Role of microRNAs in Venous Thromboembolism
Abstract
1. Introduction
2. Epidemiological Studies: miRNAs and Venous Thromboembolism
Methodological Challenges and Future Perspectives in Epidemiological Research on miRNAs and Venous Thromboembolism
| First Author (Year) | Country | Study Population | Main Findings miRNA Expression Levels in Patients with VTE Versus Subjects without VTE | Potential Role of miRNA as Biomarker |
|---|---|---|---|---|
| Xiao et al. (2011) [43] | China | 32 acute PE patients, mean age 54.8 ± 16.2 years, 47% men 22 non-acute PE patients, mean age 62.3 ± 23.3 years, 45% men 1 32 healthy controls, NA: age and sex | ↑ miR-134 | Diagnostic biomarker for PE |
| Qin et al. (2015) [44] | China | 18 acute postoperative DVT patients, mean age 69.4 ± 8.1 years, 28% men 20 postoperative control subjects, mean age 67.6 ± 7.2 years, 20% men | ↑ miR-582, ↑ miR-195, ↑ miR-532 | Diagnostic biomarker for DVT |
| Starikova et al. (2015) [54] | Norway | 20 patients with a history of first unprovoked VTE (1–5 years prior to inclusion in the study), mean age 56.4 ± 14.8 years, 50% men 20 healthy controls, mean age 56.3 ± 14.4 years, 50% men | ↑ miR-10b-5p, ↑ miR-320a, ↑ miR-320b, ↑ miR−424-5p, ↑ miR−423-5p ↓ miR-103a-3p, ↓ miR−191-5p, ↓ miR−301a-3p, ↓ miR-199b-3p | Predictive biomarker for unprovoked VTE |
| Wang et al. (2016) [45] | Sweden | 53 patients with DVT, mean age 59.8 ± 19.1 years, 40% men 185 patients without DVT, mean age 58.1 ± 16.8 years, 38% men | ↑ miR-424-5p, ↓ miR-136–5p | Diagnostic biomarker for DVT |
| Kessler et al. (2016) [46] | Germany | 30 acute PE patients, mean age 62.0 ± 14.0 years, 57% men 30 acute non-ST-segment elevation myocardial infarction patients, mean age 64.0 ± 13.0 years, 57% men 12 healthy controls, mean age 31.0 ± 6.0 years, 50% men | ↑ miR-1233 | Diagnostic biomarker for PE |
| Zhou et al. (2016) [47] | China | 37 PE patients, mean age 42.0 ± 11.0 years, 57% men 37 healthy controls, mean age 41.0 ± 8.0 years, 54% men | ↑ miR-28-3p | Diagnostic biomarker for PE |
| Sahu et al. (2017) [27] | India | 20 VTE patients, median age 31.5 years, 100% men 20 controls, NA: age and sex | ↓ miR-145 | Diagnostic biomarker for VTE |
| Li et al. (2017) [49] | China | 45 DVT patients with bone trauma, mean age 53 ± 8.6 years, 60% men 40 healthy controls, NA: age and sex | ↓ miR-26a | Diagnostic biomarker for DVT |
| Wang et al. (2018) [50] | China | 78 acute PE patients, mean age 61.0 ± 11.9 years, NA: sex 70 controls, mean age 62.0 ± 10.2 years, NA: sex | ↑ miR-27a, ↑ miR-27b | Diagnostic biomarker for PE |
| Jiang et al. (2018) [51] | China | 30 DVT patients, mean age 52.6 ± 15.4 years, 53% men 30 healthy controls, mean age 51.6 ± 12.7 years, 67% men | ↑ miR-320a, ↑ miR-320b | Diagnostic biomarker for DVT |
| Liu et al. (2018) [48] | China | 60 acute PE patients, mean age 55.8 ± 7.5 years, 58% men 50 healthy controls, mean age 55.2 ± 7.0 years, 56% men | ↑ miR-221 | Diagnostic biomarker for PE |
