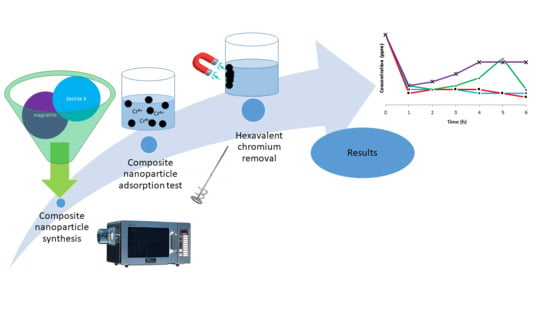
A Study on Magnetic Removal of Hexavalent Chromium from Aqueous Solutions Using Magnetite/Zeolite-X Composite Particles as Adsorbing Material
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analysis
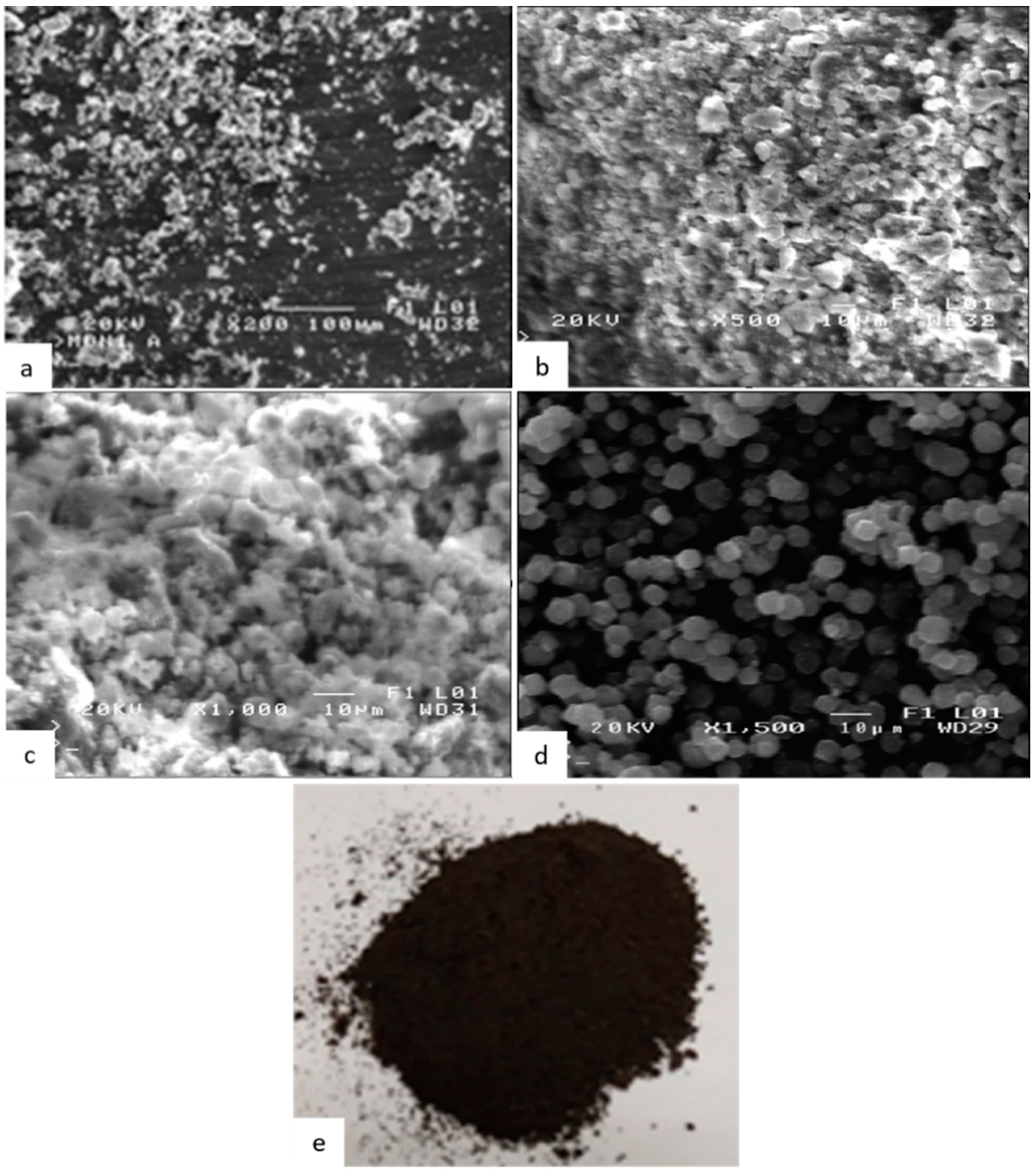
2.1.1. Scanning Electron Microscopy (SEM)
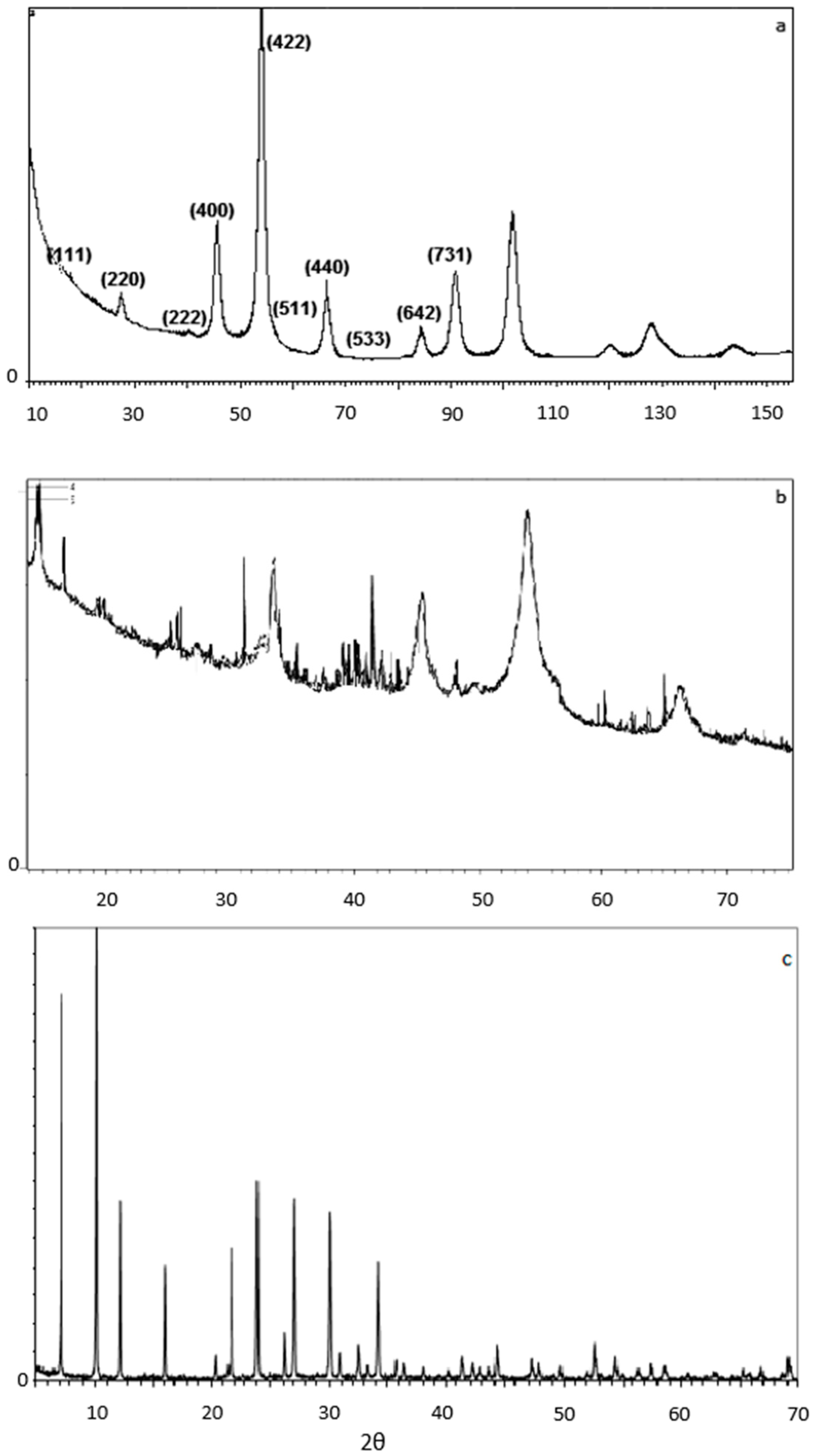
2.1.2. X-ray Diffraction (XRD)
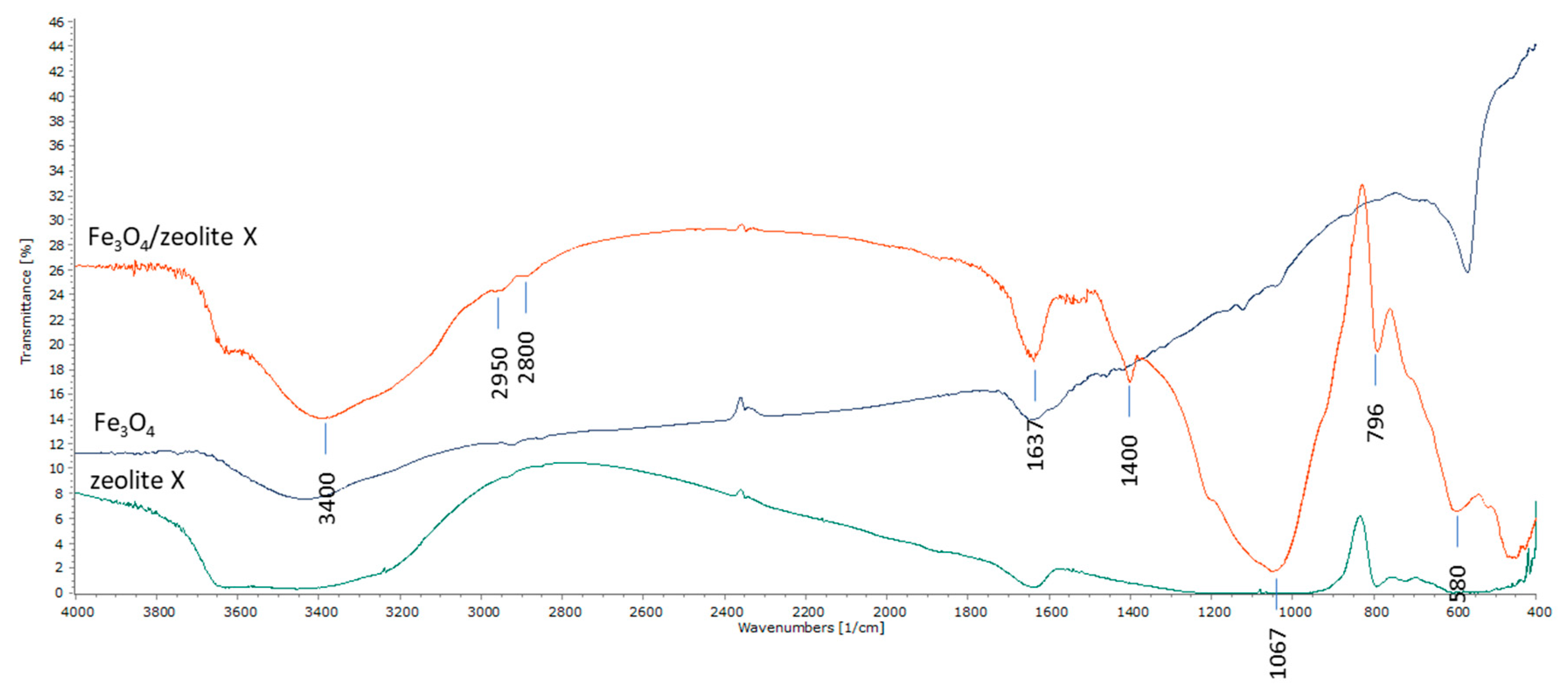
2.1.3. Fourier Transformation Infrared (FTIR)
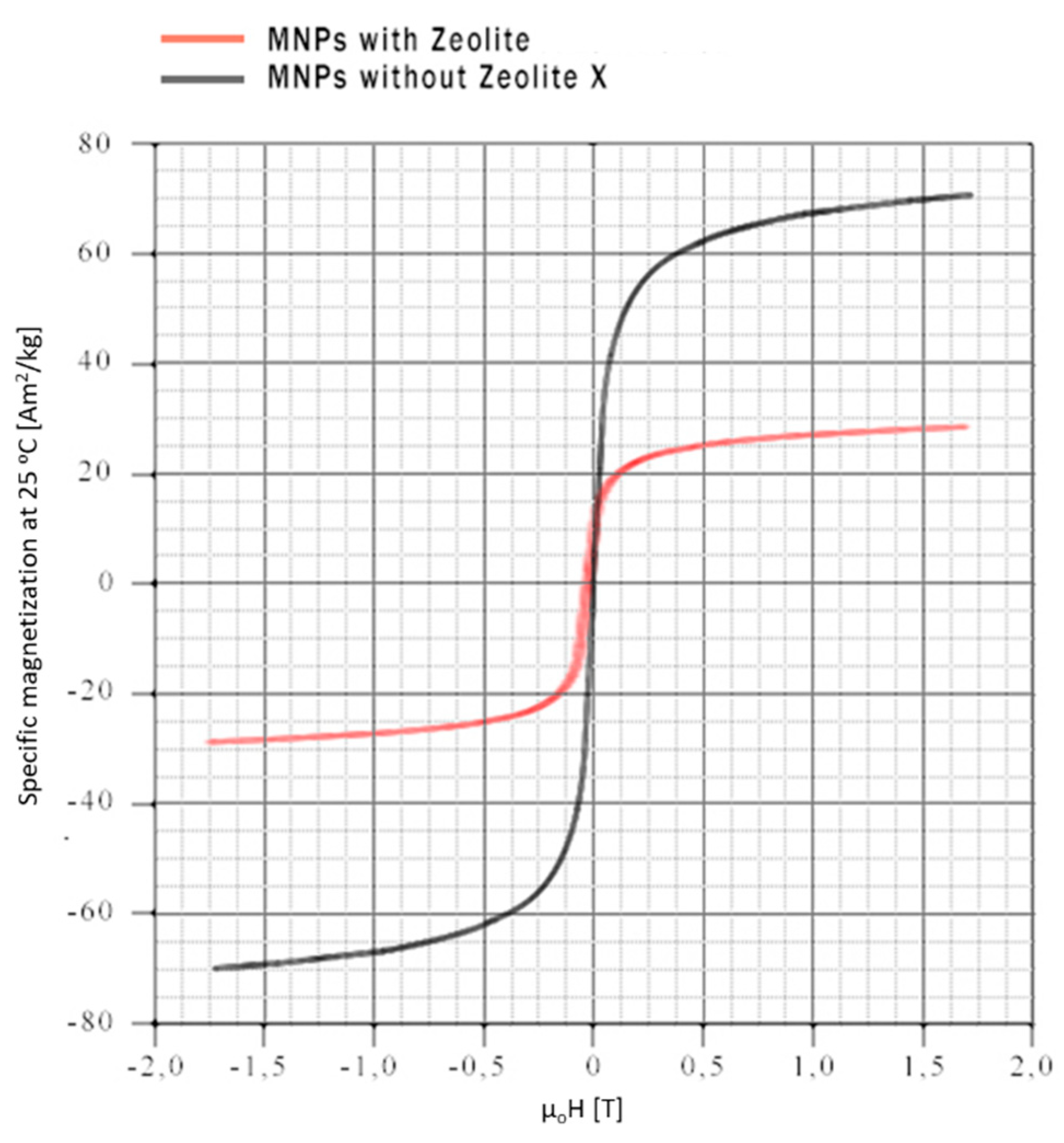
2.1.4. Vibrating Sample Magnetization
