson, J.F. Lymphedema after complete axillary node dissection for melanoma: Assessment using a new, objective definition. Ann. Surg. 2004, 240, 866–874. [Google Scholar] [CrossRef]
- Gross, J.P.; Whelan, T.J.; Parulekar, W.R.; Chen, B.E.; Rademaker, A.W.; Helenowski, I.B.; Donnelly, E.D.; Strauss, J.B. Development and validation of a nomogram to predict lymphedema after axillary surgery and radiation therapy in women with breast cancer from the NCIC CTG MA.20 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 165–173. [Google Scholar] [CrossRef]
- Bevilacqua, J.L.; Kattan, M.W.; Changhong, Y.; Koifman, S.; Mattos, I.E.; Koifman, R.J.; Bergmann, A. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann. Surg. Oncol. 2012, 19, 2580–2589. [Google Scholar] [CrossRef]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Brayton, K.M.; Hirsch, A.T.; Patricia, J.O.; Brien, A.C.; Karaca-Mandic, P.; Rockson, S.G. Lymphedema prevalence and treatment benefits in cancer: Impact of a therapeutic intervention on health outcomes and costs. PLoS ONE 2014, 9, e114597. [Google Scholar] [CrossRef] [PubMed]
- Uzkeser, H.; Karatay, S.; Erdemci, B.; Koc, M.; Senel, K. Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: A randomized controlled trial. Breast Cancer 2015, 22, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Huang, M.S.; Li, S.H.; Chen, I.R.; Wei, T.S.; Kuo, S.J.; Chen, S.T.; Hsu, J.C. Complex decongestive physiotherapy for patients with chronic cancer-associated lymphedema. J. Formos. Med. Assoc. 2004, 103, 344–348. [Google Scholar] [PubMed]
- Szuba, A.; Cooke, J.P.; Yousuf, S.; Rockson, S.G. Decongestive lymphatic therapy for patients with cancer-related or primary lymphedema. Am. J. Med. 2000, 109, 296–300. [Google Scholar] [CrossRef]
- Foldi, E. The treatment of lymphedema. Cancer 1998, 83, 2833–2834. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; DeSnyder, S.M.; Klimberg, S.; Alatriste, M.; Boccardo, F.; Smith, M.L.; Staley, A.C.; Thiruchelvam, P.T.; Hutchison, N.A.; Mendez, J.; et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema, recommendations from an expert panel: Part 2: Preventive and therapeutic options. Ann. Surg. Oncol. 2017, 24, 2827–2835. [Google Scholar] [CrossRef]
- Bozkurt, M.; Palmer, L.J.; Guo, Y. Effectiveness of decongestive lymphatic therapy in patients with lymphedema resulting from breast cancer treatment regardless of previous lymphedema treatment. Breast J. 2017, 23, 154–158. [Google Scholar] [CrossRef]
- Garza, R., 3rd; Skoracki, R.; Hock, K.; Povoski, S.P. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017, 17, 468. [Google Scholar] [CrossRef]
- Granzow, J.W.; Soderberg, J.M.; Kaji, A.H.; Dauphine, C. An effective system of surgical treatment of lymphedema. Ann. Surg. Oncol. 2014, 21, 1189–1194. [Google Scholar] [CrossRef]
- Winters, H.; Tielemans, H.J.; Hameeteman, M.; Paulus, V.A.; Beurskens, C.H.; Slater, N.J.; Ulrich, D.J. The efficacy of lymphaticovenular anastomosis in breast cancer-related lymphedema. Breast Cancer Res. Treat. 2017, 165, 321–327. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at memorial sloan-kettering cancer center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Stainton, M.C.; Slaytor, E.K.; Jaconelli, C.; Watts, S.; MacKenzie, P. Aetiology and prevalence of lower limb lymphoedema following treatment for gynaecological cancer. Aust. N. Z. J. Obstet. Gynaecol. 2003, 43, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar]
- Sharma, A.; Schwartz, R.A. Stewart-Treves syndrome: Pathogenesis and management. J. Am. Acad. Dermatol. 2012, 67, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Scholl, J.; Uecker, M.; Detmar, M. prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess lymphangiogenesis cooperatively induced by macrophages and CD4(+) T cells drives the pathogenesis of lymphedema. J. Investig. Dermatol. 2016, 136, 706–714. [Google Scholar] [CrossRef]
- Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Mehrara, B.J. Small numbers of CD4+ T cells can induce development of lymphedema. Plast. Reconstr. Surg. 2019, 143, 518e–526e. [Google Scholar] [CrossRef]
- Scallan, P.J.; Davis, M.J. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J. Physiol. 2013, 591, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cheng, G.; Conner, D.A.; Huang, Y.; Kucherlapati, R.S.; Munn, L.L.; Ruddle, N.H.; Jain, R.K.; Fukumura, D.; Padera, T.P. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. USA 2011, 108, 18784–18789. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Pan, W.R.; Taylor, G.I. Changes in the lymph structure of the upper limb after axillary dissection: Radiographic and anatomical study in a human cadaver. Plast. Reconstr. Surg. 2007, 120, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Clavin, N.W.; Avraham, T.; Fernandez, J.; Daluvoy, S.V.; Soares, M.A.; Chaudhry, A.; Mehrara, B.J. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2113–H2127. [Google Scholar] [CrossRef]
- Avraham, T.; Clavin, N.W.; Daluvoy, S.V.; Fernandez, J.; Soares, M.A.; Cordeiro, A.P.; Mehrara, B.J. Fibrosis is a key inhibitor of lymphatic regeneration. Plast. Reconstr. Surg. 2009, 124, 438–450. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Ly, C.L.; Nores, G.D.; Kataru, R.P.; Mehrara, B.J. T helper 2 differentiation is necessary for development of lymphedema. Transl. Res. 2019, 206, 57–70. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Torrisi, J.S.; Cuzzone, D.A.; Ghanta, S.; Albano, N.J.; Gardenier, J.C.; Joseph, W.J.; Mehrara, B.J. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H165–H172. [Google Scholar] [CrossRef]
- Shin, K.; Kataru, R.P.; Park, H.J.; Kwon, B.I.; Kim, T.W.; Hong, Y.K.; Lee, S.H. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun. 2015, 6, 6196. [Google Scholar] [CrossRef]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Ziyou, Y.; Liu, N.F. Pathological changes of lymphedematous skin: increased mast cells, related proteases, and activated transforming growth factor-beta1. Lymphat. Res. Biol. 2016, 14, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L.; Wilson, M.S., 3rd; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Narushima, M.; Yamamoto, T.; Ogata, F.; Yoshimatsu, H.; Mihara, M.; Koshima, I. Indocyanine green lymphography findings in limb lymphedema. J. Reconstr. Microsurg. 2016, 32, 72–79. [Google Scholar]
- Sosa-Pineda, B.; Wigle, J.T.; Oliver, G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 2000, 25, 254–255. [Google Scholar] [CrossRef]
- Escobedo, N.; Proulx, S.T.; Karaman, S.; Dillard, M.E.; Johnson, N.; Detmar, M.; Oliver, G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI insight 2016, 1, e85096. [Google Scholar] [CrossRef]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef]
- Nores, G.G.; Cuzzone, D.A.; Albano, N.J.; Hespe, G.E.; Kataru, R.P.; Torrisi, J.S.; Gardenier, J.C.; Savetsky, I.L.; Aschen, S.Z.; Nitti, M.D.; et al. Obesity but not high-fat diet impairs lymphatic function. Int. J. Obes. 2016, 40, 1582–1590. [Google Scholar] [CrossRef]
- Aschen, S.; Zampell, J.C.; Elhadad, S.; Weitman, E.; Andrade, M.D.; Mehrara, B.J. Regulation of adipogenesis by lymphatic fluid stasis: Part II. Expression of adipose differentiation genes. Plast. Reconstr. Surg. 2012, 129, 838–847. [Google Scholar] [CrossRef]