| Sun et al. (2020) [52] | China | 81 acute DVT patients, mean age 45.5 ± 9.1 years, 40% men 20 healthy controls, mean age 44.5 ± 6.8 years, 40% men | ↓ miR-103a-3p | Diagnostic biomarker for DVT |
| Wang et al. (2019) [55] | Sweden | 39 VTE patients with recurrent VTE (cases), median age 65.3 (IQR 11.7), 59% men 39 VTE patients without recurrent VTE (controls), median age 65.1 (IQR 11.9), 59% men | VTE patients with recurrence versus VTE patients without recurrence: ↑ miR-15b-5p, ↑ miR-222-3p, ↑ miR-26b-5p, ↑ miR-532-5p, ↑ miR-21-5p, ↑ miR-30c-5p, ↑ miR-146b-5p, ↑ miR-22-3p ↓ miR-106a-5p, ↓ miR-197-3p, ↓ miR-652-3p, ↓ miR-361-5p, ↓ miR-27b-3p, ↓ miR-103a-3p | Predictive biomarker for VTE recurrence |
| Zhang et al. (2020) [53] | China | 36 DVT patients with symptom duration ≤ 21 days, mean age 57.3 ± 9.9 years, 47% men 36 healthy controls, mean age 54.1 ± 8.7 years, 44% men | ↓ miR-338-5p | Diagnostic biomarker for DVT |
3. Modulation of miRNA Activity in Animal Models of Venous Thrombosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef]
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: A major contributor to the global disease burden. J. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef]
- Schulman, S.; Lindmarker, P.; Holmström, M.; Lärfars, G.; Carlsson, A.; Nicol, P.; Svensson, E.; Ljungberg, B.; Viering, S.; Nordlander, S.; et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J. Thromb. Haemost. 2006, 4, 734–742. [Google Scholar] [CrossRef]
- Arshad, N.; Bjøri, E.; Hindberg, K.; Isaksen, T.; Hansen, J.B.; Braekkan, S.K. Recurrence and mortality after first venous thromboembolism in a large population-based cohort. J. Thromb. Haemost. 2017, 15, 295–303. [Google Scholar] [CrossRef]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Huang, W.; Goldberg, R.J.; Anderson, F.A.; Kiefe, C.I.; Spencer, F.A. Secular trends in occurrence of acute venous thromboembolism: The Worcester VTE study (1985–2009). Am. J. Med. 2014, 127, 829–839.e5. [Google Scholar] [CrossRef]
- Arshad, N.; Isaksen, T.; Hansen, J.B.; Brækkan, S.K. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur. J. Epidemiol. 2017, 32, 299–305. [Google Scholar] [CrossRef]
- Munster, A.M.; Rasmussen, T.B.; Falstie-Jensen, A.M.; Harboe, L.; Stynes, G.; Dybro, L.; Hansen, M.L.; Brandes, A.; Grove, E.L.; Johnsen, S.P. A changing landscape: Temporal trends in incidence and characteristics of patients hospitalized with venous thromboembolism 2006–2015. Thromb. Res. 2019, 176, 46–53. [Google Scholar] [CrossRef]
- Stein, P.D.; Hull, R.D.; Kayali, F.; Ghali, W.A.; Alshab, A.K.; Olson, R.E. Venous thromboembolism according to age: The impact of an aging population. Arch. Intern. Med. 2004, 164, 2260–2265. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.L.; Schmittgen, T.D.; et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Kirschner, M.B.; van Zandwijk, N.; Reid, G. Cell-free microRNAs: Potential biomarkers in need of standardized reporting. Front. Genet. 2013, 4, 56. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Tiao, J.; Hughes, Q.; Jorritsma, J.; Gilmore, G.; Baker, R. Circulating MicroRNA as thrombosis sentinels: Caveats and considerations. Semin. Thromb. Hemost. 