Molecular Functionality of Plant Proteins from Low- to High-Solid Systems with Ligand and Co-Solute
Abstract
1. Introduction
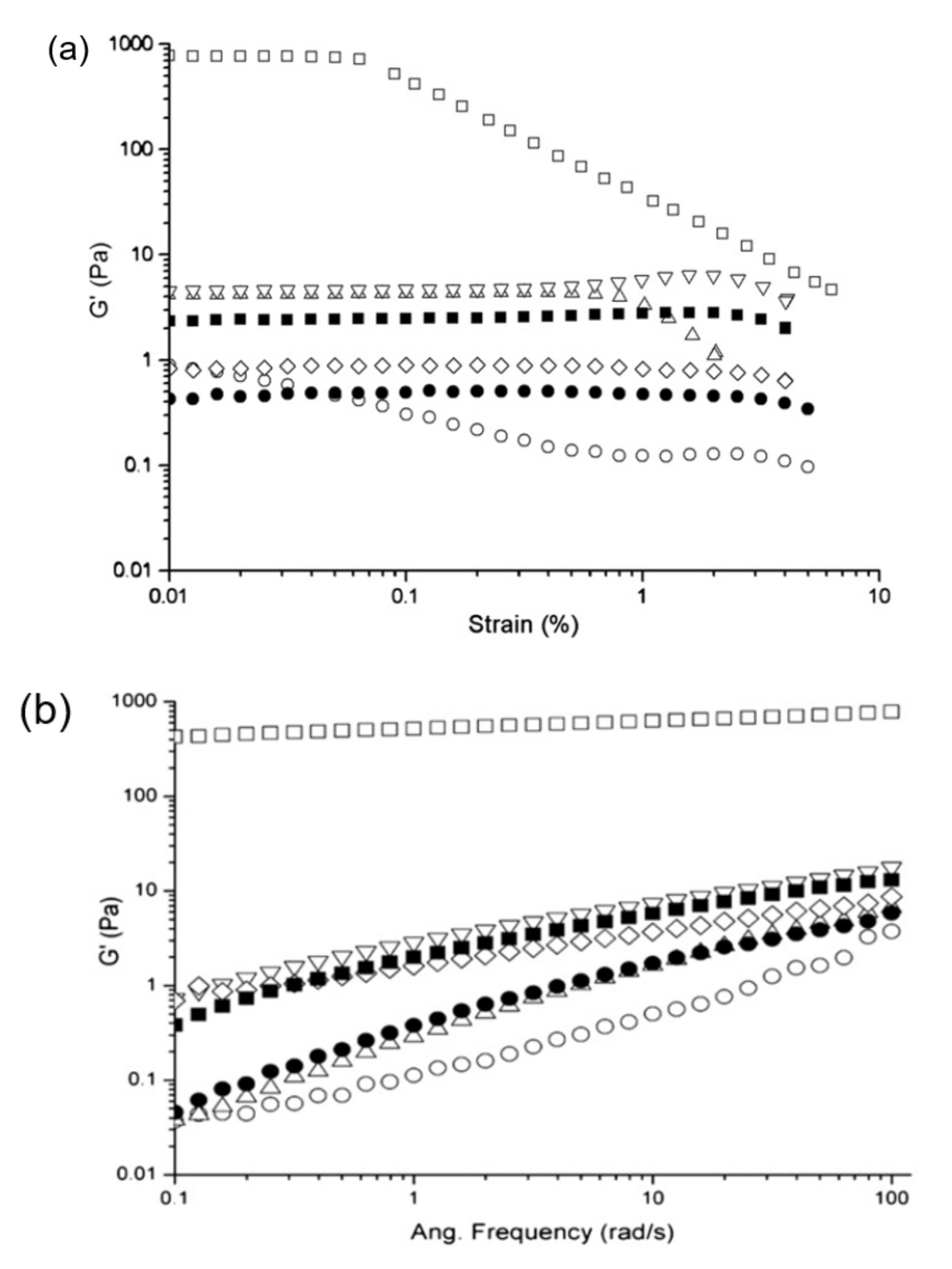
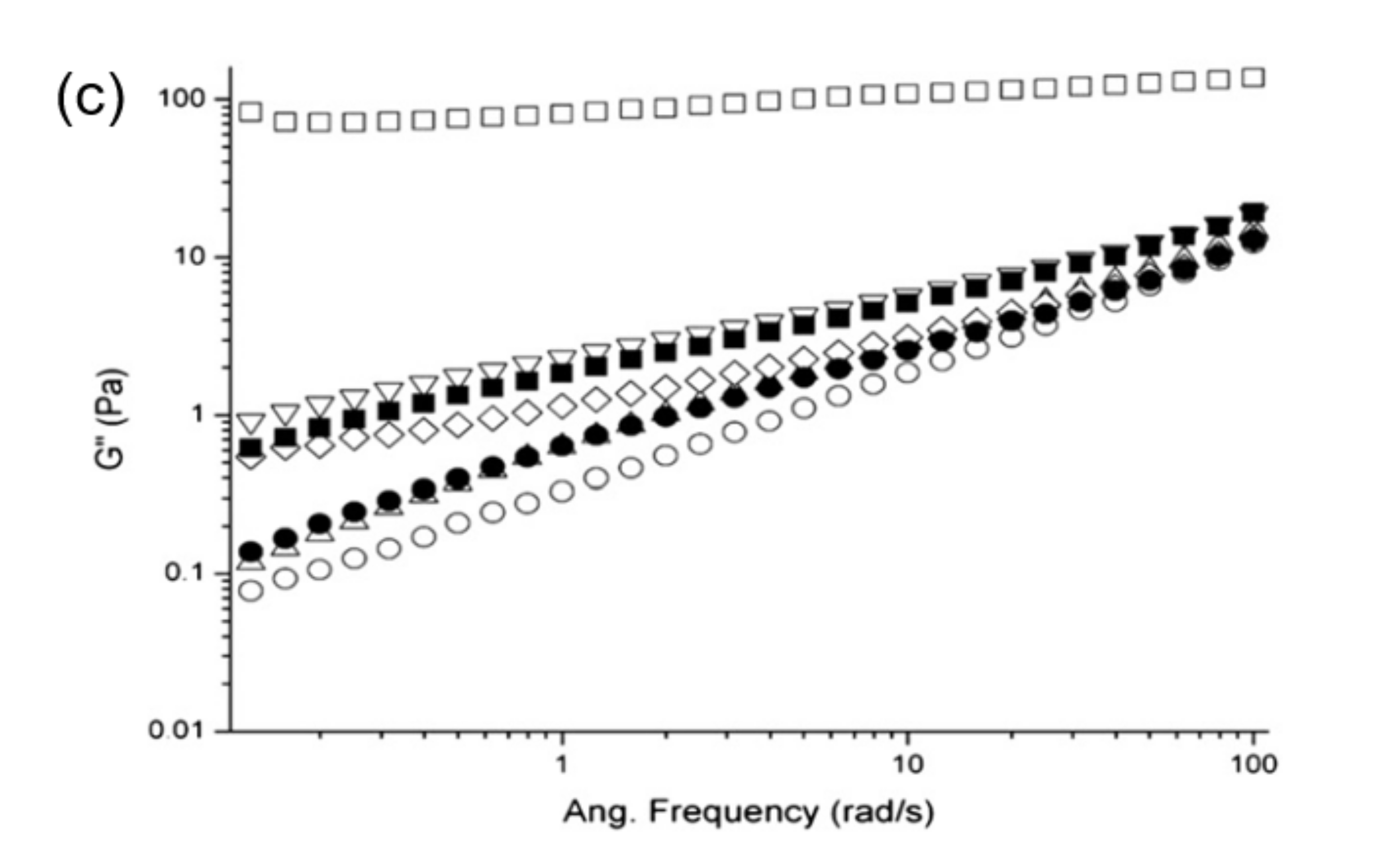
2. Structural Functionality of Plant Proteins from Low to High Solid Systems
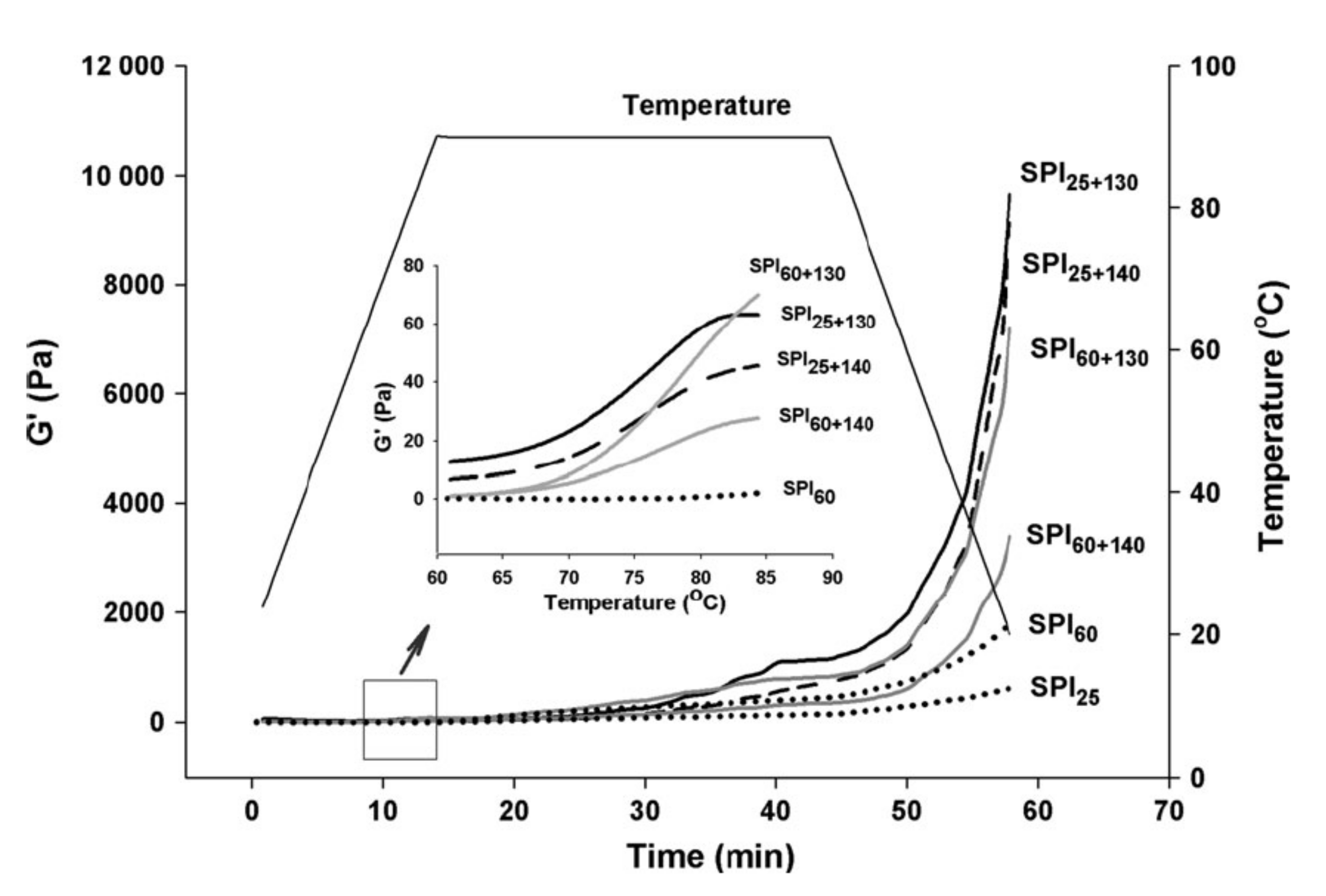
2.1. Effect of Heating
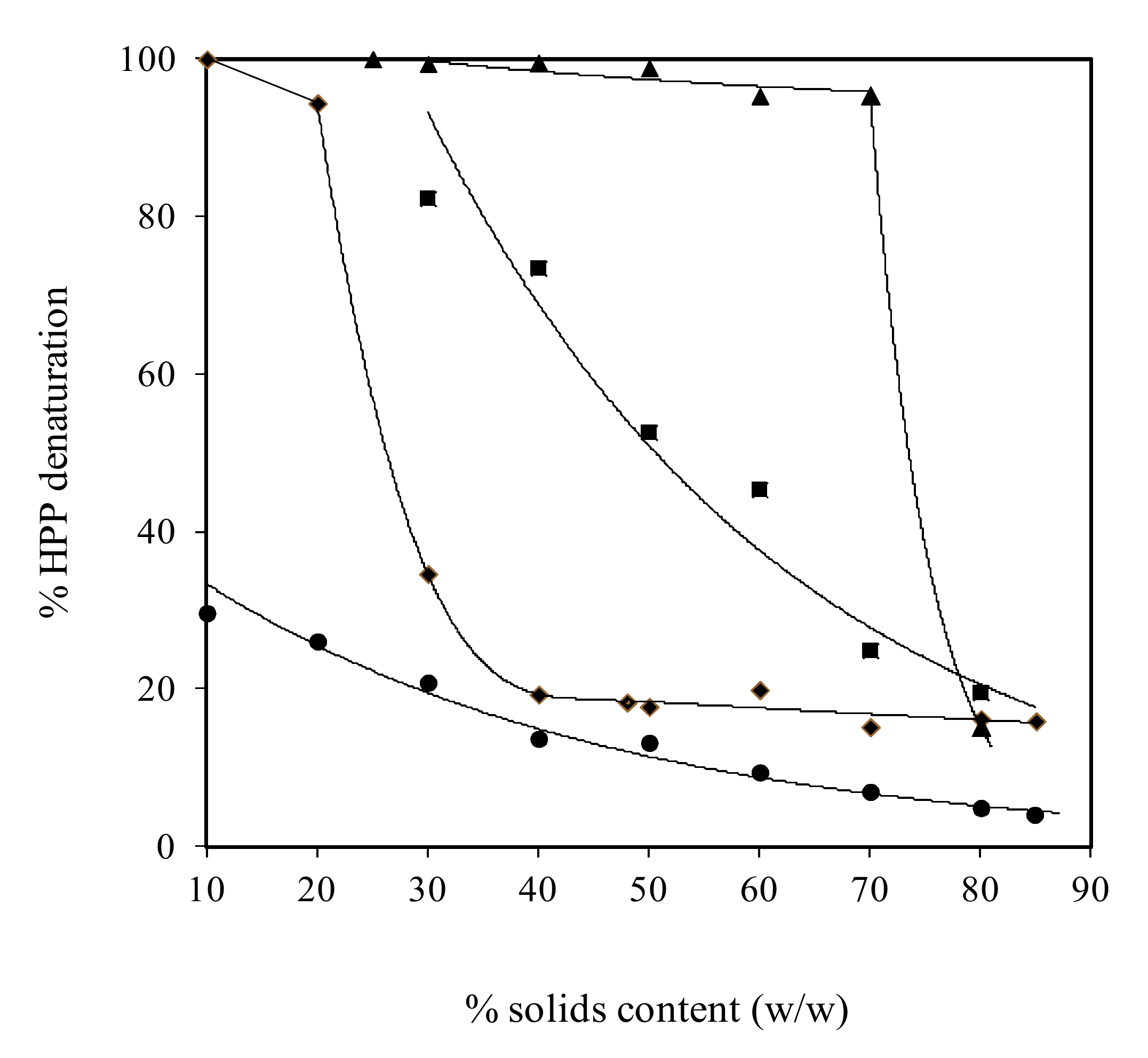
2.2. Effect of High Pressure
2.3. Effect of Ultra-High Temperature (UHT) Processing
2.4. Effect of Ultrasound Treatment
3. Protein-Ligand Interactions
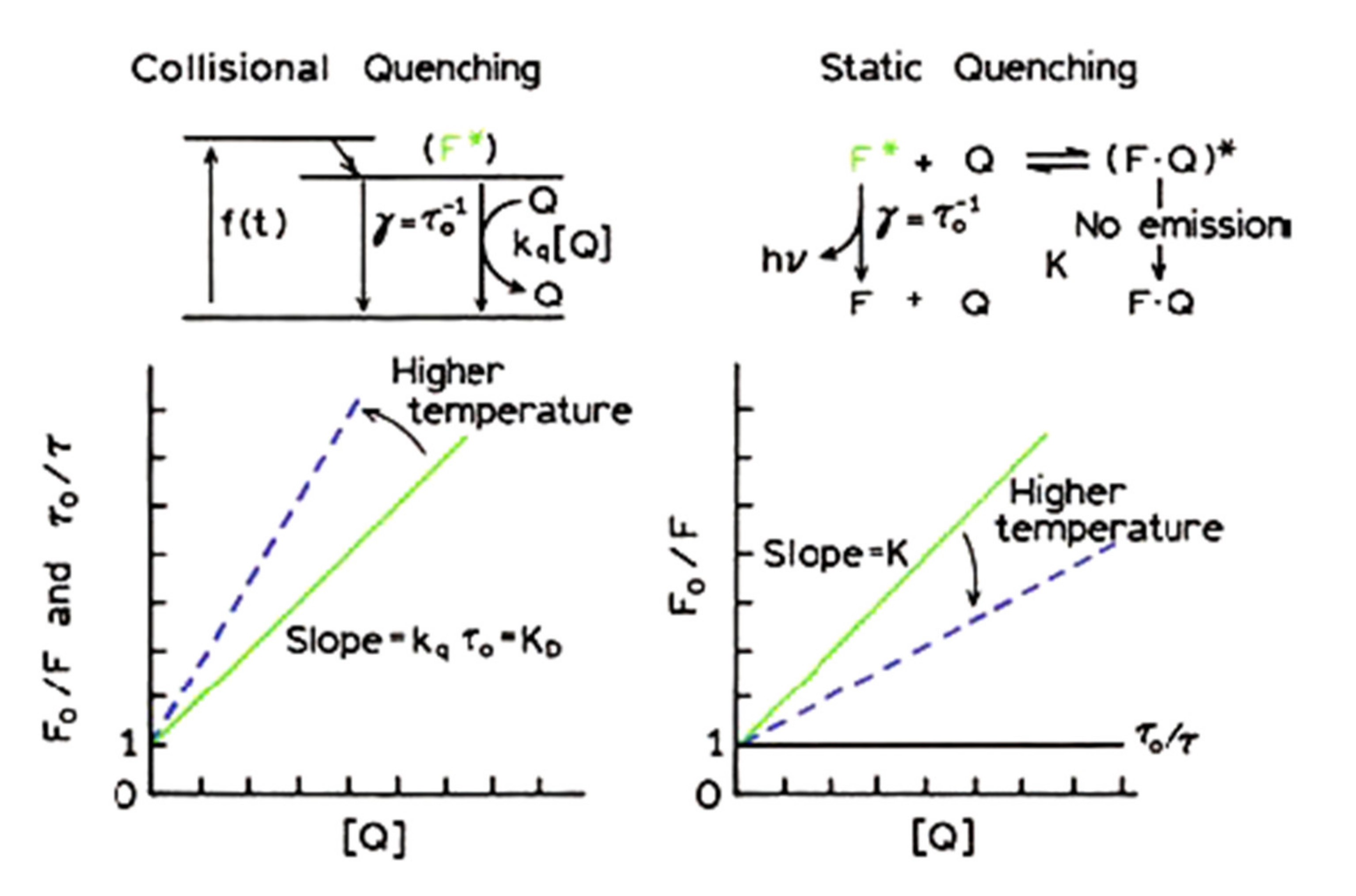
3.1. Overview of the Kinetic and Thermodynamic Approach on Protein-Ligand Interactions
- (1)
- ΔH > 0 and ΔS > 0, mainly hydrophobic/entropic forces
- (2)
- ΔH < 0 and ΔS < 0, van der Waals interactions and hydrogen bonds
- (3)
- ΔH < 0 and ΔS > 0, mainly electrostatic interactions
3.2. Pinpointing the Biding Sies with Molecular Docking Simulations
4. Transport Phenomena of Bioactive Compounds in Plant Protein Matrices
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biswas, A.K.; Mandal, P.K. Meat-borne Pathogens and Use of Natural Antimicrobials for Food Safety. In Foodborne Pathogens and Antibiotic Resistance; Singh, O.V., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; de Oliveira, B.A.P.; Barbin, R.; Marchini, J.S.; Junior, W.S.; Nonino, C.B. Red meat intolerance in patients submitted to gastric bypass: A 4-year follow-up study. Surg. Obes. Relat. Dis. 2015, 11, 842–846. [Google Scholar] [CrossRef] [PubMed]
- De Lima Binsfeld, B.; Pastorino, A.C.; Castro, A.P.B.; Yonamine, G.H.; Gushken, A.K.F.; Jacob, C.M.A. Knowledge of industrialized dairy products labels by parents of patients allergic to cow’s milk. Rev Paul Pediatr. 2009, 27, 296–302. [Google Scholar]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Johnston, S.P.; Nickerson, M.T.; Low, N.H. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J. Food Sci. Tech. 2015, 52, 4135–4145. [Google Scholar] [CrossRef]
- Condés, M.C.; Speroni, F.; Mauri, A.; Añón, M.C. Physicochemical and structural properties of amaranth protein isolates treated with high pressure. Innov. Food Sci. Emerg. Technol. 2012, 14, 11–17. [Google Scholar] [CrossRef]
- Jian, H.; Xiong, Y.L.; Guo, F.; Huang, X.; Adhikari, B.; Chen, J. Gelation enhancement of soy protein isolate by sequential low- and ultrahigh-temperature two-stage preheating treatments. Int. J. Food Sci. Technol. 2014, 49, 2529–2537. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Kasapis, S. High pressure effects on the structural functionality of condensed globular-protein matrices. Int. J. Biol. Macromol. 2016, 88, 433–442. [Google Scholar] [CrossRef]
- Verbeek, C.J.R.; van den Berg, L.E. Extrusion Processing and Properties of Protein-Based Thermoplastics. Macromol. Mater. Eng. 2010, 295, 10–21. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Bannikova, A.; Mantri, N.; Kasapis, S. Structural modification in condensed soy glycinin systems following application of high pressure. Food Hydrocoll. 2016, 53, 115–124. [Google Scholar] [CrossRef]
- Gueguen, J.; Viroben, G.; Noireaux, P.; Subirade, M. Influence of plasticizers and treatments on the properties of films from pea proteins. Ind. Crops Prod. 1998, 7, 149–157. [Google Scholar] [CrossRef]
- Choi, W.S.; Han, J.H. Film-forming mechanism and heat denaturation effects on the physical and chemical properties of pea-protein-isolate edible films. J. Food Sci. 2002, 67, 1399–1406. [Google Scholar] [CrossRef]
- Brückner-Gühmann, M.; Banovic, M.; Drusch, S. Towards an increased plant protein intake: Rheological properties, sensory perception and consumer acceptability of lactic acid fermented, oat-based gels. Food Hydrocoll. 2019, 96, 201–208. [Google Scholar] [CrossRef]
- Jain, A.; Prakash, M.; Radha, C. Extraction and evaluation of functional properties of groundnut protein concentrate. J. Food Sci. Technol. 2015, 52, 6655–6662. [Google Scholar] [CrossRef]
- Shi, W.; Dumont, M.-J. bio-based films from zein, keratin, pea, and rapeseed protein feedstocks. J. Mater. Sci. 2014, 49, 1915–1930. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef]
- Panyoyai, N.; Bannikova, A.; Small, D.M.; Shanks, R.A.; Kasapis, S. Diffusion of nicotinic acid in spray-dried capsules of whey protein isolate. Food Hydrocoll. 2016, 52, 811–819. [Google Scholar] [CrossRef]
- Teimouri, S.; Morrish, C.; Panyoyai, N.; Small, D.M.; Kasapis, S. Diffusion and relaxation contributions in the release of vitamin B6 from a moving boundary of genipin crosslinked gelatin matrices. Food Hydrocoll. 2019, 87, 839–846. [Google Scholar] [CrossRef]
