Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation
Abstract
:1. Introduction
2. Results
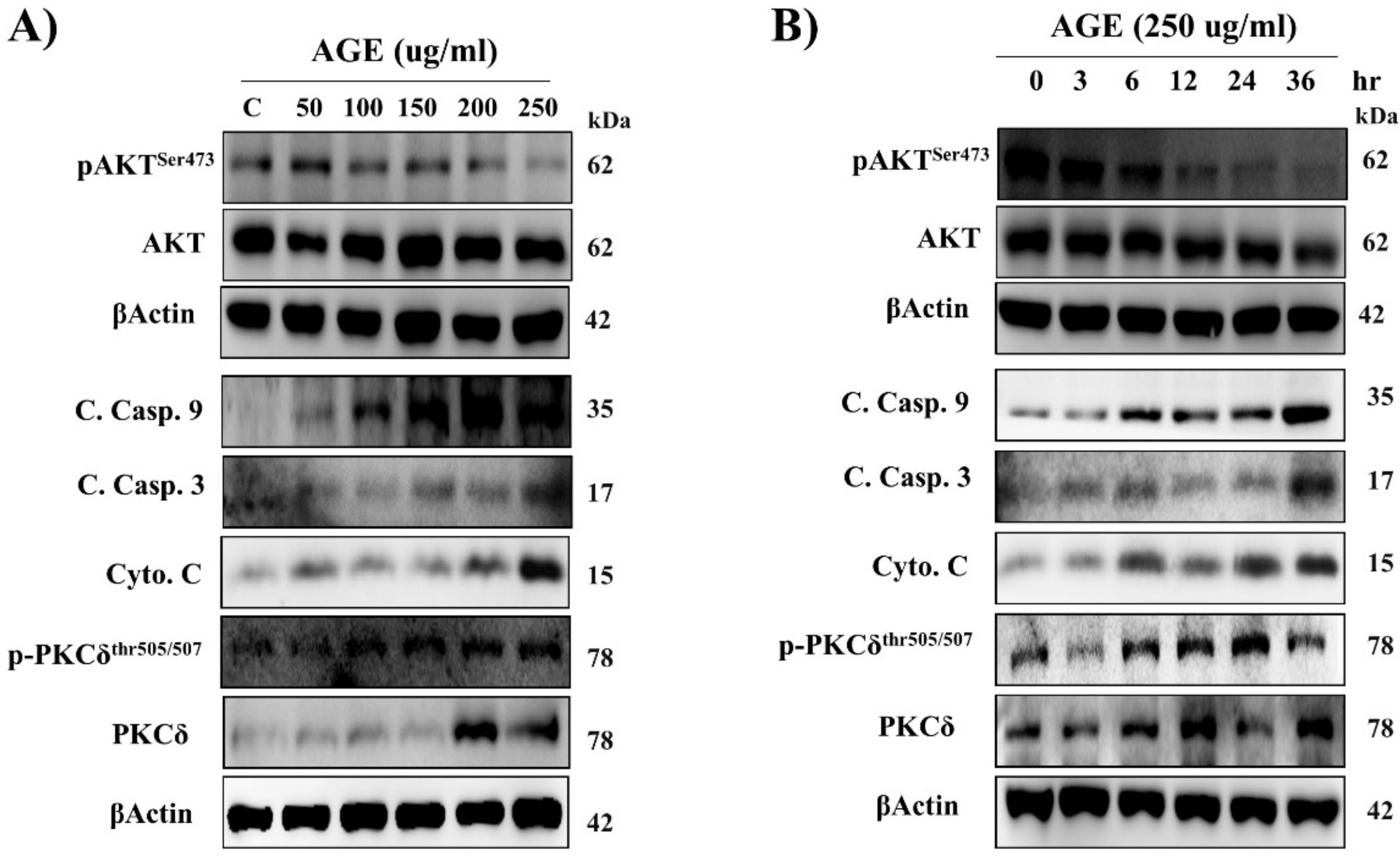
2.1. AGE Induced Cardiac PKCδ Protein Expression, Phosphorylation, and Apoptosis in a Dose- and Time-Dependent Manner
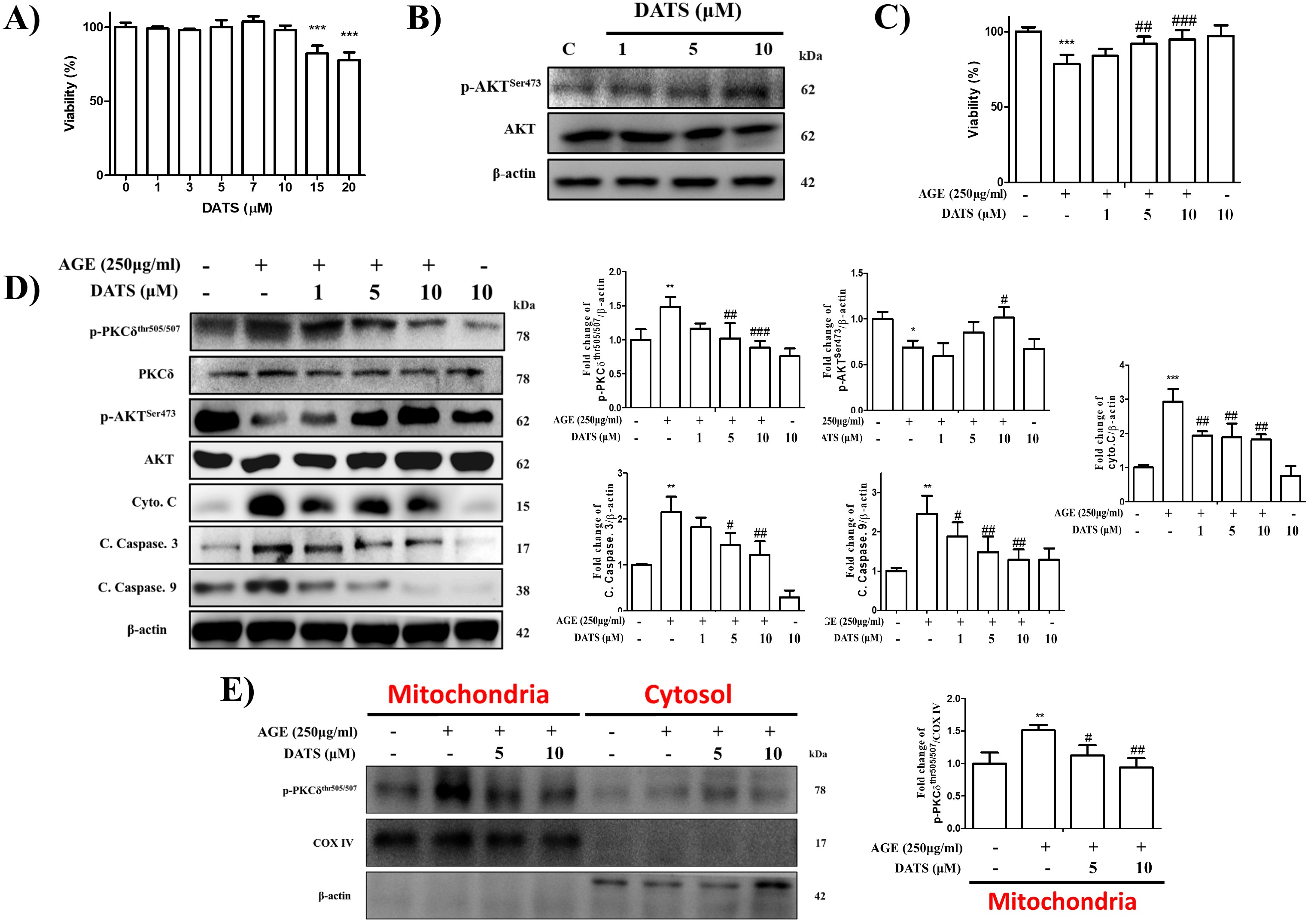
2.2. Inhibitory Effect of DATS on AGE-Induced Cardiac Apoptosis and PKCδ Mitochondrial Translocation
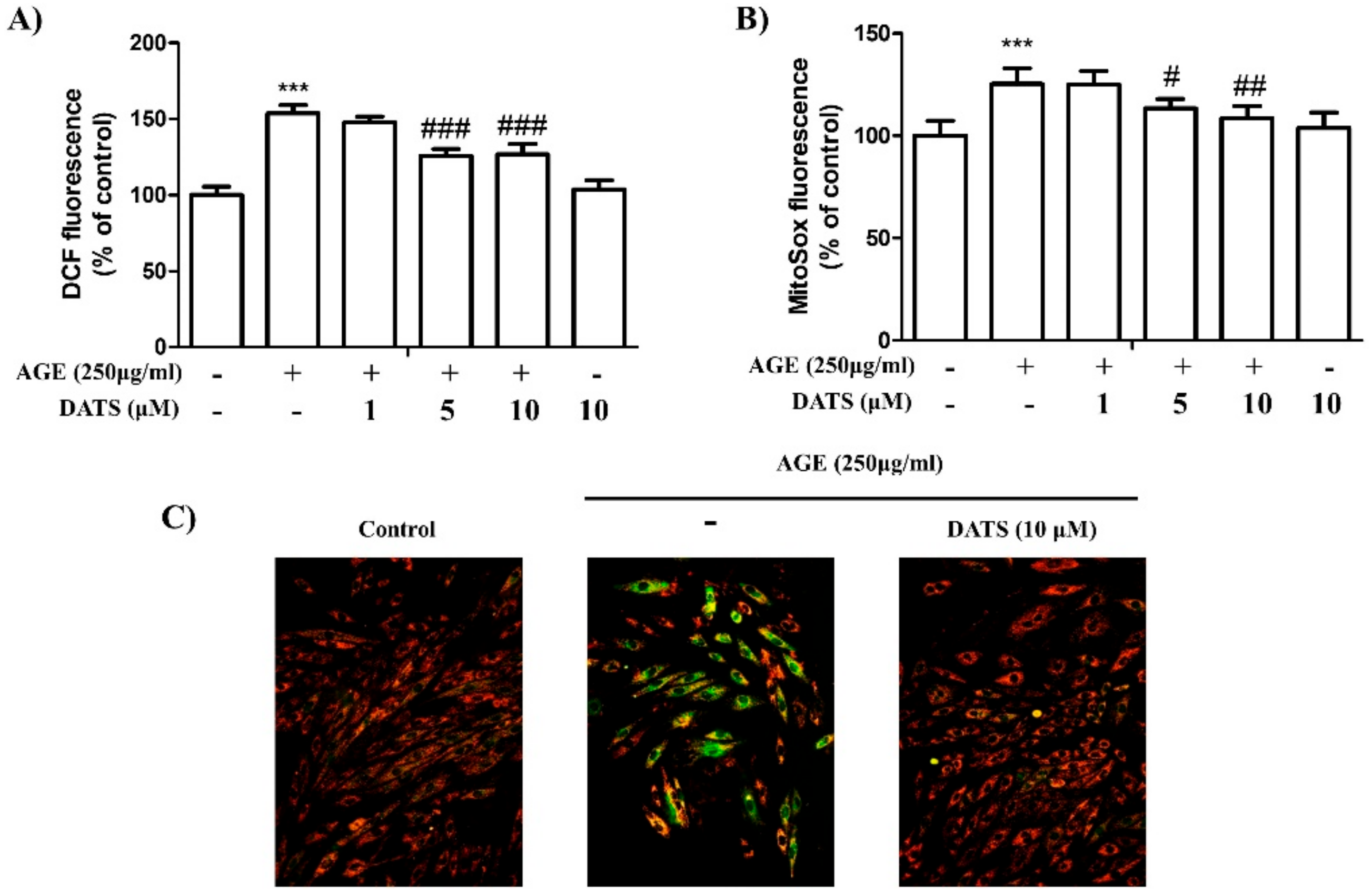
2.3. Antioxidant Effect of DATS on AGE-Induced Cardiac ROS Generation
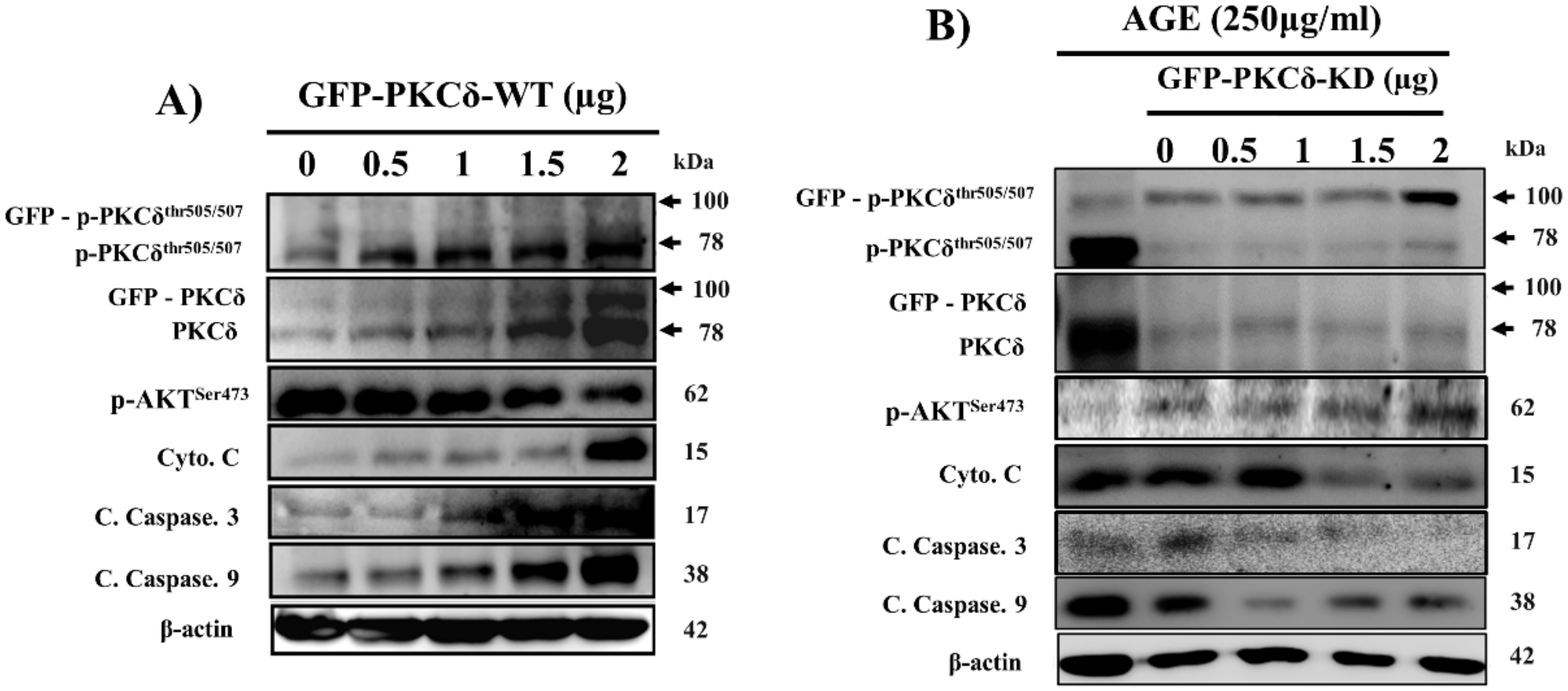
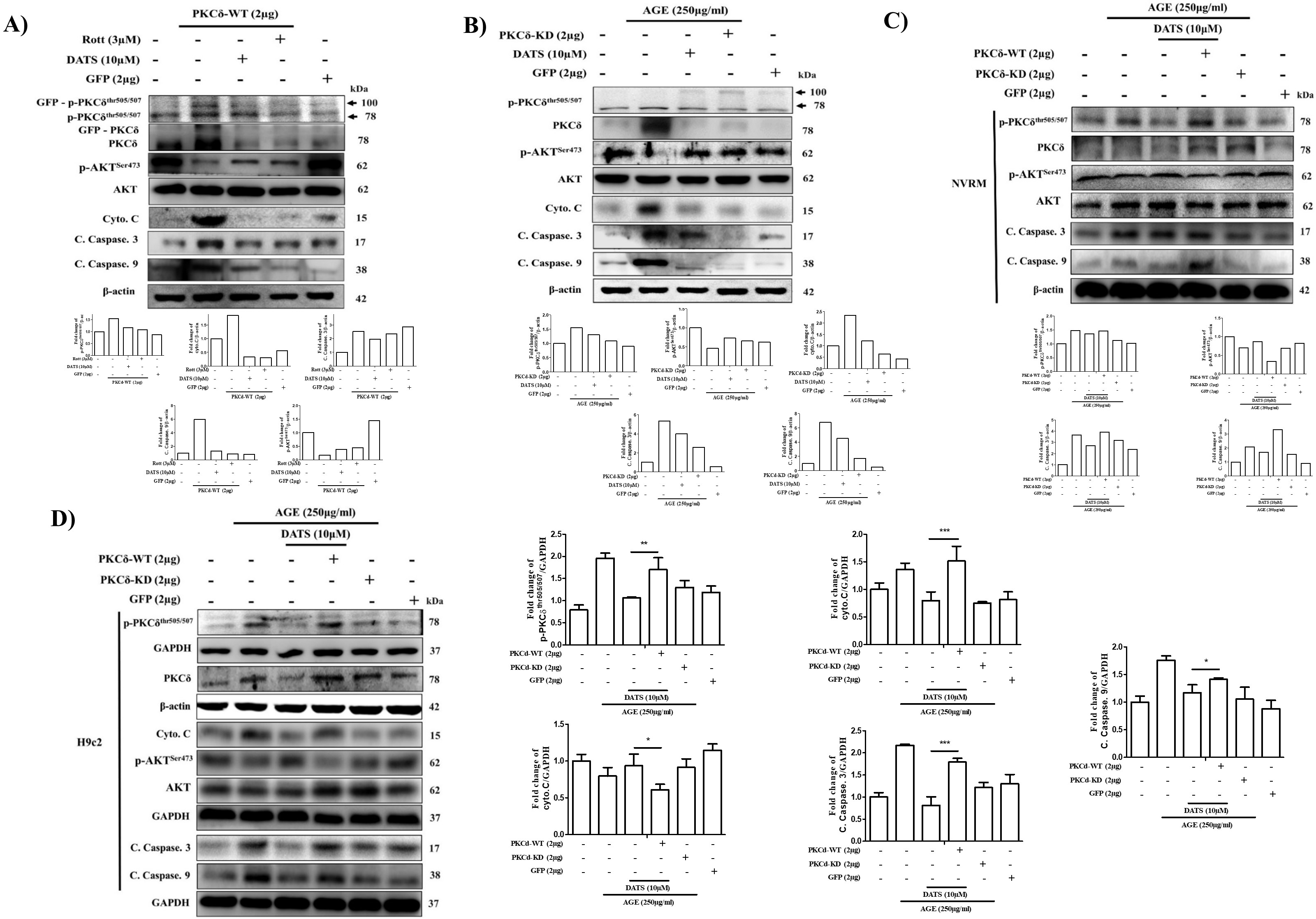
2.4. Cardiac PKCδ-Dependent Apoptosis Induced by AGE is Inhibited by GFP-PKCδ-KD
2.5. DATS Suppresses Cardiac Apoptosis by Inhibiting PKCδ Activation and its Downstream Apoptosis-Related Proteins Following AGE Exposure
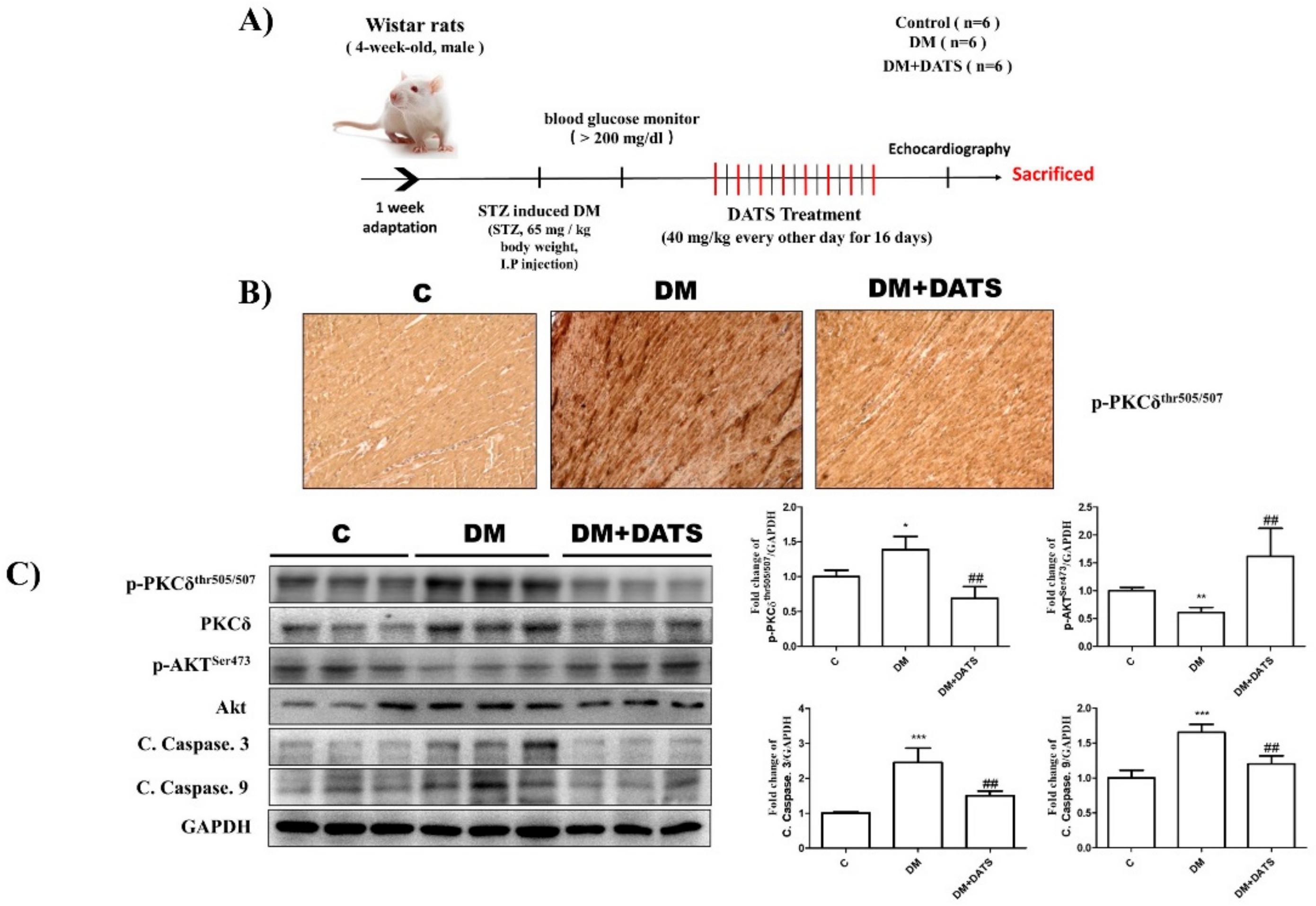
2.6. DATS Suppresses Cardiac Apoptosis by Inhibiting Activation of PKCδ and Apoptosis-Related Signaling Pathways in STZ-Induced Diabetic Rats
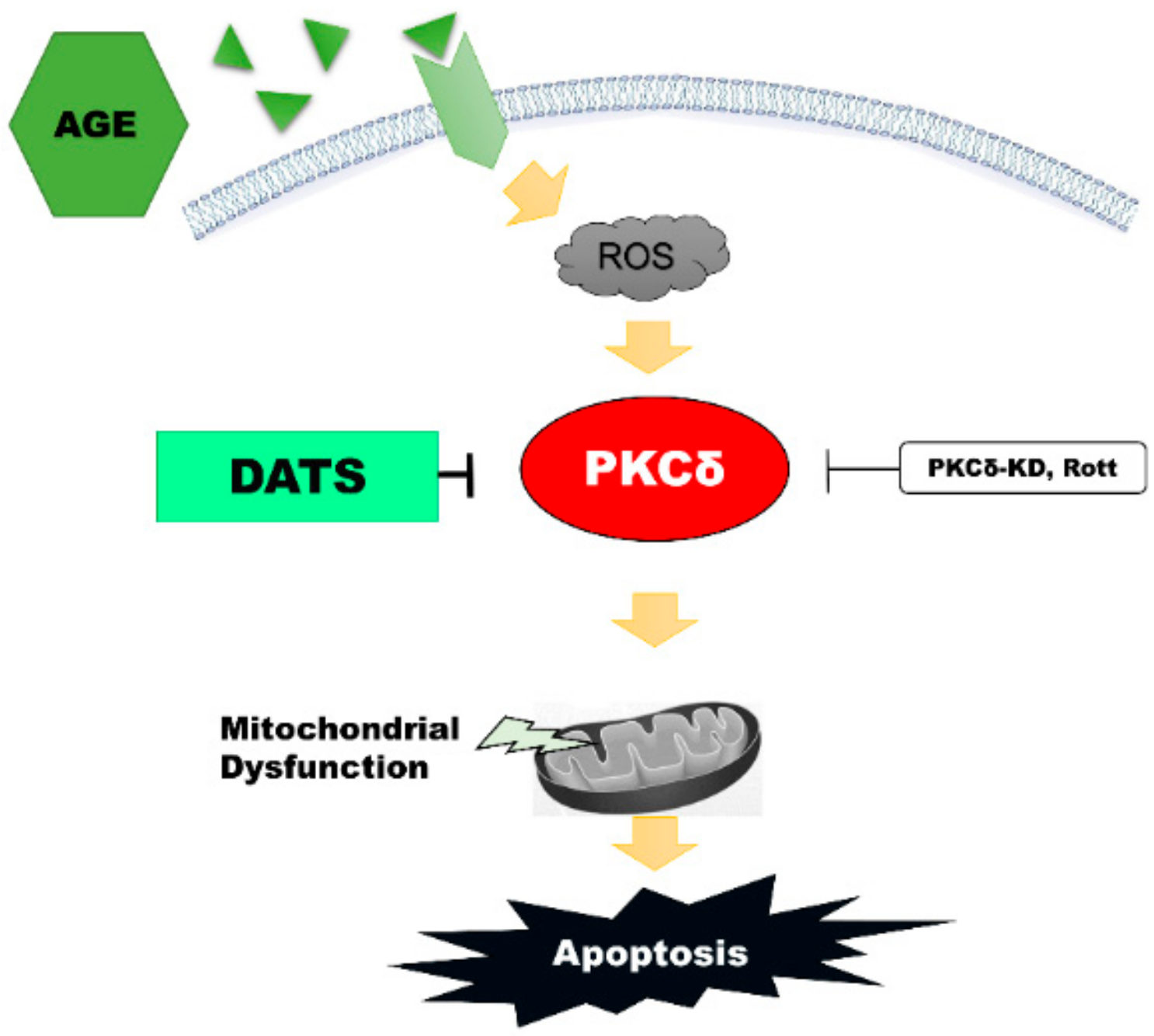
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Neonatal Rat Ventricular Myocyte (NRVM) Primary Culture