2018, 44, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.B.; de Los Reyes-García, A.M.; Teruel-Montoya, R.; Vicente, V.; González-Conejero, R.; Martínez, C. microRNAs in the haemostatic system: More than witnesses of thromboembolic diseases? Thromb. Res. 2018, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fort, A.; Borel, C.; Migliavacca, E.; Antonarakis, S.E.; Fish, R.J.; Neerman-Arbez, M. Regulation of fibrinogen production by microRNAs. Blood 2010, 116, 2608–2615. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Ren, J.; Geng, Q.; Song, J.; Lee, C.; Cao, C.; Zhang, J.; Xu, N. MicroRNA-223 inhibits tissue factor expression in vascular endothelial cells. Atherosclerosis 2014, 237, 514–520. [Google Scholar] [CrossRef]
- Sahu, A.; Jha, P.K.; Prabhakar, A.; Singh, H.D.; Gupta, N.; Chatterjee, T.; Tyagi, T.; Sharma, S.; Kumari, B.; Singh, S.; et al. MicroRNA-145 impedes thrombus formation via targeting tissue factor in venous thrombosis. EBioMedicine 2017, 26, 175–186. [Google Scholar] [CrossRef]
- Eisenreich, A.; Raugh, U. Regulation of the tissue factor isoform expression and thrombogenicity of HMEC-1 by miR-126 and miR-19a. Cell Biol. Res. Ther. 2013, 2. [Google Scholar] [CrossRef]
- Li, S.; Ren, J.; Xu, N.; Zhang, J.; Geng, Q.; Cao, C.; Lee, C.; Song, J.; Li, J.; Chen, H. MicroRNA-19b functions as potential anti-thrombotic protector in patients with unstable angina by targeting tissue factor. J. Mol. Cell. Cardiol. 2014, 75, 49–57. [Google Scholar] [CrossRef]
- Witkowski, M.; Weithauser, A.; Tabaraie, T.; Steffens, D.; Kränkel, N.; Witkowski, M.; Stratmann, B.; Tschoepe, D.; Landmesser, U.; Rauch-Kroehnert, U. Micro-RNA-126 reduces the blood thrombogenicity in diabetes mellitus via targeting of tissue factor. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1263–1271. [Google Scholar] [CrossRef]
- Salloum-Asfar, S.; Teruel-Montoya, R.; Arroyo, A.B.; García-Barberá, N.; Chaudhry, A.; Schuetz, E.; Luengo-Gil, G.; Vicente, V.; González-Conejero, R.; Martínez, C. Regulation of coagulation factor XI expression by microRNAs in the human liver. PLoS ONE 2014, 9, e111713. [Google Scholar] [CrossRef] [PubMed]
- Sennblad, B.; Basu, S.; Mazur, J.; Suchon, P.; Martinez-Perez, A.; van Hylckama Vlieg, A.; Truong, V.; Li, Y.; Gådin, J.R.; Tang, W.; et al. Genome-wide association study with additional genetic and post-transcriptional analyses reveals novel regulators of plasma factor XI levels. Hum. Mol. Genet. 2017, 26, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.W.; Romeo, G.; Hughes, Q.W.; Baker, R.I. Micro-ribonucleic Acid 494 regulation of protein S expression. J. Thromb. Haemost. 2013, 11, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.O.; Arroyo, A.B.; González-Conejero, R.; Stavik, B.; Iversen, N.; Sandset, P.M.; Martínez, C.; Skretting, G. The role of microRNA-27a/b and microRNA-494 in estrogen-mediated downregulation of tissue factor pathway inhibitor alpha. J. Thromb. Haemost. 2016, 14, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Proust, C.; Morange, P.E.; Lompré, A.M.; Trégouët, D.A. miR-421 and miR-30c inhibit SERPINE 1 gene expression in human endothelial cells. PLoS ONE 2012, 7, e44532. [Google Scholar] [CrossRef]