2.2. Ion Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
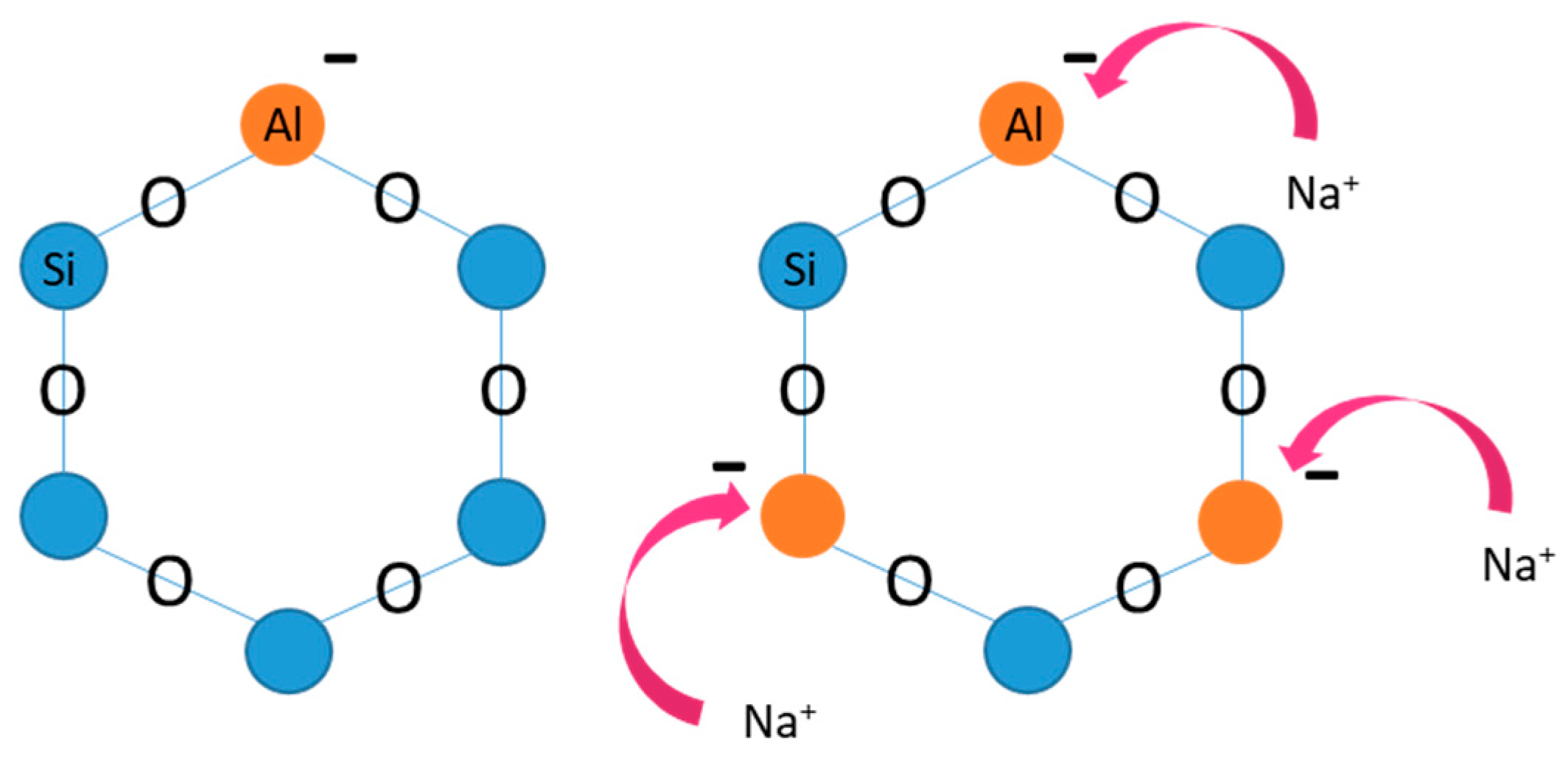
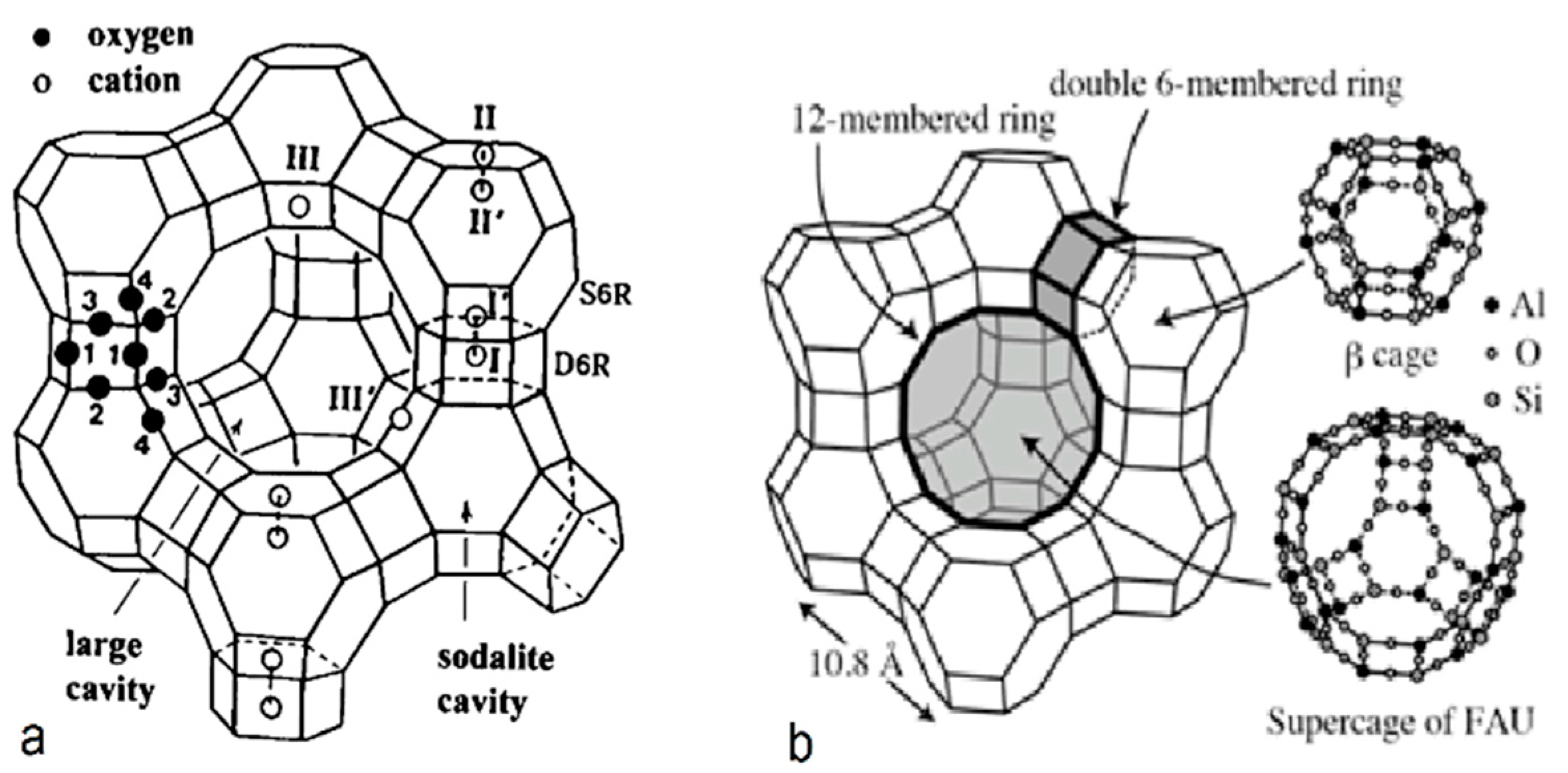
3.2.1. Synthesis of Zeolite-X (Linde Type X)
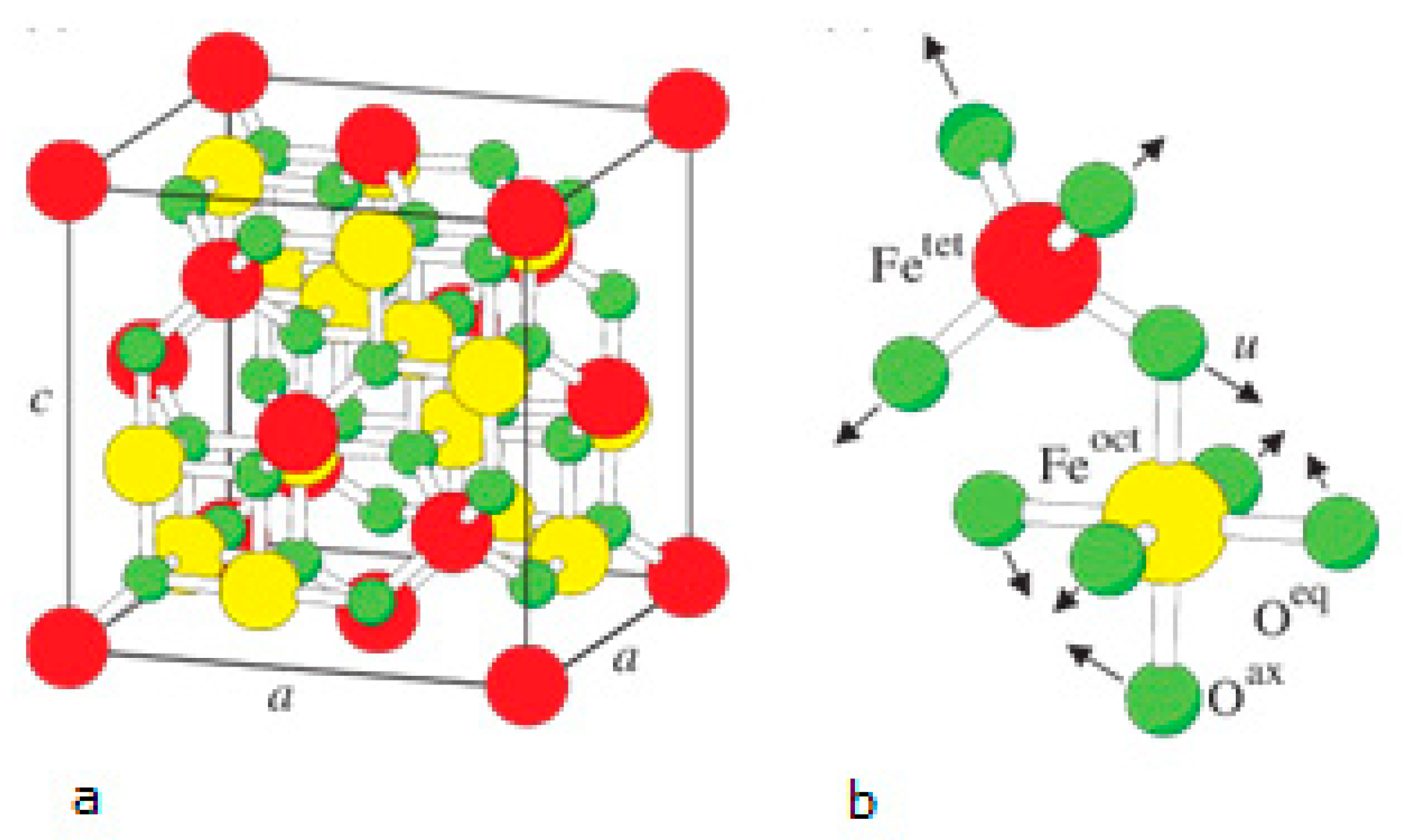
3.2.2. Synthesis of Fe3O4 Nanoparticles
3.2.3. Synthesis of Fe3O4/Zeolite-X Composite Particles
3.2.4. Ion Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Da̧browski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- United States EPA. Summary Report: Control and Treatment Technology for the Metal Finishing Industry, Ion Exchange, Environ. Prot. Agency 1980, 625, 8–80.