- Assadpour, E.; Maghsoudlou, Y.; Jafari, S.-M.; Ghorbani, M.; Aalami, M. Optimization of folic acid nano-emulsification and encapsulation by maltodextrin-whey protein double emulsions. Int. J. Biol. Macromol. 2016, 86, 197–207. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Whey protein gels for the entrapment of bioactive anthocyanins from bilberry extract. Int. Dairy J. 2011, 21, 703–710. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yermand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with k-carrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Abbasi, A.; Emam-Djomeh, Z.; Mousavi, M.A.E.; Davoodi, D. Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate. Food Chem. 2014, 143, 379–383. [Google Scholar] [CrossRef]
- Liang, L.; Leung Sok Line, V.; Remondetto, G.E.; Subirade, M. In vitro release of α-tocopherol from emulsion-loaded β-lactoglobulin gels. Int. Dairy J. 2010, 20, 176–181. [Google Scholar] [CrossRef]
- Paramita, V.D.; Lo Piccolo, J.D.; Kasapis, S. Effect of co-solute concentration on the diffusion of linoleic acid from whey protein matrices. Food Hydrocoll. 2017, 70, 277–285. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chem. 1998, 75, 1–9. [Google Scholar] [CrossRef]
- Wan, Z.-L.; Guo, J.; Yang, X.-Q. Plant protein-based delivery systems for bioactive ingredients in foods. Food Funct. 2015, 6, 2876–2889. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Bannikova, A.; Kasapis, S. Glass Transition of Globular Proteins from Thermal and High Pressure Perspectives. In Glass Transition and Phase Transitions in Food and Biological Materials; Jasim, A., Ed.; Wiley: New York, NY, USA, 2017; pp. 49–117. [Google Scholar]
- Ma, L.; Cui, X.; Cai, W.; Shao, X. Understanding the function of water during the gelation of globular proteins by temperature-dependent near infrared spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 20132–20140. [Google Scholar] [CrossRef]
- Schmidt, R.H. Gelation and Coagulation. In Protein Functionality in Foods; American Chemical Society: Washington, DC, USA, 1981; Volume 147, pp. 131–147. [Google Scholar]
- Nicolai, T.; Chassenieux, C. Heat-induced gelation of plant globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Tay, S.L.; Kasapis, S.; Perera, C.O.; Barlow, P.J. Functional and Structural Properties of 2S Soy Protein in Relation to Other Molecular Protein Fractions. J. Agric. Food Chem. 2006, 54, 6046–6053. [Google Scholar] [CrossRef]
- Berghout, J.A.M.; Boom, R.M.; van der Goot, A.J. Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocoll. 2015, 43, 465–472. [Google Scholar] [CrossRef]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- German, B.; Damodaran, S.; Kinsella, J.E. Thermal dissociation and association behavior of soy proteins. J. Agric. Food Chem. 1982, 30, 807–811. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Koehler, P.; Arendt, E.K. Heat-denaturation and aggregation of quinoa (Chenopodium quinoa) globulins as affected by the pH value. Food Chem. 2016, 196, 17–24. [Google Scholar] [CrossRef]
- Wong, D.; Vasanthan, T.; Ozimek, L. Synergistic enhancement in the co-gelation of salt-soluble pea proteins and whey proteins. Food Chem. 2013, 141, 3913–3919. [Google Scholar] [CrossRef]
- Ersch, C.; ter Laak, I.; van der Linden, E.; Venema, P.; Martin, A. Modulating fracture properties of mixed protein systems. Food Hydrocoll. 2015, 44, 59–65. [Google Scholar] [CrossRef]
- Tay, S.L.; Kasapis, S.; Han, A.T.K. Phase model interpretation of the structural properties of two molecular soy protein fractions. J. Agric. Food Chem. 2008, 56, 2490–2497. [Google Scholar] [CrossRef]
- Silva, J.V.C.; Cochereau, R.; Schmitt, C.; Chassenieux, C.; Nicolai, T. Heat-induced gelation of mixtures of micellar caseins and plant proteins in aqueous solution. Food Res. Int. 2019, 116, 1135–1143. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Ruwaih, N.; Mulla, M.; Rahman, M.H. Effect of high pressure treatment on functional, rheological and structural properties of kidney bean protein isolate. LWT 2018, 91, 191–197. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N. Amaranth protein films prepared with high-pressure treated proteins. J. Food Eng. 2015, 166, 38–44. [Google Scholar] [CrossRef]
- Peyrano, F.; Speroni, F.; Avanza, M.V. Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2016, 33, 38–46. [Google Scholar] [CrossRef]
- He, X.-H.; Liu, H.Z.; Liu, L.; Zhao, G.L.; Wang, Q.; Chen, Q.-L. Effects of high pressure on the physicochemical and functional properties of peanut protein isolates. Food Hydrocoll. 2014, 36, 123–129. [Google Scholar] [CrossRef]