- Patel, N.; Tahara, S.M.; Malik, P.; Kalra, V.K. Involvement of miR-30c and miR-301a in immediate induction of plasminogen activator inhibitor-1 by placental growth factor in human pulmonary endothelial cells. Biochem. J. 2011, 434, 473–482. [Google Scholar] [CrossRef]
- Villadsen, S.B.; Bramsen, J.B.; Ostenfeld, M.S.; Wiklund, E.D.; Fristrup, N.; Gao, S.; Hansen, T.B.; Jensen, T.I.; Borre, M.; Ørntoft, T.F.; et al. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br. J. Cancer 2012, 106, 366–374. [Google Scholar] [CrossRef]
- Nourse, J.; Braun, J.; Lackner, K.; Hüttelmaier, S.; Danckwardt, S. Large-scale identification of functional microRNA targeting reveals cooperative regulation of the hemostatic system. J. Thromb. Haemost. 2018, 16, 2233–2245. [Google Scholar] [CrossRef]
- De Los Reyes-García, A.M.; Arroyo, A.B.; Teruel-Montoya, R.; Vicente, V.; Lozano, M.L.; González-Conejero, R.; Martínez, C. MicroRNAs as potential regulators of platelet function and bleeding diatheses. Platelets 2019, 30, 803–808. [Google Scholar] [CrossRef]
- Wells, P.S.; Owen, C.; Doucette, S.; Fergusson, D.; Tran, H. Does this patient have deep vein thrombosis? JAMA 2006, 295, 199–207. [Google Scholar] [CrossRef]
- Verhovsek, M.; Douketis, J.D.; Yi, Q.; Shrivastava, S.; Tait, R.C.; Baglin, T.; Poli, D.; Lim, W. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann. Intern. Med. 2008, 149, 481–490. [Google Scholar] [CrossRef]
- Lippi, G.; Bonfanti, L.; Saccenti, C.; Cervellin, G. Causes of elevated D-dimer in patients admitted to a large urban emergency department. Eur. J. Intern. Med. 2014, 25, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jing, Z.C.; Ellinor, P.T.; Liang, D.; Zhang, H.; Liu, Y.; Chen, X.; Pan, L.; Lyon, R.; Liu, Y.; et al. MicroRNA-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. J. Transl. Med. 2011, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liang, H.; Shi, D.; Dai, J.; Xu, Z.; Chen, D.; Chen, X.; Jiang, Q. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J. Thromb. Thrombolysis 2015, 39, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sundquist, K.; Elf, J.L.; Strandberg, K.; Svensson, P.J.; Hedelius, A.; Palmer, K.; Memon, A.A.; Sundquist, J.; Zöller, B. Diagnostic potential of plasma microRNA signatures in patients with deep-vein thrombosis. Thromb. Haemost. 2016, 116, 328–336. [Google Scholar] [CrossRef]
- Kessler, T.; Erdmann, J.; Vilne, B.; Bruse, P.; Kurowski, V.; Diemert, P.; Schunkert, H.; Sager, H.B. Serum microRNA-1233 is a specific biomarker for diagnosing acute pulmonary embolism. J. Transl. Med. 2016, 14, 120. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Qian, J.; Li, H.; Jiang, T.; Wang, W.; Cheng, W.; Wang, F.; Qi, L.; et al. MiR-28-3p as a potential plasma marker in diagnosis of pulmonary embolism. Thromb. Res. 2016, 138, 91–95. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, F. Plasma Levels of microRNA-221 (miR-221) are increased in patients with acute pulmonary embolism. Med. Sci. Monit. 2018, 24, 8621–8626. [Google Scholar] [CrossRef]