- Gaikwad, R.W.; Sapkal, V.S.; Sapkal, R.S. Ion exchange system design for removal of heavy metals from acid mine drainage wastewater. Acta Montan. Slovaca Ročník 2010, 15, 298–304. [Google Scholar]
- Beker, U.G.; Guner, F.S.; Dizman, M.; Erciyes, A.T. Heavy Metal Removal by Ion Exchanger Based on Hydroxyethyl Cellulose. J. Appl. Polym. Sci. 1999, 74, 3501–3506. [Google Scholar] [CrossRef]
- Tomáš, B.; Búgel, M.; Gajdošová, L. Heavy Metal Removal Using Reverse Osmosis. Acta Montan. Slovaca Ročník 2009, 14, 250–253. [Google Scholar]
- Algureiri, A.H.; Abdulmajeed, Y.R. Abdulmajeed, Removal of Heavy Metals from Industrial Wastewater by Using RO Membrane. Iraqi J. Chem. Pet. Eng. 2016, 17, 125–136. [Google Scholar]
- Thaçi, B.S.; Gashi, S.T. Reverse Osmosis Removal of Heavy Metals from Wastewater Effluents Using Biowaste Materials Pretreatment. Polish J. Environ. Stud. 2019, 28, 337–341. [Google Scholar] [CrossRef]
- Bobade, V.; Eshtiagi, N. Heavy Metals Removal from Wastewater by Adsorption Process: A Review. In Proceedings of the APCChE 2015 Congress Incorporating Chemeca, Melbourne, Australia, 27 September–1 October 2015. [Google Scholar]
- Algureiri, A.H.; Abdulmajeed, Y.R. Removal of Heavy Metals via Adsorption using Natural Clay Material. J. Environ. Earth Sci. 2014, 4, 19. [Google Scholar]
- Javaid, A.; Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass- Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.M.; Ahmad, M. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, K.S. Adsorption of Heavy Metals: A Review. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5. [Google Scholar] [CrossRef]
- Search ResultsWeb resultsUnited States Environmental Protection Agency. Toxicological Review of Hexavalent Chromium; EPA: Washington, DC, USA, 2010.