- Zhao, Z.-K.; Mu, T.-H.; Zhang, M.; Richel, A. Effect of salts combined with high hydrostatic pressure on structure and gelation properties of sweet potato protein. LWT 2018, 93, 36–44. [Google Scholar] [CrossRef]
- Manassero, C.A.; Vaudagna, S.R.; Añón, M.C.; Speroni, F. High hydrostatic pressure improves protein solubility and dispersion stability of mineral-added soybean protein isolate. Food Hydrocoll. 2015, 43, 629–635. [Google Scholar] [CrossRef]
- Puppo, C.; Chapleau, N.; Speroni, F.; de Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Wang, X.-S.; Tang, C.-H.; Li, B.-S.; Yang, X.-Q.; Li, L.; Ma, C.-Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R.; Khedkar, C.D.; Jana, A.H. UHT milk processing and effect of plasmin activity on shelf life: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 251–268. [Google Scholar] [CrossRef]
- Zheng, H.-G.; Yang, X.-Q.; Tang, C.-H.; Li, L.; Ahmad, I. Preparation of soluble soybean protein aggregates (SSPA) from insoluble soybean protein concentrates (SPC) and its functional properties. Food Res. Int. 2008, 41, 154–164. [Google Scholar] [CrossRef]
- Nagano, T.; Fukuda, Y.; Akasaka, T. Dynamic Viscoelastic Study on the Gelation Properties of β-Conglycinin-Rich and Glycinin-Rich Soybean Protein Isolates. J. Agric. Food Chem. 1996, 44, 3484–3488. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Bao Chau, N.H.; Trivedi, J.; Dissanayake, M.; Vasiljevic, T. Impact of selected process parameters on solubility and heat stability of pea protein isolate. LWT 2019, 102, 246–253. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Uniacke-Lowe, T.; O’Mahony, J.A.; Arendt, E.K. Physicochemical and acid gelation properties of commercial UHT-treated plant-based milk substitutes and lactose free bovine milk. Food Chem. 2015, 168, 630–638. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Cruz, N.S.; Capellas, M.; Jayamillo, D.P.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Soymilk treated by ultra high-pressure homogenization: Acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll. 2009, 23, 490–496. [Google Scholar] [CrossRef]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Zhang, C.; Kong, X.; Hua, Y. Heat-induced inactivation mechanisms of Kunitz trypsin inhibitor and Bowman-Birk inhibitor in soymilk processing. Food Chem. 2014, 154, 108–116. [Google Scholar] [CrossRef]
- Kwok, K.-C.; Niranjan, K. Review: Effect of thermal processing on soymilk. Int. J. Food Sci. Technol. 1995, 30, 263–295. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.C.; Liu, Z.; Xu, B. Elimination of Trypsin Inhibitor Activity and Beany Flavor in Soy Milk by Consecutive Blanching and Ultrahigh-Temperature (UHT) Processing. J. Agric. Food Chem. 2008, 56, 7957–7963. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.-C.; Liang, H.-H.; Niranjan, K. Optimizing Conditions for Thermal Processes of Soy Milk. J. Agric. Food Chem. 2002, 50, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Arora, A. Off-Flavor Precursors in Soy Protein Isolate and Novel Strategies for their Removal. Annu. Rev. Food Sci. Technol. 2013, 4, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard Alsoe, K.; Adler-Nissen, J. Thermal denaturation of lipoxygenase from soya beans in the presence of its natural substrate; Dénaturation thermique de la lipoxygénase de soja en présence de son substrat naturel. Leb. - Wiss. + Technol. 1988, 21, 8–12. [Google Scholar]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Alqahtani, N.K.; Ashton, J.; Katopo, L.; Gorczyca, E.; Kasapis, S. Shelf-life studies of flavour characteristics in model UHT liquid systems enriched with wholegrain oat. Heliyon 2018, 4, e00566. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, J.; Zhou, Y.; Huand, X.; Liu, F.; Pan, S.; Hu, H. Effect of high intensity ultrasound on physicochemical and functional properties of soybean glycinin at different ionic strengths. Innov. Food Sci. Emerg. Technol. 2016, 34, 205–213. [Google Scholar] [CrossRef]
- Flores-Jiménez, N.T.; Ulloa, J.A.; Silvas, J.E.U.; Ramírez, J.C.R.; Ulloa, P.R.; Rosales, P.U.B.; Camillo, Y.S.; Leyva, R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019, 121, 947–956. [Google Scholar]
- Hu, H.; Cheung, I.W.Y.; Pan, S.; Li-Chan, E.C.Y. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015, 45, 102–110. [Google Scholar]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Peressini, D.; Debeaufort, F.; Sensidoni, A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. Technol. 2010, 11, 451–457. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, C.Y.; Chen, G. Effect of ultrasound treatment on properties of soy proteins film. Appl. Mech. Mater. 2012, 117, 513–516. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, B.; Gao, Y.; Liu, J.; Gao, W.; Gai, X.; Bao, W. Process optimization of ultrasound-assisted treatment for soya bean protein isolate/polyacrylamide composite film. R. Soc. Open Sci. 2018, 5, 180213. [Google Scholar] [CrossRef]