- Zampell, J.C.; Aschen, S.; Weitman, E.S.; Yan, A.; Elhadad, S.; Andrade, M.D.; Mehrara, B.J. Regulation of adipogenesis by lymphatic fluid stasis: Part I. Adipogenesis, fibrosis, and inflammation. Plast. Reconstr. Surg. 2012, 129, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Cuzzone, D.A.; Weitman, E.S.; Albano, N.J.; Ghanta, S.; Savetsky, I.L.; Gardenier, J.C.; Joseph, W.J.; Torrisi, J.S.; Bromberg, J.F.; Olszewski, W.L.; et al. IL-6 regulates adipose deposition and homeostasis in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1426–H1434. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.S.; McCormick, B.; Petrek, J.; Cox, L.; Cirrincione, C.; Gray, J.R.; Yahalom, J. Arm edema in conservatively managed breast cancer: Obesity is a major predictive factor. Radiology 1991, 180, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.S.; Karaman, S.; Proulx, S.T.; Ochsenbein, A.M.; Luciani, P.; Leroux, J.C.; Wolfrum, C.; Detmar, M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS ONE 2014, 9, e94713. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Karaman, S.; Proulx, S.T.; Leu, K.; Buschle, D.; Detmar, M. High-fat diet in the absence of obesity does not aggravate surgically induced lymphoedema in mice. Eur. Surg. Res. 2017, 58, 180–192. [Google Scholar] [CrossRef]
- Arngrim, N.; Simonsen, L.; Holst, J.J.; Bülow, J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: A possible link between obesity and local tissue inflammation? Int. J. Obes. (London) 2013, 37, 748–750. [Google Scholar] [CrossRef]
- Szuba, A.; Shin, W.S.; Strauss, H.W.; Rockson, S. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J. Nucl. Med. 2003, 44, 43–57. [Google Scholar]
- Greene, A.K.; Grant, F.D.; Slavin, S.A.; Maclellan, R.A. Obesity-induced lymphedema: Clinical and lymphoscintigraphic features. Plast. Reconstr. Surg. 2015, 135, 1715–1719. [Google Scholar] [CrossRef]
- Torrisi, J.S.; Hespe, G.E.; Cuzzone, D.A.; Savetsky, I.L.; Nitti, M.D.; Gardenier, J.C.; Nores, G.D.; Jowhar, D.; Kataru, R.P.; Mehrara, B.J. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci. Rep. 2016, 6, 19817. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Troxel, A.B.; Dean, L.T.; DeMichele, A.; Brown, J.C.; Sturgeon, K.; Zhang, Z.; Evangelisti, M.; Spinelli, B.; Kallan, M.J.; et al. Effect of home-based exercise and weight loss programs on breast cancer–related lymphedema outcomes among overweight breast cancer survivors: The WISER survivor randomized clinical trial. JAMA Oncol. 2019, 5, 1605–1613. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Ahmed, R.L.; Troxel, A.; Cheville, A.; Smith, R.; Lewis-Grant, L.; Bryan, C.J.; Williams-Smith, C.T.; Greene, Q.P. Weight lifting in women with breast-cancer-related lymphedema. N. Engl. J. Med. 2009, 361, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Mortimer, P.; Judd, P.A. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer 2007, 110, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Williams, A.F.; Badger, C.; Jeffs, E.; Bosanquet, N.; Mortimer, P.S. Lymphoedema: An underestimated health problem. Qjm 2003, 96, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H.; Deng, J.; Fu, M.R.; Radina, E.; Thiadens, S.R.; Weiss, J.; Dietrich, M.S.; Cormier, J.N.; Tuppo, C.M.; Armer, J.M. Symptom burden and infection occurrence among individuals with extremity lymphedema. Lymphology 2012, 45, 113–123. [Google Scholar] [PubMed]
- Connor, P.M.; Gamelli, R. Challenges of cellulitis in a lymphedematous extremity: A case report. Cases J. 2009, 2, 9377. [Google Scholar]
- Campanholi, L.L.; Neto, J.P.D.; Fregnani, J.H.T.G. Mathematical model to predict risk for lymphoedema after treatment of cutaneous melanoma. Int. J. Surg. 2011, 9, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Soo, J.K.; Bicanic, T.A.; Heenan, S.; Mortimer, P.S. Lymphatic abnormalities demonstrated by lymphoscintigraphy after lower limb cellulitis. Br. J. Dermatol. 2008, 158, 1350–1353. [Google Scholar] [CrossRef]
- Valente, A.; Camacho, E.L.; Paiva, E.V. Lymphoscintigraphic evaluation in patients after erysipelas. Lymphology 2000, 33, 177–180. [Google Scholar]