- Meena, A.K.; Mishra, G.; Rai, P.; Rajagopal, C.; Nagar, P. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Santhy, K.; Selvapathy, P. Removal of Heavy Metals from Wastewater by Adsorption on Coir Pith Activated Carbon. Sep. Sci. Technol. 2004, 39, 3331–3351. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015, 13, 699–708. [Google Scholar] [CrossRef]
- Zayat, M.E.; Smith, E. Removal of Heavy Metals by Using Activated Carbon Produced from Cotton Stalks. Can. J. Environ. Constr. Civil Eng. 2010, 1, 4. [Google Scholar]
- Burakov, A.; Romantsova, I.; Kucherova, A.; Tkachev, A. Removal of Heavy-Metal Ions from Aqueous Solutions Using Activated Carbons: Effect of Adsorbent Surface Modification with Carbon Nanotubes. Adsorpt. Sci. Technol. 2014, 32, 737–747. [Google Scholar] [CrossRef]
- Cervera, M.L.; Arnal, M.C.; De La Guardia, M. Removal of heavy metals by using adsorption on alumina or chitosan. Anal. Bioanal. Chem. 2003, 375, 820–825. [Google Scholar]
- Szatyłowicz, E.; Skoczko, I. The Use of Activated Alumina and Magnetic Field for the Removal Heavy Metals from Water. J. Ecol. Eng. 2018, 19, 61–67. [Google Scholar] [CrossRef]
- Marzouk, I.; Hannachi, C.; Dammak, L.; Hamrouni, B. Removal of chromium by adsorption on activated alumina. Desalin. Water Treat. 2011, 26, 279–286. [Google Scholar] [CrossRef]
- Yarkandi, N.H. Removal of toxic copper ions using alumina. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 415–431. [Google Scholar]
- Tzvetkova, P.; Nickolov, R. Modified and unmodified silica gel used for heavy metal ions removal from aqueous solutions. J. Univ. Chem. Technol. Metall. 2012, 47, 498–504. [Google Scholar]
- Noei, M.; Salari, A.-A.; Nafar, S.; Ebrahimikia, M.; Anaraki–Ardekani, H. Removal of Heavy Metal Ions from Aqueous Solutions using Silica Aerogel as Adsorbent. J. Sci. Technol. 2005, 112, 161–170. [Google Scholar]
- Ouafi, R.; Rais, Z.; Taleb, M.; Benabbou, M.; Asri, M. Sawdust in the treatment of heavy metals contaminated wastewater. Environ. Res. J. 2017, 11, 1935–3049. [Google Scholar]
- Sahmoune, M.N.; Yeddou, A.R. Potential of sawdust materials for the removal of dyes and heavy metals: Examination of isotherms and kinetics. Desalin. Water Treat. 2016, 57, 24019–24034. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Removal of heavy metals from aqueous solution by sawdust adsorption. J. Environ. Sci. 2007, 19, 160–166. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis, 1st ed.; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Iskandar, F.; Fitriani, P.; Merissa, S.; Mukti, R.R.; Khairurrijal, K.; Abdullah, M. Fe3O4/Zeolite nanocomposites synthesized by microwave assisted coprecipitation and its performance in reducing viscosity of heavy oil. In Proceedings of the 5th Nanoscience and Nanotechnology Symposium (Nns2013), Surabaya, Indonesia, 23–25 October 2013; pp. 132–135. [Google Scholar]
- Pati, S.; Kalyani, S.; Mahendran, V.; Philip, J. Microwave assisted synthesis of magnetite nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 5790–5797. [Google Scholar] [CrossRef]
- Kalyani, S.; Sangeetha, J.; Philip, J. Microwave Assisted Synthesis of Ferrite Nanoparticles: Effect of Reaction Temperature on Particle Size and Magnetic Properties. J. Nanosci. Nanotechnol. 2015, 15, 5768–5774. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cejka, J.; van Bekkum, H. (Eds.) Zeolites and Ordered Mesoporous Materials: Progress and Prospects; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Maesen, T.; Marcus, B. Introduction to Zeolite Science and Practice, 2nd ed.; Van Bekkum, E.M.H., Flanigen, P.A., Jacobs, J.C., Eds.; Jansen Elsevier: Amsterdam, The Netherlands, 2001; p. 137. [Google Scholar]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Dyer, A. An Introduction to Zeolite Molecular Sieves, Chichester; John Wiley: Hoboken, NJ, USA, 1988; p. 49. [Google Scholar]