4.3. Glucose-Derived AGE Preparation
4.4. Transient Transfection of Plasmid DNA
4.5. Western Blot Analysis
4.6. MTT Assay
4.7. Intracellular and Mitochondrial Reactive Oxygen Species (ROS) Production
4.8. Measurement of Mitochondrial Membrane Potential (MMP)
4.9. Isolation of Mitochondria
4.10. Animals and Treatment Protocol
4.11. Immunohistochemistry
4.12. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Wendt, T.; Tanji, N.; Guo, J.; Hudson, B.I.; Bierhaus, A.; Ramasamy, R.; Arnold, B.; Nawroth, P.P.; Yan, S.F.; D’Agati, V.; et al. Glucose, glycation, and RAGE: Implications for amplification of cellular dysfunction in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1383–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Marra, G.; Cotroneo, P.; Pitocco, D.; Manto, A.; Di Leo, M.A.; Ruotolo, V.; Caputo, S.; Giardina, B.; Ghirlanda, G.; Santini, S.A. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: A case for gender difference. Diabetes Care 2002, 25, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992, 23, 1–34. [Google Scholar]
- Xie, J.; Mendez, J.D.; Mendez-Valenzuela, V.; Aguilar-Hernandez, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Nozynski, J.; Zakliczynski, M.; Konecka-Mrowka, D.; Zakliczynska, H.; Pijet, M.; Zembala-Nozynska, E.; Lange, D.; Zembala, M. Advanced glycation end products and lipofuscin deposits share the same location in cardiocytes of the failing heart. Exp. Gerontol. 2013, 48, 223–228. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Receptor for AGE (RAGE): Signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 2011, 1243, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhao, Y.; Xu, S.; Ding, F.; Jin, C.; Fu, G.; Weng, S. Advanced Glycation End Product (AGE)-AGE Receptor (RAGE) System Upregulated Connexin43 Expression in Rat Cardiomyocytes via PKC and Erk MAPK Pathways. Int. J. Mol. Sci. 2013, 14, 2242–2257. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Regulation of the ABC kinases by phosphorylation: Protein kinase C as a paradigm. Biochem. J. 2003, 370, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, L.E.; Standage, S.W.; Li, H.; Raj, N.R.; Korchak, H.M.; Wolfson, M.R.; Deutschman, C.S. Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-delta (delta-PKC). J. Leukoc. Biol. 2011, 89, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xia, L.; Chen, G.Q. Protein kinase cdelta in apoptosis: A brief overview. Arch. Immunol. Ther. Exp. 2012, 60, 361–372. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar] [CrossRef] [Green Version]
- Churchill, E.N.; Szweda, L.I. Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005, 439, 194–199. [Google Scholar] [CrossRef]
- Konishi, H.; Tanaka, M.; Takemura, Y.; Matsuzaki, H.; Ono, Y.; Kikkawa, U.; Nishizuka, Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 1997, 94, 11233–11237. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Guo, J.; Cong, L.; Rybin, V.O.; Gertsberg, Z.; Steinberg, S.F. Protein kinase C-{delta} regulates the subcellular localization of Shc in H2O2-treated cardiomyocytes. Am. J. Physiol. Cell Physiol. 2010, 299, C770–C778. [Google Scholar] [CrossRef] [Green Version]
- Churchill, E.N.; Mochly-Rosen, D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem. Soc. Trans. 2007, 35, 1040–1042. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tsai, C.Y.; Chen, C.L.; Kuo, C.H.; Hou, C.W.; Cheng, S.Y.; Aneja, R.; Huang, C.Y.; Kuo, W.W. Pkcdelta Activation is Involved in ROS-Mediated Mitochondrial Dysfunction and Apoptosis in Cardiomyocytes Exposed to Advanced Glycation End Products (Ages). Aging Dis. 2018, 9, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: nature’s protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Lowe, G.M. Garlic and cardiovascular disease: A critical review. J. Nutr. 2006, 136, 736S–740S. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef] [Green Version]
- Ou, H.C.; Tzang, B.S.; Chang, M.H.; Liu, C.T.; Liu, H.W.; Lii, C.K.; Bau, D.T.; Chao, P.M.; Kuo, W.W. Cardiac contractile dysfunction and apoptosis in streptozotocin-induced diabetic rats are ameliorated by garlic oil supplementation. J. Agric. Food Chem. 2010, 58, 10347–10355. [Google Scholar] [CrossRef]
- Huang, Y.T.; Yao, C.H.; Way, C.L.; Lee, K.W.; Tsai, C.Y.; Ou, H.C.; Kuo, W.W. Diallyl trisulfide and diallyl disulfide ameliorate cardiac dysfunction by suppressing apoptotic and enhancing survival pathways in experimental diabetic rats. J. Appl. Physiol. 2013, 114, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.W.; Wang, W.J.; Tsai, C.Y.; Way, C.L.; Hsu, H.H.; Chen, L.M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFkappaB signaling via attenuating ROS generation. Int. J. Cardiol. 