A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models
Abstract
1. Introduction
RPE Replacements as Experimental Therapies in AMD
2. Results
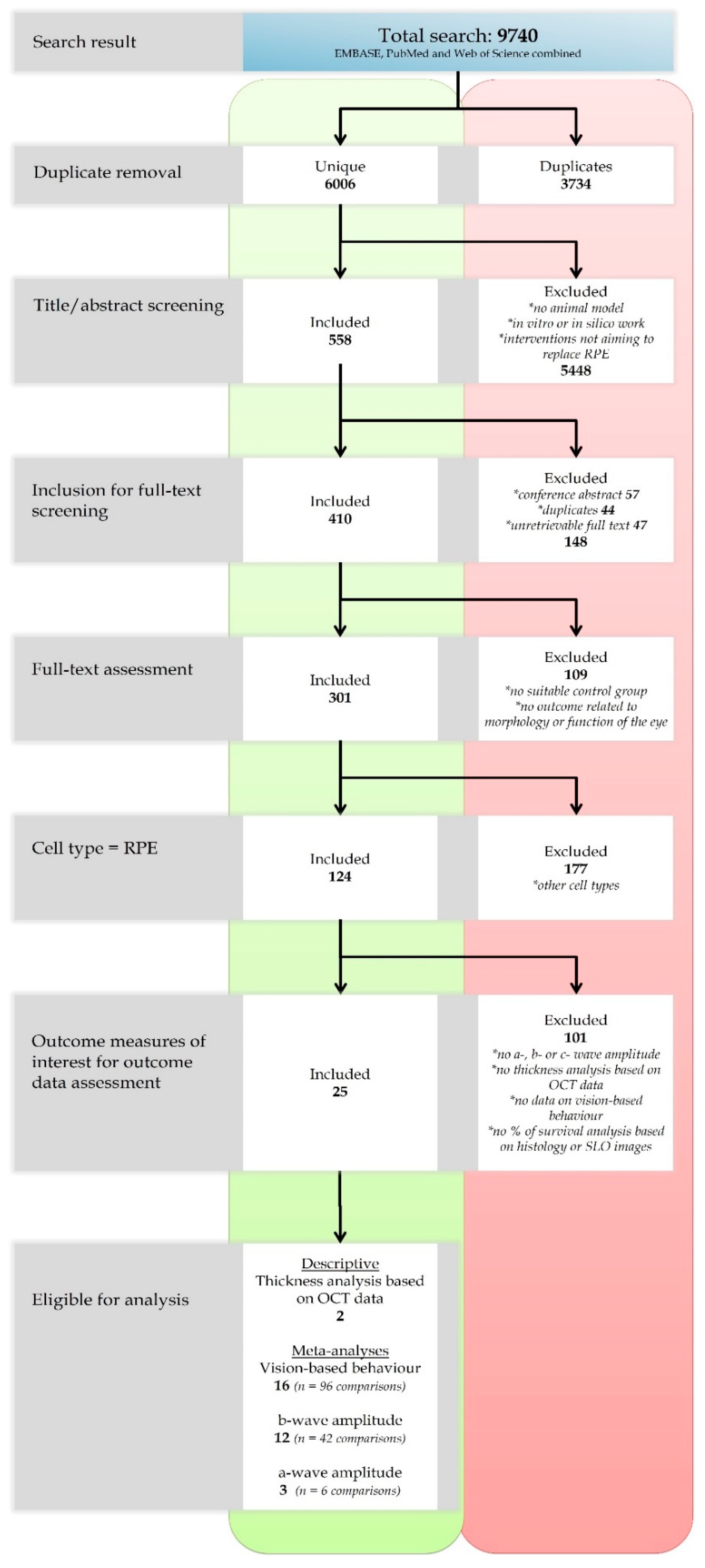
2.1. Search and Study Selection
2.2. Characteristics of the Included Studies
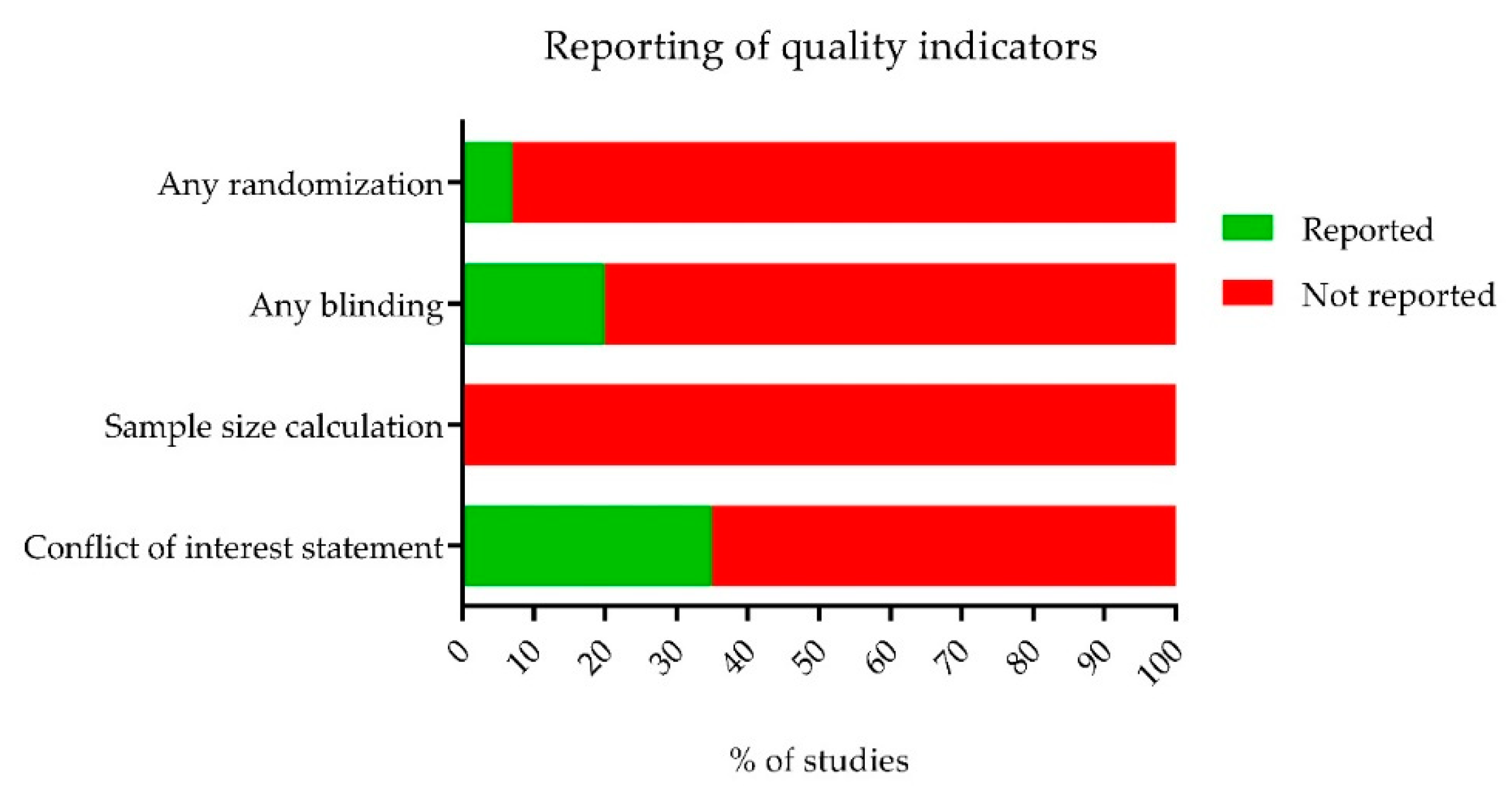