- Lafarga, T.; Álvarez, C.; Bobo, G.; Aguiló-Aguayo, I. Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT 2018, 98, 106–112. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Park, M.; Beevers, J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016, 69, 77–84. [Google Scholar] [CrossRef]
- De Wolf, F.A.; Brett, G.M. Ligand-Binding Proteins: Their Potential for Application in Systems for Controlled Delivery and Uptake of Ligands. Pharmacol. Rev. 2000, 52, 207–236. [Google Scholar] [PubMed]
- Schreiber, G.; Keating, A.E. Protein binding specificity versus promiscuity. Curr. Opin. Struct. Biol. 2011, 21, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.; Schaffhausen, B.; Günther, U.L. Direct Observation of Protein−Ligand Interaction Kinetics. Biochemistry 2003, 42, 11128–11136. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Condict, L.; Kaur, J.; Hung, A.; Ashton, J.; Kasapis, S. Combined spectroscopic, molecular docking and quantum mechanics study of β-casein and ferulic acid interactions following UHT-like treatment. Food Hydrocoll. 2019, 89, 351–359. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, T.; Li, T.; Huang, K.; Li, Y.; Liu, C.; Chen, J. Investigation on the binding interaction between rice glutelin and epigallocatechin-3-gallate using spectroscopic and molecular docking simulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 215–222. [Google Scholar] [CrossRef]
- Rahimi Yazdi, S.; Corredig, M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry 1973, 12, 4171–4179. [Google Scholar] [CrossRef]
- Van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, T.; Huang, K.; Liang, L.; Liu, C.; Chen, J. Analyses on the binding interaction between rice glutelin and conjugated linoleic acid by multi-spectroscopy and computational docking simulation. J. Food Sci. Technol. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bronowska, A.K. Thermodynamics of ligand-protein interactions: Implications for molecular design. In Thermodynamics-Interaction Studies-Solids, Liquids and Gases; IntechOpen: London, UK, 2011. [Google Scholar]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Olsson, T.S.G.; Williams, M.A.; Pitt, W.R.; Ladbury, J.E. The Thermodynamics of Protein–Ligand Interaction and Solvation: Insights for Ligand Design. J. Mol. Biol. 2008, 384, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Chen, X.Q.; Jiang, X.Y.; Hilczer, M.; Tachiya, M. Probing the interaction of trans-resveratrol with bovine serum albumin: A fluorescence quenching study with Tachiya model. J. Fluoresc. 2008, 18, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Roche, D.B.; Brackenridge, D.A.; McGuffin, L.J. Proteins and their interacting partners: An introduction to protein–ligand binding site prediction methods. Int. J. Mol. Sci. 2015, 16, 29829–29842. [Google Scholar] [CrossRef]
- Tao, X.; Huang, Y.; Wang, C.; Chen, F.; Yang, L.; Ling, L.; Che, Z.; Chen, X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Roslak, D.; Belica, S.; den-Haan, H.; Pena-García, J.; Pérez-Sánchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT - Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Glaab, E. Building a virtual ligand screening pipeline using free software: A survey. Brief. Bioinform. 2016, 17, 352–366. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghante, M.H.; Ajgunde, B.R. Software based approaches for drug designing and development: A systematic review on commonly used software and its applications. Bull. Fac. Pharm. Cairo Univ. 2017, 5, 203–210. [Google Scholar]
- Blaszczyk, M.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. Protein–peptide docking using CABS-dock and contact information. Brief. Bioinform. 2019, 20, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Morency, L.P.; Gaudreault, F.; Najmanovich, R. Applications of the NRGsuite and the Molecular Docking Software FlexAID in Computational Drug Discovery and Design. In Computational Drug Discovery and Design; Humana Press: New York, NY, USA, 2018; pp. 367–388. [Google Scholar]
- Lee, H.; Seok, C. Template-based prediction of protein-peptide interactions by using GalaxyPepDock. In Modeling Peptide-Protein Interactions; Humana Press: New York, NY, USA, 2017; pp. 37–47. [Google Scholar]