- Li, Z.; Ni, J. Role of microRNA-26a in the diagnosis of lower extremity deep vein thrombosis in patients with bone trauma. Exp. Ther. Med. 2017, 14, 5069–5074. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Ma, J.; Jiang, Z.; Wu, F.; Ping, J.; Ming, L. Diagnostic value of circulating microRNA-27a/b in patients with acute pulmonary embolism. Int. Angiol. 2018, 37, 19–25. [Google Scholar]
- Jiang, Z.; Ma, J.; Wang, Q.; Wu, F.; Ping, J.; Ming, L. Combination of circulating miRNA-320a/b and D-dimer improves diagnostic accuracy in deep vein thrombosis patients. Med. Sci. Monit. 2018, 24, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chai, S.; Zhang, F.; Lu, L. Overexpressed microRNA-103a-3p inhibits acute lower-extremity deep venous thrombosis via inhibition of CXCL12. IUBMB Life 2020, 72, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Wei, R.; Miao, X.; Sun, S.; Liang, G.; Chu, C.; Zhao, L.; Zhu, X.; Guo, Q.; et al. IL (Interleukin)-6 contributes to deep vein thrombosis and is negatively regulated by miR-338-5p. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Starikova, I.; Jamaly, S.; Sorrentino, A.; Blondal, T.; Latysheva, N.; Sovershaev, M.; Hansen, J.B. Differential expression of plasma miRNAs in patients with unprovoked venous thromboembolism and healthy control individuals. Thromb. Res. 2015, 136, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sundquist, K.; Svensson, P.J.; Rastkhani, H.; Palmér, K.; Memon, A.A.; Sundquist, J.; Zöller, B. Association of recurrent venous thromboembolism and circulating microRNAs. Clin. Epigenetics 2019, 11, 28. [Google Scholar] [CrossRef]
- Van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.M.; Mahé, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef]
- Oto, J.; Navarro, S.; Larsen, A.C.; Solmoirago, M.J.; Plana, E.; Hervás, D.; Fernández-Pardo, Á.; España, F.; Kristensen, S.R.; Thorlacius-Ussing, O.; et al. MicroRNAs and neutrophil activation markers predict venous thrombosis in pancreatic ductal adenocarcinoma and distal Extrahepatic Cholangiocarcinoma. Int. J Mol. Sci. 2020, 21, 840. [Google Scholar] [CrossRef]
- Sunderland, N.; Skroblin, P.; Barwari, T.; Huntley, R.P.; Lu, R.; Joshi, A.; Lovering, R.C.; Mayr, M. MicroRNA biomarkers and platelet reactivity: The clot thickens. Circ. Res. 2017, 120, 418–435. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, H.X.; Wang, Z.; Liu, Z.Y.; Xie, Q.F.; Hu, K.; Zhang, Z.; Mu, G.Y.; Ma, L.Y.; Jiang, J.; et al. The predictive value of circulating microRNAs for venous thromboembolism diagnosis: A systematic review and diagnostic meta-analysis. Thromb. Res. 2019, 181, 127–134. [Google Scholar] [CrossRef]
- Van Rooij, E.; Purcell, A.L.; Levin, A.A. Developing microRNA therapeutics. Circ. Res. 2012, 110, 496–507. [Google Scholar] [CrossRef]