- Colella, C. Ion exchange equilibria in zeolite minerals. Mineralium Deposita. 1996, 31, 554–562. [Google Scholar] [CrossRef]
- Database of Zeolite Structures, IZA Comission of Natural Zeolites. Available online: http://asia.iza-structure.org/IZA-SC/material_tm.php?STC=FAU (accessed on 13 April 2020).
- Bae, D.; Seff, K. Crystal structure of zeolite X nickel(II) exchanged at pH 4.3 and partially dehydrated, Ni2(NiOH)35(Ni4AlO4)2(H3O)46Si101Al91O384. Microporous Mesoporous Mater. 2000, 40, 219–232. [Google Scholar] [CrossRef]
- Igarashi, M.; Nakano, T.; Thi, P.T.; Nozue, Y.; Goto, A.; Hashi, K.; Ohki, S.; Shimizu, T.; Krajnc, A.; Jeglič, P.; et al. NMR study of thermally activated paramagnetism in metallic low-silica X zeolite filled with sodium atoms. Phys. Rev. B 2013, 87, 075138. [Google Scholar] [CrossRef]
- Lee, B. Magnetite (Fe3O4): Properties, Synthesis, and Applications. Lehigh Univ. Lehigh Preserv. 2007, 15, 5. [Google Scholar]
- Martin, F.; Arno, S.; Matthias, S. Ab initio study of the half-metal to metal transition in strained magnetite. New J. Phys. 2007, 9, 5. [Google Scholar] [CrossRef]
- Ghezelbash, M.; Darbani, S.M.R.; Majd, A.E.; Ghasemi, A. Temperature Dependence of Magnetic Hysteresis Loop of NdFeB with Uniaxial Anisotropy by LIBS Technique. J. Supercond. Nov. Magnag. 2017, 30, 1893–1898. [Google Scholar] [CrossRef]
- Sato, T.; Nagaoka, K.; Kobayashi, S.; Manjannaa, J.; Murakami, T. Temperature dependence of magnetic hysteresis scaling for cubic Fe3O4 nanoparticles. AIP Adv. 2017, 7, 056319. [Google Scholar] [CrossRef]
- Yu, Y.; Tauxe, L.; Moskowitz, B.M. Temperature dependence of magnetic hysteresis. Geochem. Geophys. Geosystems 2004, 5, 11. [Google Scholar] [CrossRef]
- Kong, I.; Ahmad, S.H.; Abdullah, M.H.; Yusoff, A.N. The Effect of Temperature on Magnetic Behavior of Magnetite Nanoparticles and Its Nanocomposites. In Proceedings of the Nanoscience and Nanotechnology: International Conference on Nanoscience and Nanotechnology, Lucknow, India, 3–5 March 2008; pp. 830–834. [Google Scholar]
- Ghasemi, A. Particle size dependence of magnetic features for Ni0.6−xCuxZn0.4Fe2O4 spinel nanoparticles. J. Magn. Magn. Mater. 2014, 360, 41–47. [Google Scholar] [CrossRef]
- He, X.; Zhong, W.; Au, C.T.; Du, Y. Size dependence of the magnetic properties of Ni nanoparticles prepared by thermal decomposition method. Nanoscale Res. Lett. 2013, 8, 446. [Google Scholar] [CrossRef]
- Bui, T.Q.; Ton, S.N.-C.; Duong, A.T.; Tran, H.T. Size-dependent magnetic responsiveness of magnetite nanoparticles synthesised by co-precipitation and solvothermal methods. J. Sci. Adv. Mater. Devices 2018, 3, 107–112. [Google Scholar] [CrossRef]
- Kumar, E.R.; Srinivas, C.; Seehra, M.S.; Deepty, M.; Pradeep, I.; Kamzin, A.S.; Mehar, M.V.K.; Mohan, N.K. Particle size dependence of the magnetic, dielectric and gas sensing properties of Cosubstituted NiFe2O4 nanoparticles. Sens. Actuators A Phys. 2018, 279, 10–16. [Google Scholar] [CrossRef]
- Widyawati, Y.; Notodarmojo, S.; Hidayat, S.; Helmy, Q. Removal of Hexavalent Chromium from Raw Drinking Water by Zeolite-FeO Composite Supported Carbon: Case Study Citarum River. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 012067. [Google Scholar] [CrossRef]