- Jiménez-García, B.; Roel-Touris, J.; Romero-Durana, M.; Vidal, M.; Jiménez-González, D.; Fernández-Recio, J. LightDock: A new multi-scale approach to protein–protein docking. Bioinformatics 2018, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.S.; Gautham, N. MOLS 2.0: Software package for peptide modeling and protein–ligand docking. J. Mol. Model. 2016, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Li, R.; Liu, C.; Liu, W.; Li, T.; Chen, J.; Kharat, M.; McClements, D.J. Effect of rice glutelin-resveratrol interactions on the formation and stability of emulsions: A multiphotonic spectroscopy and molecular docking study. Food Hydrocoll. 2019, 97, 105234. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Chen, G.; Wang, S.; Feng, B.; Jiang, B.; Miao, M. Interaction between soybean protein and tea polyphenols under high pressure. Food Chem. 2019, 277, 632–638. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Stănciuc, N.; Grigore-Gurgu, L.; Aprodu, I. Investigation on the interaction of heated soy proteins with anthocyanins from cornelian cherry fruits. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118114. [Google Scholar] [CrossRef]
- Dai, T.; Yan, X.; Li, Q.; Li, T.; Liu, C.; McClements, D.J.; Chen, J. Characterization of binding interaction between rice glutelin and gallic acid: Multi-spectroscopic analyses and computational docking simulation. Food Res. Int. 2017, 102, 274–281. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Vesic, J.; Stambolic, I.; Apostolovic, D.; Milcic, M.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Complexes of green tea polyphenol, epigalocatechin-3-gallate, and 2S albumins of peanut. Food Chem. 2015, 185, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, F.; Gao, Y. Quercetagetin loaded in soy protein isolate–κ-carrageenan complex: Fabrication mechanism and protective effect. Food Res. Int. 2016, 83, 31–40. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Y.; Huang, G.; Liu, J.; Slavin, M.; Yu, L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K.; Siger, A.; Kachlicki, P.; Neunert, G.; lampart-Szczapa, E.; Nogala-Kalucka, M. Release of flavonoids from lupin globulin proteins during digestion in a model system. J. Agric. Food Chem. 2012, 60, 1830–1836. [Google Scholar] [CrossRef]
- Budryn, G.; Palecz, B.; Rachwal-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Belica, S.; Navano-Gonzáles, I.; Mesequer, J.M.; Pérez-Sánchez, H. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in β-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. [Google Scholar] [CrossRef]
- Ren, C.; Xiong, W.; Peng, D.; He, Y.; Zhou, P.; Li, J.; Li, B. Effects of thermal sterilization on soy protein isolate/polyphenol complexes: Aspects of structure, in vitro digestibility and antioxidant activity. Food Res. Int. 2018, 112, 284–290. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Guo, S. The binding mechanism of lecithin to soybean 11S and 7S globulins using fluorescence spectroscopy. Food Sci. Biotechnol. 2014, 23, 1785–1791. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Zhong, J.; Luo, L.; Liu, C.; Luo, S.; Chen, L. Binding interaction between rice glutelin and amylose: Hydrophobic interaction and conformational changes. Int. J. Biol. Macromol. 2015, 81, 942–950. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U. Microencapsulation of bioactive food ingredients and controlled release-A review. MOJ Food Process. Technol. 2016, 2, 00059. [Google Scholar]
- Paramita, V.; Kasapis, S. Molecular dynamics of the diffusion of natural bioactive compounds from high-solid biopolymer matrices for the design of functional foods. Food Hydrocoll. 2019, 88, 301–319. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Hernández-Munoz, P.; Gavara, R. Novel antimicrobial zein film for controlled release of lauroyl arginate (LAE). Food Hydrocoll. 2016, 61, 547–554. [Google Scholar] [CrossRef]
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Murial-Gallet, V.; Gavara, R.; Hernández-Munoz, P. Zein films and coatings as carriers and release systems of Zataria multiflora Boiss. essential oil for antimicrobial food packaging. Food Hydrocoll. 2017, 70, 260–268. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surfaces B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Wang, Q. Development of Zein Nanoparticles Coated with Carboxymethyl Chitosan for Encapsulation and Controlled Release of Vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Martinez-Abad, A.; Ocio, M.J.; Lagaron, J.M. Stabilization of a Nutraceutical Omega-3 Fatty Acid by Encapsulation in Ultrathin Electrosprayed Zein Prolamine. J. Food Sci. 2010, 75, N69–N79. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Development of flexible zein–wax composite and zein–fatty acid blend films for controlled release of lysozyme. Food Res. Int. 2013, 51, 208–216. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Lawaneta, E.; González-Navarro, C.J.; Gamazo, C.; Irache, J.M. Zein-Based Nanoparticles Improve the Oral Bioavailability of Resveratrol and Its Anti-inflammatory Effects in a Mouse Model of Endotoxic Shock. J. Agric. Food Chem. 2015, 63, 5603–5611. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Georget, D.M.R.; Barker, S.A.; Belton, P.S. A study on maize proteins as a potential new tablet excipient. Eur. J. Pharm. Biopharm. 2008, 69, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Lucio, D.; Martinez-Ohárriz, M.C.; Jaras, G.; Arancz, P.; González-Navarro, C.J.; Raduescu, A.; Irache, J.M. Optimization and evaluation of zein nanoparticles to improve the oral delivery of glibenclamide. In vivo study using C. elegans. Eur. J. Pharm. Biopharm. 2017, 121, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.A.; Alric, I.; Brouillet, F.; Peydecastaing, J.; Fullana, S.G.; Durrieu, V. Soy protein microparticles for enhanced oral ibuprofen delivery: Preparation, characterization, and in vitro release evaluation. AAPS PharmSciTech 2018, 19, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Tabletted soy protein cold-set hydrogels as carriers of nutraceutical substances. Food Hydrocoll. 2010, 24, 518–524. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Wang, T.; Zhang, B.; Wang, Q. Development and Application of Nanoparticles Synthesized with Folic Acid Conjugated Soy Protein. J. Agric. Food Chem. 2013, 61, 2556–2564. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Comparative study of encapsulation of vitamins with native and modified soy protein. Food Hydrocoll. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Alamilla-Beltrán, L.; Lartundo-Rojas, L.; de Perea-Flores, M.; Yánez-Fernández, J.; Palacois-González, E.; Gutiérrez-López, G.F. Chemical components distribution and morphology of microcapsules of paprika oleoresin by microscopy and spectroscopy. Food Hydrocoll 2018, 81, 6–14. [Google Scholar] [CrossRef]
- He, N.; Chen, S.; Wang, L.; Wen, J.; Li, Y.; Cao, Q.; Liu, Z.; Li, B. Fabrication of Composite Hydrogels Based on Soy Protein Isolate and their Controlled Globular Protein Delivery. Glob. Challenges 2019, 3, 1900030. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, S.; Wang, Y.; Wang, X.; Xu, N.; Jiang, L. Stability and in vitro digestion simulation of soy protein isolate-vitamin D3 nanocomposites. LWT 2020, 117, 108647. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.T.; Niakousari, M.; Hosseini, S.M.H.; Ohrani, B.; Lopez-Rubio, A. Active food packaging coatings based on hybrid electrospun gliadin nanofibers containing ferulic acid/hydroxypropyl-beta-cyclodextrin inclusion complexes. Nanomaterials 2018, 8, 919. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, P.; Balaguer, M.P.; Gomez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Chemically modified gliadins as sustained release systems for lysozyme. Food Hydrocoll. 2014, 41, 53–59. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Wang, R.; Chen, Z.; Zhong, Q. Self-emulsification of eugenol by modified rice proteins to design nano delivery systems for controlled release of caffeic acid phenethyl ester. RSC Adv. 2017, 7, 49953–49961. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Z.; Chen, L. Nano-encapsulations liberated from barley protein microparticles for oral delivery of bioactive compounds. Int. J. Pharm. 2011, 406, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.M.; Nunes, J.C.; Lima, B.N.; Pedrosa, C.; Calado, V.; Torres, A.; Pierucci, A.P. Effective stabilization of CLA by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Lu, L.; Unsworth, L.; Guan, L.L.; Chen, L. protein-shellac beads: Superior protection and delivery carriers for sensitive bioactive compounds. Food Hydrocoll. 2018, 77, 754–763. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Chien, K.B.; Chung, E.J.; Shah, R.N. Investigation of soy protein hydrogels for biomedical applications: Materials characterization, drug release, and biocompatibility. J. Biomater. Appl. 2013, 28, 1085–1096. [Google Scholar] [CrossRef]
- Paramita, V.D.; Kasapis, S. The role of structural relaxation in governing the mobility of linoleic acid in condensed whey protein matrices. Food Hydrocoll. 2018, 76, 184–193. [Google Scholar] [CrossRef]
- Mehrer, H. Random Walk Theory and Atomic Jump Process. Diffus. Solids Fundam. Methods Mater. Diffus. Process. 2007, 155, 55–67. [Google Scholar]