- Van Rooij, E.; Kauppinen, S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014, 6, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Diaz, J.A.; Obi, A.T.; Myers, D.D., Jr.; Wrobleski, S.K.; Henke, P.K.; Mackman, N.; Wakefield, T.W. Critical review of mouse models of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Li, W.; Kong, L.; Qian, A.; Hu, N.; Meng, Q.; Li, X. MiR-150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb. Res. 2014, 133, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, W.; Yu, X.; Li, W.; Kong, L.; Qian, A.; Li, C.; Li, X. Upregulation of MicroRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J. Cell. Biochem. 2015, 116, 1613–1623. [Google Scholar] [CrossRef]
- Kong, L.; Du, X.; Hu, N.; Li, W.; Wang, W.; Wei, S.; Zhuang, H.; Li, X.; Li, C. Downregulation of let-7e-5p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis via targeting FASLG. Thromb. Res. 2016, 138, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Hu, N.; Du, X.; Wang, W.; Chen, H.; Li, W.; Wei, S.; Zhuang, H.; Li, X.; Li, C. Upregulation of miR-483-3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J. Transl. Med. 2016, 14, 23. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, X.; Du, X.; Xu, A.; Yuan, X.; Zhan, Y.; Liu, M.; Wang, S. MiR-150 promotes angiogensis and proliferation of endothelial progenitor cells in deep venous thrombosis by targeting SRCIN1. Microvasc. Res. 2019, 123, 35–41. [Google Scholar] [CrossRef]
- Du, X.; Hong, L.; Sun, L.; Sang, H.; Qian, A.; Li, W.; Zhuang, H.; Liang, H.; Song, D.; Li, C.; et al. miR-21 induces endothelial progenitor cells proliferation and angiogenesis via targeting FASLG and is a potential prognostic marker in deep venous thrombosis. J. Transl. Med. 2019, 17, 270. [Google Scholar] [CrossRef]
- Ramasamy, S.; Duraisamy, S.; Barbashov, S.; Kawano, T.; Kharbanda, S.; Kufe, D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol. Cell 2007, 27, 992–1004. [Google Scholar] [CrossRef]
- Takenaka, Y.; Fukumori, T.; Raz, A. Galectin-3 and metastasis. Glycoconj. J. 2004, 19, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D., Jr.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3-binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
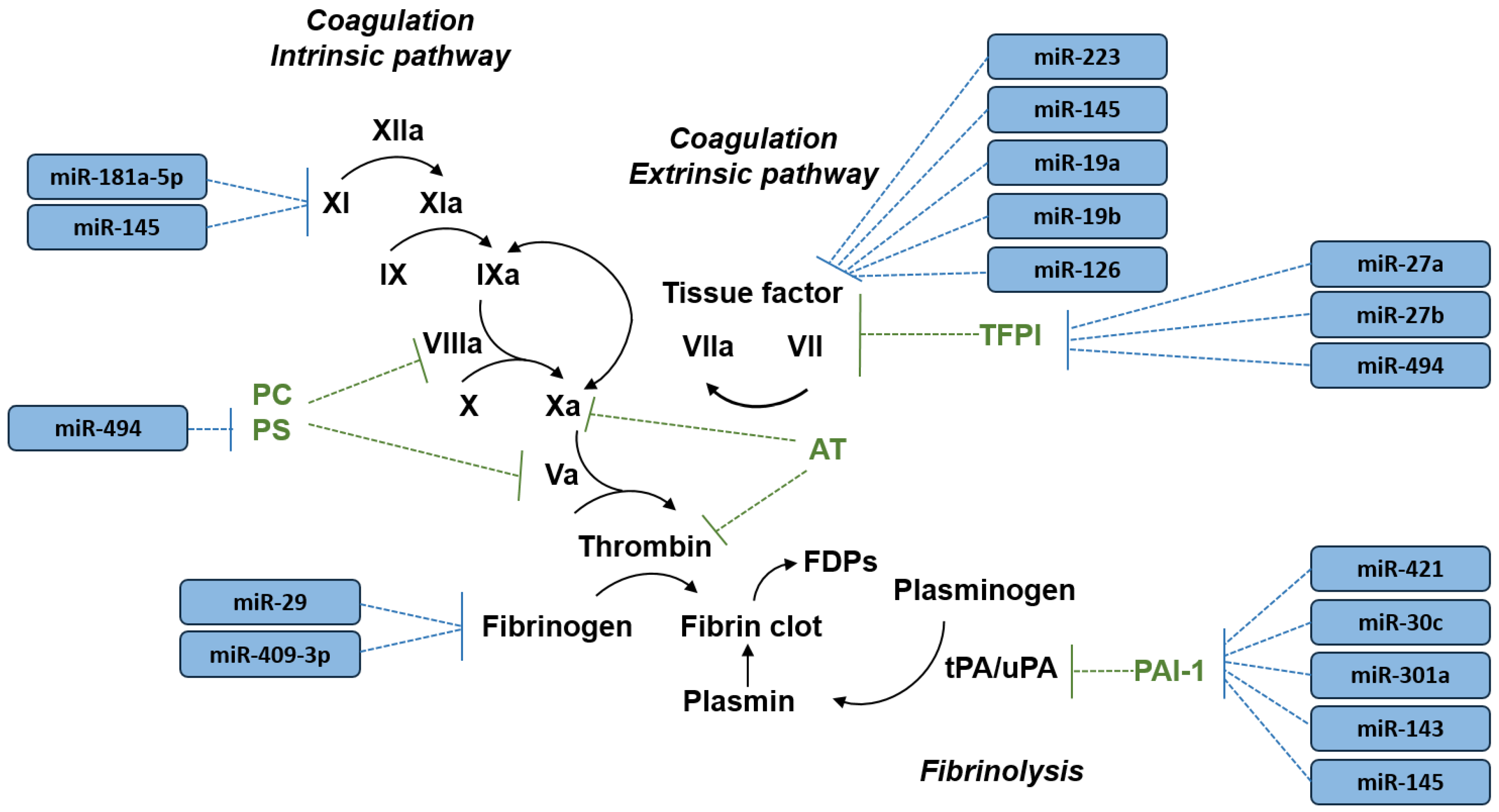
| Target Gene | Protein | miRNA 1 |
|---|---|---|
| FGA, FGB, FGG | Fibrinogen | miR-29 [25] miR-409-3p [25] |
| F3 | Tissue factor | miR-223 [26] miR-145 [27] miR-19a [28] miR-19b [29] miR-126 [28,30] |
| F11 | Factor XI | miR-181a-5p [31,32] miR-145 [32] |
| PROS1 | Protein S | miR-494 [33] |
| TFPI | Tissue factor pathway inhibitor | miR-27a [34] miR-27b [34] miR-494 [34] |
| SERPINE1 | Plasminogen activator inhibitor-1 | miR-421 [35] miR-30c [35,36] miR-301a [36] miR-143 [37] miR-145 [37] |
| First Author (year) | Country | Model Experimental DVT | miRNA Studied | Main Findings Impact of Modulation of miRNA Activity on Experimental DVT | Potential Target Genes Associated with the Mechanism of Experimental DVT 1 |
|---|---|---|---|---|---|
| Wang et al. (2014) [64] | China | Rat model of DVT by IVC ligation (IVC stasis model) | miR-150 | Intravenous injection of viral vector expressing miR-150 resulted in enhanced EPC homing and venous thrombus recanalization and resolution | c-Myb (c-Myb proto-oncogene) |