- Tashauoei, H.R.; Attar, H.; Kamali, M.; Amin, D.M.; Nikaeen, M. Removal of hexavalent chromium (VI) from aqueous solutions using surface modified nanozeolite A. Int. J. Environ. Res. 2010, 4, 491–500. [Google Scholar]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Othman, M.H.D.; Ismail, A.; Rahman, M.; Othman, M.H.D. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef]
- Mintova, S.; Barrier, N. Verified syntheses of zeolitic materials, third revised edition. In Proceedings of the Synthesis Commission of the International Zeolite Association, Selandor, Malaysia, 25–29 February 2008; ISBN 978-0-692-68539-6. [Google Scholar]


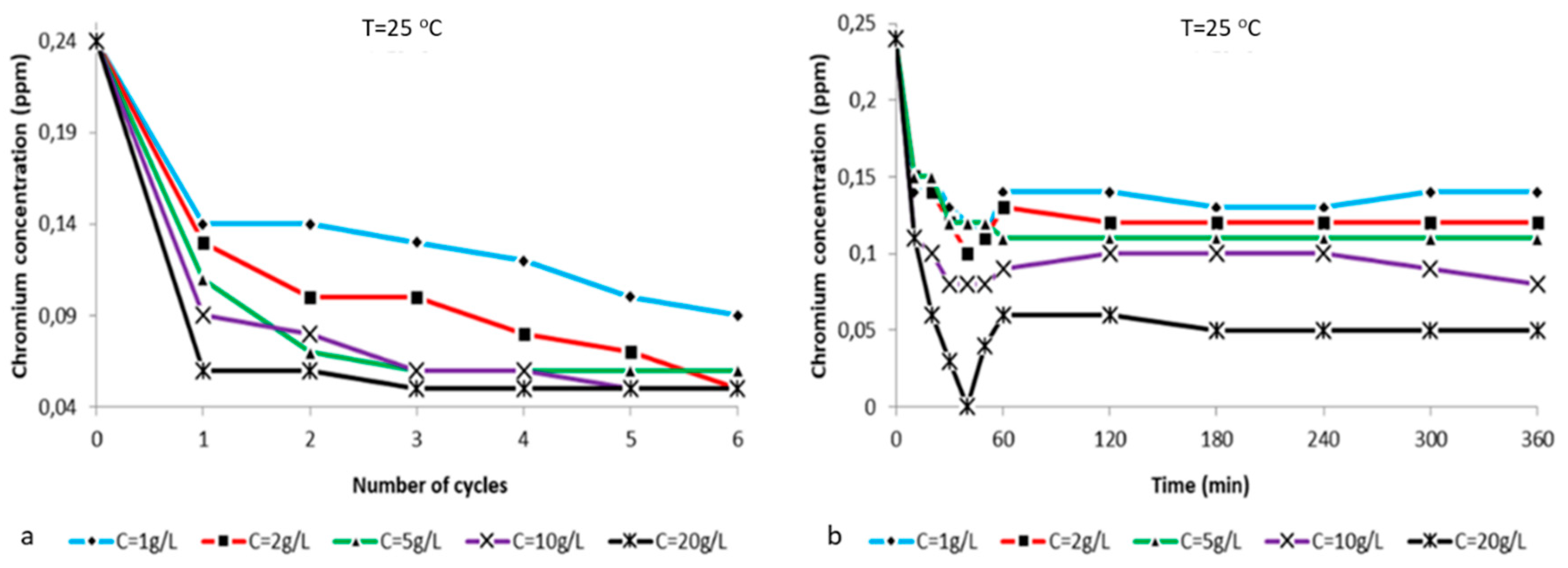
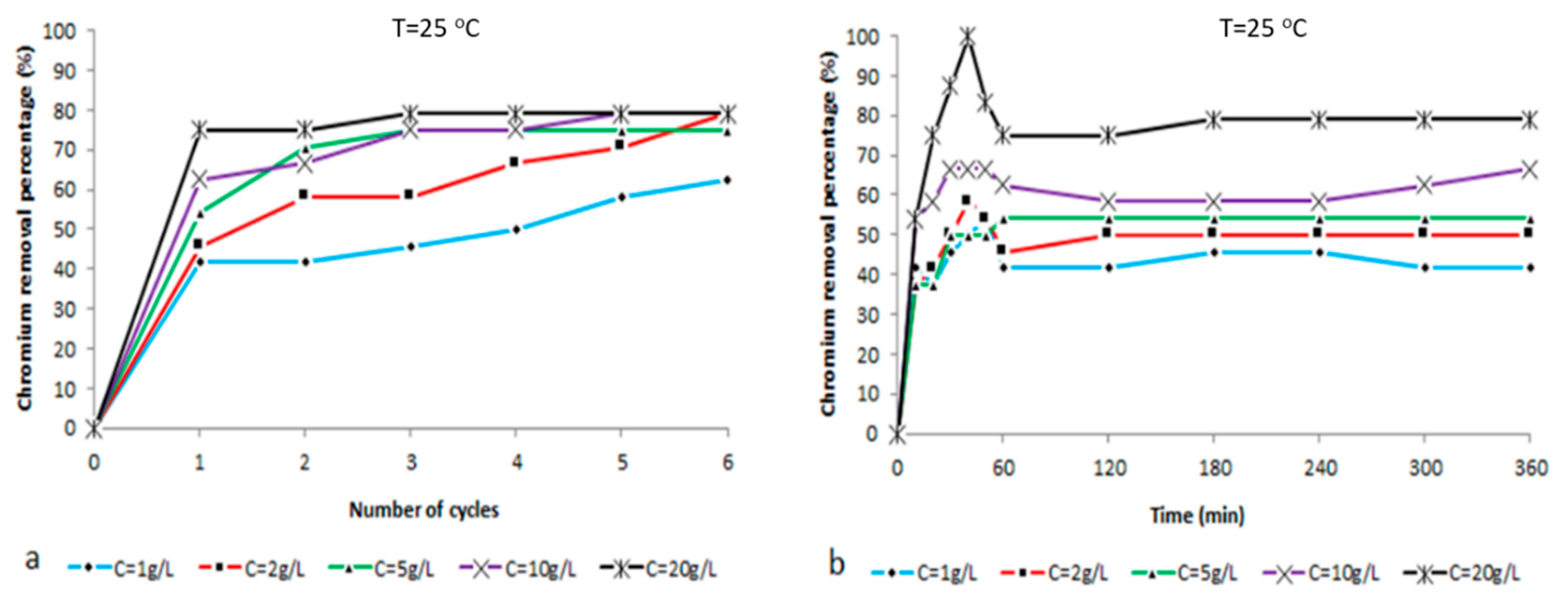
| T = 25 °C | % [Cr6+] Reduction | ||||
|---|---|---|---|---|---|
| Time (Hours) | C = 1 g/L | C = 2 g/L | C = 5 g/L | C = 10 g/L | C = 20 g/L |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 42 | 46 | 54 | 63 | 75 |
| 2 | 42 | 58 | 71 | 67 | 75 |
| 3 | 46 | 58 | 75 | 75 | 79 |
| 4 | 50 | 67 | 75 | 75 | 79 |
| 5 | 58 | 71 | 75 | 79 | 79 |
| 6 | 63 | 79 | 75 | 79 | 79 |
| T = 25 °C | % [Cr6+] Reduction | ||||
|---|---|---|---|---|---|
| Time (min) | C = 1 g/L | C = 2 g/L | C = 5 g/L | C = 10 g/L | C = 20 g/L |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 42 | 38 | 38 | 54 | 54 |
| 20 | 38 | 42 | 38 | 58 | 75 |
| 30 | 46 | 50 | 50 | 67 | 88 |
| 40 | 50 | 58 | 50 | 67 | 100 |
| 50 | 54 | 54 | 50 | 67 | 83 |
| 60 | 42 | 46 | 54 | 63 | 75 |
| 120 | 42 | 50 | 54 | 58 | 75 |
| 180 | 46 | 50 | 54 | 58 | 79 |
| 240 | 46 | 50 | 54 | 58 | 79 |
| 300 | 42 | 50 | 54 | 63 | 79 |
| 360 | 42 | 50 | 54 | 67 | 79 |
| Parameter | Experimental Change |
|---|---|
| Time | 1–6 h |
| Temperature | 5 °C, 25 °C, 50 °C, 70 °C |
| Zeolite-X concentration | 1 g/L, 2 g/L, 5 g/L, 10 g/L, 20 g/L |
| Zeolite-X Concentration (g/L) | Temperature (°C) | Time (h) |
|---|---|---|
| 1 | 5 | 1–6 |
| 25 | 1–6 | |
| 50 | 1–6 | |
| 70 | 1–6 | |
| 2 | 5 | 1–6 |
| 25 | 1–6 | |
| 50 | 1–6 | |
| 70 | 1–6 | |
| 5 | 5 | 1–6 |
| 25 | 1–6 | |
| 50 | 1–6 | |
| 70 | 1–6 | |
| 10 | 5 | 1–6 |
| 25 | 1–6 | |
| 50 | 1–6 | |
| 70 | 1–6 | |
| 20 | 5 | 1–6 |
| 25 | 1–6 | |
| 50 | 1–6 | |
| 70 | 1–6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouli, M.-E.; Banis, G.; Savvidou, M.G.; Ferraro, A.; Hristoforou, E. A Study on Magnetic Removal of Hexavalent Chromium from Aqueous Solutions Using Magnetite/Zeolite-X Composite Particles as Adsorbing Material. Int. J. Mol. Sci. 2020, 21, 2707. https://doi.org/10.3390/ijms21082707
Kouli M-E, Banis G, Savvidou MG, Ferraro A, Hristoforou E. A Study on Magnetic Removal of Hexavalent Chromium from Aqueous Solutions Using Magnetite/Zeolite-X Composite Particles as Adsorbing Material. International Journal of Molecular Sciences. 2020; 21(8):2707. https://doi.org/10.3390/ijms21082707
Chicago/Turabian StyleKouli, Maria-Elisavet, George Banis, Maria G. Savvidou, Angelo Ferraro, and Evangelos Hristoforou. 2020. "A Study on Magnetic Removal of Hexavalent Chromium from Aqueous Solutions Using Magnetite/Zeolite-X Composite Particles as Adsorbing Material" International Journal of Molecular Sciences 21, no. 8: 2707. https://doi.org/10.3390/ijms21082707
APA StyleKouli, M.-E., Banis, G., Savvidou, M. G., Ferraro, A., & Hristoforou, E. (2020). A Study on Magnetic Removal of Hexavalent Chromium from Aqueous Solutions Using Magnetite/Zeolite-X Composite Particles as Adsorbing Material. International Journal of Molecular Sciences, 21(8), 2707. https://doi.org/10.3390/ijms21082707