- Chalier, P.; Ben Arfa, A.; Guillard, V.; Gontard, N. Moisture and Temperature Triggered Release of a Volatile Active Agent from Soy Protein Coated Paper: Effect of Glass Transition Phenomena on Carvacrol Diffusion Coefficient. J. Agric. Food Chem. 2009, 57, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Paramita, V.D.; Kasapis, S. Rate of fatty acid transport in glassy biopolymers: A free volume based predictive approach. Food Hydrocoll. 2018, 78, 128–131. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Paramita, V.D.; Teimouri, S.; Young, S.; Kasapis, S. Controlled release of ascorbic acid from genipin-crosslinked gelatin matrices under moving boundary conditions. Food Hydrocoll. 2019, 89, 171–179. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Guo, J.; He, Z.; Wu, S.; Zeng, M.; Chen, J. Effects of concentration of flavor compounds on interaction between soy protein isolate and flavor compounds. Food Hydrocoll. 2020, 100, 105388. [Google Scholar] [CrossRef]
- Dórame-Miranda, R.F.; Rodriguez-Félix, D.E.; López-Ahumada, G.A.; Castro-Enriquez, D.D.; Quiroz-Castillo, J.M.; Márquez-Ríos, F.; Rodriguez-Félix, F. Effect of pH and temperature on the release kinetics of urea from wheat-gluten membranes obtained by electrospinning. Polym. Bull. 2018, 75, 5305–5319. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef]

| Protein | Ligand | Method | Main Findings | References |
|---|---|---|---|---|
| a. Polyphenols | ||||
| Soy glycinin and soy trypsin inhibitor | Chlorogenic acid, caffeic acid, gallic acid, flavonoids, flavone, apigenin, kaempferol, quercetin and myricetin | FUV-CD IF FLQ DSC | -Secondary and tertiary structure of protein changed (protein surface became more hydrophilic) -Formation of new non-covalent forces (inter- and intramolecular interactions, e.g. ionic/hydrogen bonds) by the introduction of the carboxylic groups (following the covalent attachment of the phenolic acids) and by the hydroxy groups (in flavonoids) with protein, for all samples but flavone, apigenin and kaempferol | [112] |
| Soybean protein | Tea polyphenol | IF CD FS MDS | -High pressure treatment (400 MPa) increased β-sheet and reduced α-helix and polyphenol protex helix structure -Interactions were mostly hydrophobic and hydrogen bounding with polyphenol binding to the 7s and 11s protein fractions | [113] |
| Glycinin, β-conglycinin and soy protein isolate | Anthocyanins (mainly CYG and CYR) | FS MDS | -The Trp residue of soy protein shifted to a more hydrophilic environment due to protein-ligand interaction -Glycinin has higher affinity towards CYG and CYR compared to β-conglycinin, but native β-conglycinin can bind one CYG and three CYR molecules simultaneously, while other soy proteins can accommodate one ligand only | [114] |
| Rice glutelin | Resveratrol | CD FS MDS | Binding of resveratrol to protein was spontaneous and mainly driven by hydrophobic interactions | [111] |
| Rice glutelin | Gallic acid | CD FS MDS | The hydrogen bonds and van der Waals forces were the main factors affecting protein-ligand interactions which led to conformational changes in the protein structure | [115] |
| Rice glutelin | EGCG | CD FS MDS | Hydrogen bonding and hydrophobic associations cause changes in secondary structure of protein | [90] |
| Zein | EGCG, Quercetagetin and chlorogenic acid | FUV-CD FS DSC | Secondary and tertiary structure of protein changed depending on nature of interaction (covalent and non-covalent) | [116] |
| Zein-BSA-CA conjugate | Resveratrol | FTIR | Protein-resveratrol interactions occur via hydrogen bonds, hydrophobic interactions, or electrostatic interactions | [117] |
| 2S albumins in peanuts | EGCG | CD FLQ ITC | Complex formation followed by change in protein secondary structure | [118] |
| b. Flavonoids | ||||
| Soy protein isolate–κ-carrageen | Quercetagetin | CD | -Interaction between the quercetagetin and protein was through the hydrophobic interaction hydrogen bonding | [119] |
| Zein-CAS NPs | Curcumin | DSC FTIR | Zein-CAS NPs interacts with curcumin via hydrogen bonding and hydrophobic interactions | [120] |
| Lupin seed globulin | Flavonoid (apigenin glycosides) | SSF FL | -Lupin seed globulins bind with phenolic compounds through electrostatic attraction or hydrogen bonding. Pepsin digestion caused release of apigenin glycosides (mainly 7-O-β-apiofuranosyl- 6,8-di-C-β-glucopyranoside) | [121] |
| c. Hydroxycinnamic acid and chlorogenic acids | ||||
| Soy protein isolate | HCA (caffeic, ferulic acids), and CHA (chlorogenic acids) from green coffee | ITC MDS | -Significant proportion of HCA and CHA were bound to proteins through electrostatic, hydrogen bonds and hydrophobic interactions -pH affected binding affinity of ligand to soy protein, reduced as pH beyond pI (4.5) -Complexed ligands with β-cyclodextrin limit their binding to protein | [122] |