| Meng et al. (2015) [65] | China | Rat model of DVT by IVC ligation (IVC stasis model) | miR-126 | Intravenous injection of viral vector expressing miR-126 resulted in enhanced EPC homing and venous thrombus recanalization and resolution | PIK3R2 (phosphoinositide-3-kinase regulatory subunit 2) |
| Kong et al. (2016) [66] | China | Rat model of DVT by IVC ligation (IVC stasis model) | let-7e-5p | Intravenous injection of viral vector expressing let-7e-5p resulted in enhanced EPC homing and venous thrombus revascularization | FASLG (Fas ligand) |
| Kong et al. (2016) [67] | China | Rat model of DVT by IVC ligation (IVC stasis model) | miR-483-3p | Intravenous injection of viral vector expressing miR-483-3p inhibitor resulted in enhanced EPC homing and venous thrombus recanalization and resolution | SRF (serum response factor) |
| Sahu et al. (2017) [27] | India | Rat model of DVT by IVC ligation (IVC stasis model) | miR-145 | Intravenous injection of miR-145 mimics resulted in decreased tissue factor mRNA levels and activity, and reduced venous thrombus formation | F3 (coagulation factor III, tissue factor) |
| Wang et al. (2019) [68] | China | Rat model of DVT by IVC ligation (IVC stasis model) | miR-150 | Intravenous injection of EPCs transfected with miR-150 mimics resulted in enhanced venous thrombus resolution | SRCIN1 (SRC kinase signaling inhibitor 1) |
| Du et al. (2019) [69] | China | Rat model of DVT by IVC ligation (IVC stasis model) | miR-21 | Injection within the thrombus of viral vector expressing miR-21 resulted in enhanced venous thrombus resolution | FASLG (Fas ligand) |
| Sun et al. (2020) [52] | China | Mouse model of DVT by IVC stenosis (IVC stenosis model) | miR-103a-3p | Intravenous injection of viral vector expressing miR-103a-3p resulted in decreased inflammatory cell infiltration and venous thrombus formation | CXCL12 (C-X-C motif chemokine ligand 12) |
| Zhang et al. (2020) [53] | China | Mouse model of DVT by IVC stenosis (IVC stenosis model) | miR-338-5p | Intravenous injection of miR-338-5p mimics resulted in decreased interleukin-6 expression and venous thrombus formation | IL6 (interleukin 6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, V.M.; Brækkan, S.K.; Hansen, J.-B. Role of microRNAs in Venous Thromboembolism. Int. J. Mol. Sci. 2020, 21, 2602. https://doi.org/10.3390/ijms21072602
Morelli VM, Brækkan SK, Hansen J-B. Role of microRNAs in Venous Thromboembolism. International Journal of Molecular Sciences. 2020; 21(7):2602. https://doi.org/10.3390/ijms21072602
Chicago/Turabian StyleMorelli, Vânia M., Sigrid K. Brækkan, and John-Bjarne Hansen. 2020. "Role of microRNAs in Venous Thromboembolism" International Journal of Molecular Sciences 21, no. 7: 2602. https://doi.org/10.3390/ijms21072602
APA StyleMorelli, V. M., Brækkan, S. K., & Hansen, J.-B. (2020). Role of microRNAs in Venous Thromboembolism. International Journal of Molecular Sciences, 21(7), 2602. https://doi.org/10.3390/ijms21072602