2013, 168, 270–280. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, H.M.; Wei, X.; Gong, X.; Lu, Z.Y.; Huang, Z.H. Diallyl trisulfide attenuates hyperglycemia-induced endothelial apoptosis by inhibition of Drp1-mediated mitochondrial fission. Acta Diabetol. 2019, 56, 1177–1189. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis 2017, 22, 942–954. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.R.; Bhattacharya, S.K.; Saul, I.; DeFazio, R.A.; Dezfulian, C.; Lin, H.W.; Raval, A.P.; Perez-Pinzon, M.A. Activation of protein kinase C delta following cerebral ischemia leads to release of cytochrome C from the mitochondria via bad pathway. PLoS ONE 2011, 6, e22057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.F.; Chen, Y.C.; Liu, C.Y.; Wei, Y.H. Involvement of protein kinase C delta in the alteration of mitochondrial mass in human cells under oxidative stress. Free Radic. Biol. Med. 2006, 40, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.; Nawroth, P.P.; King, G.; Moller, W.; Tritschler, H.J.; Packer, L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 2001, 17, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.O.; Zweier, J.L.; Sollott, S.J. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Griendling, K.K.; Harrison, D.G. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol. Sci. 2003, 24, 471–478. [Google Scholar] [CrossRef]
- Tan, A.L.; Forbes, J.M.; Cooper, M.E. AGE, RAGE, and ROS in diabetic nephropathy. Semin. Nephrol. 2007, 27, 130–143. [Google Scholar] [CrossRef]
- Forbes, J.M.; Yee, L.T.; Thallas, V.; Lassila, M.; Candido, R.; Jandeleit-Dahm, K.A.; Thomas, M.C.; Burns, W.C.; Deemer, E.K.; Thorpe, S.R.; et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 2004, 53, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhashi, T.; Vlassara, H.; Founds, H.W.; Li, Y.M. Standardizing the immunological measurement of advanced glycation endproducts using normal human serum. J. Immunol. Methods 1997, 207, 79–88. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsieh, Y.T.; Chen, H.C. Phosphorylation of adducin by protein kinase Cdelta promotes cell motility. J. Cell Sci. 2007, 120, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.Y.; Fan, C.W.; Maa, M.C.; Leu, T.H. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, D.J.-Y.; Ng, S.-C.; Zeng, R.-Y.; Padma, V.V.; Huang, C.-Y.; Kuo, W.-W. Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation. Int. J. Mol. Sci. 2020, 21, 2608. https://doi.org/10.3390/ijms21072608
Hsieh DJ-Y, Ng S-C, Zeng R-Y, Padma VV, Huang C-Y, Kuo W-W. Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation. International Journal of Molecular Sciences. 2020; 21(7):2608. https://doi.org/10.3390/ijms21072608
Chicago/Turabian StyleHsieh, Dennis Jine-Yuan, Shang-Chuan Ng, Ren-You Zeng, Viswanadha Vijaya Padma, Chih-Yang Huang, and Wei-Wen Kuo. 2020. "Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation" International Journal of Molecular Sciences 21, no. 7: 2608. https://doi.org/10.3390/ijms21072608
APA StyleHsieh, D. J.-Y., Ng, S.-C., Zeng, R.-Y., Padma, V. V., Huang, C.-Y., & Kuo, W.-W. (2020). Diallyl Trisulfide (DATS) Suppresses AGE-Induced Cardiomyocyte Apoptosis by Targeting ROS-Mediated PKCδ Activation. International Journal of Molecular Sciences, 21(7), 2608. https://doi.org/10.3390/ijms21072608