| Soy protein hydrolysates | HCA from green coffee | ITC MDS | -The interactions were mostly hydrogen bonds and electrostatic forces being largely stable under proteolytic digestion -Complex ligands with β-cyclodextrin limit their binding to protein hydrolysates | [103] |
| d. Others | ||||
| Soy protein isolate | Black soybean seed coat extract | CD FS | -heat treatment led to protein unfolding and enhanced the binding capacity of protein with polyphenols through hydrophobic interaction | [123] |
| Soybeen 11s and 7S globulin | Lecithin | FS | -Lecithin changed protein structure and enhanced protein hydrophilicity with the effect being more pronounced for 11S compared to 7S protein -Hydrophobic interaction was the major force affected binding of lecithin to 11S and 7S protein | [124] |
| Rice glutelin | Conjugated linoleic acid | CD FS MDS | -Binding of conjugated linoleic acid to rice glutelin was spontaneous and mainly driven by hydrogen bonds -Fatty acid interaction led to change in secondary structure of protein | [95] |
| Rice glutelin | Amylose | CD FS MDS | -Rice glutelin bound with amylose through hydrophobic interactions -Secondary structure of protein changed due to binding of the amylose fraction | [125] |
| Protein | Bioactive Compound | Geometry | Bioactive Release Mechanism | Reference |
|---|---|---|---|---|
| Zein-chitosan complex | α-tocopherol, | NP | Burst release within 1.5 h | [131] |
| Zein- CMCh | Vitamin D3 | NP | Burst release within 1.5 h | [132] |
| Zein | Docosahexaenoic acid (DHA) | UTC | Not specified | [133] |
| Zein | LysozymeCatechin | F | Burst release within 1 h | [134] |
| Zein | Resveratrol | NP | Fickian diffusion within 1 h and erosion/relaxation release process after 3.5 h | [135] |
| Zein | Essential oil | F | Fickian diffusion | [130] |
| Zein-BSA-CA conjugates | Resveratrol | NP | Not specified | [117] |
| Zein-caseinate composite | Curcumin | NP | Burst release within 10 min | [120] |
| Zein | Curcumin | EF | Fickian diffusion | [136] |
| Zein/SSPS | Lutein | NP | Not specified | [137] |
| Zein | Theophylline * | T | Mostly Fickian diffusion with contribution of matrix relaxation based on Peppas-Sahlin equation | [138] |
| Zein | Glibenclamide * | NP | Fickian diffusion | [139] |
| Zein | Gentamicin * | MM | Fickian diffusion | [140] |
| Soy Protein | Ibuprofen * | MP | pH sensitive release | [141] |
| Soy Protein | Riboflavin | HG | Fickian diffusion | [142] |
| Conjugated soy protein-Folic Acid | Curcumin | NP | Burst effect within 1 h | [143] |
| Soy Protein | α-Tocopherol or Ascorbic acid | MC | Not specified | [144] |
| Soy Protein Isolate | Paprika oleoresin | MC | Not specified | [145] |
| Soy Protein | Bovine serum albumin | HG | Not specified | [146] |
| Soy protein isolate and SPI-CMCh | Vitamin D3 | NCS | Fast release within 1 h | [147] |
| Gliadin | Ferulic acid with hydroxypropyl- β-cyclodextrin | EF | Burst release within 10 min | [148] |
| Wheat Gliadin | Lysozyme | F | Fickian short time diffusion | [149] |
| Modified rice Proteins with eugenol | Caffeic acid phenethyl ester | NC | First-order release, burst release within 3 h | [150] |
| Barley Glutelin Crosslinked Glutaraldehyde | β-Carotene | MC | Zero-order release kinetics following enzymatic degradation | [151] |
| Pea Protein | Conjugated linoleic acid | MC | Not specified | [152] |
| Oat protein isolate | Riboflavin | B | Non-Fickian transport | [153] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramita, V.D.; Panyoyai, N.; Kasapis, S. Molecular Functionality of Plant Proteins from Low- to High-Solid Systems with Ligand and Co-Solute. Int. J. Mol. Sci. 2020, 21, 2550. https://doi.org/10.3390/ijms21072550
Paramita VD, Panyoyai N, Kasapis S. Molecular Functionality of Plant Proteins from Low- to High-Solid Systems with Ligand and Co-Solute. International Journal of Molecular Sciences. 2020; 21(7):2550. https://doi.org/10.3390/ijms21072550
Chicago/Turabian StyleParamita, Vilia Darma, Naksit Panyoyai, and Stefan Kasapis. 2020. "Molecular Functionality of Plant Proteins from Low- to High-Solid Systems with Ligand and Co-Solute" International Journal of Molecular Sciences 21, no. 7: 2550. https://doi.org/10.3390/ijms21072550
APA StyleParamita, V. D., Panyoyai, N., & Kasapis, S. (2020). Molecular Functionality of Plant Proteins from Low- to High-Solid Systems with Ligand and Co-Solute. International Journal of Molecular Sciences, 21(7), 2550. https://doi.org/10.3390/ijms21072550