- Jones, D.; Meijer, E.F.; Blatter, C.; Liao, S.; Pereira, E.R.; Bouta, E.M.; Jung, K.; Chin, S.M.; Huang, P.; Munn, L.L.; et al. Methicillin-resistant staphylococcus aureus causes sustained collecting lymphatic vessel dysfunction. Sci. Transl. Med. 2018, 10, eaam7964. [Google Scholar] [CrossRef]
- Nores, G.D.; Ly, C.L.; Savetsky, I.L.; Kataru, R.P.; Ghanta, S.; Hespe, G.E.; Rockson, S.G.; Mehrara, B.J. Regulatory T cells mediate local immunosuppression in lymphedema. J. Investig. Dermatol. 2018, 138, 325–335. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Scholl, J.; Dionyssiou, D.; Demiri, E.; Halin, C.; Dieterich, L.C.; Detmar, M. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. JCI Insight 2016, 1, e89081. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; Rutkowski, J.M.; Pasquier, M.; Kuan, E.L.; Alitalo, K.; Randolph, G.J.; Swartz, M.A. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 2012, 189, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Gardenier, J.C.; Hespe, G.E.; Nores, G.D.; Kataru, R.P.; Ly, C.L.; Martinez-Corral, I.; Ortega, S.; Mehrara, B.J. Lymph node transplantation decreases swelling and restores immune responses in a transgenic model of lymphedema. PLoS ONE 2016, 11, e0168259. [Google Scholar] [CrossRef] [PubMed]
- Gardenier, J.C.; Hespe, G.E.; Kataru, R.P.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.; Dayan, J.J.; Chang, D.; Zampell, J.; Martínez-Corral, I.; et al. Diphtheria toxin–mediated ablation of lymphatic endothelial cells results in progressive lymphedema. JCI Insight 2016, 1, e84095. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Rockson, S.G.; Jiang, X.; Kim, J.; Begaye, A.; Shuffle, E.M.; Tu, A.B.; Cribb, M.; Nepiyushchikh, Z.; Feroze, A.H.; et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci. Transl. Med. 2017, 9, eaal3920. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 2018, 3, e123775. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Holopainen, T.; Lyytikkä, J.; Kotronen, A.; Pitkonen, M.; Abo-Ramadan, U.; Ylä-Herttuala, S.; Petrova, T.V.; Alitalo, K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 2007, 13, 1458–1466. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Moya, M.; Johannes, J.; Goldman, J.; Swartz, M.A. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 2006, 72, 161–171. [Google Scholar] [CrossRef]
- Lähteenvuo, M.; Honkonen, K.; Tervala, T.; Tammela, T.; Suominen, E.; Lähteenvuo, J.; Kholová, I.; Alitalo, K.; Ylä-Herttuala, S.; Saaristo, A. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 2011, 123, 613–620. [Google Scholar] [CrossRef]
- A Phase I Study with Lymfactin® in the Treatment of Patients with Secondary Lymphedema. Available online: https://ClinicalTrials.gov/show/NCT02994771 (accessed on 2 January 2020).
- Nakamura, K.; Radhakrishnan, K.; Wong, Y.M.; Rockson, S.G. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS ONE 2009, 4, e8380. [Google Scholar] [CrossRef]
- Ubenimex in Adult Patients with Lymphedema of the Lower Limb (ULTRA). Available online: https://ClinicalTrials.gov/show/NCT02700529 (accessed on 2 January 2020).
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.Y.; Kataru, R.P.; Mehrara, B.J. Histopathologic Features of Lymphedema: A Molecular Review. Int. J. Mol. Sci. 2020, 21, 2546. https://doi.org/10.3390/ijms21072546
Li CY, Kataru RP, Mehrara BJ. Histopathologic Features of Lymphedema: A Molecular Review. International Journal of Molecular Sciences. 2020; 21(7):2546. https://doi.org/10.3390/ijms21072546
Chicago/Turabian StyleLi, Claire Y., Raghu P. Kataru, and Babak J. Mehrara. 2020. "Histopathologic Features of Lymphedema: A Molecular Review" International Journal of Molecular Sciences 21, no. 7: 2546. https://doi.org/10.3390/ijms21072546
APA StyleLi, C. Y., Kataru, R. P., & Mehrara, B. J. (2020). Histopathologic Features of Lymphedema: A Molecular Review. International Journal of Molecular Sciences, 21(7), 2546. https://doi.org/10.3390/ijms21072546