2.3. The Methodological Quality of the Studies
2.4. Descriptive Analysis
Retina Thickness Analysis Based on OCT Data
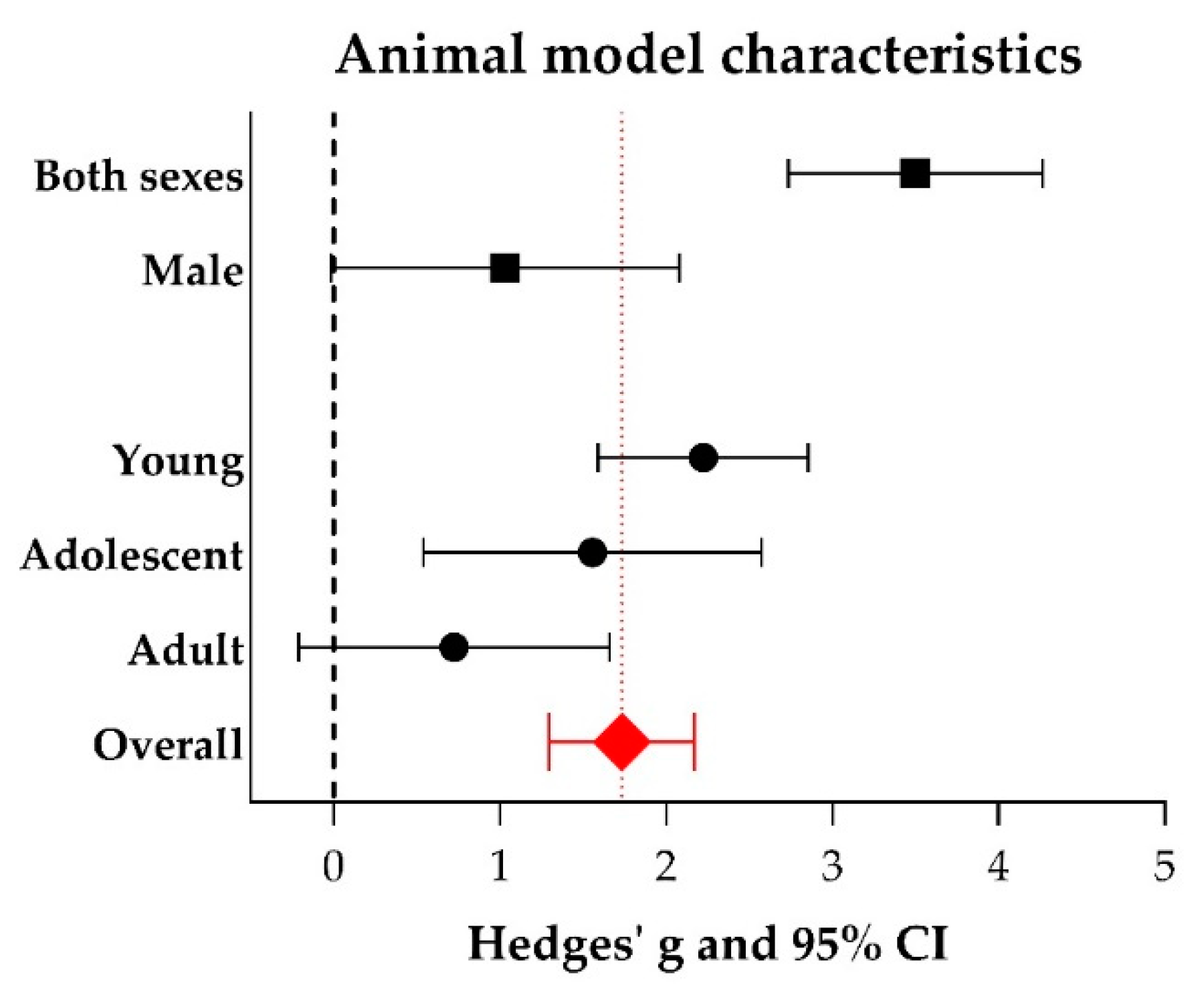
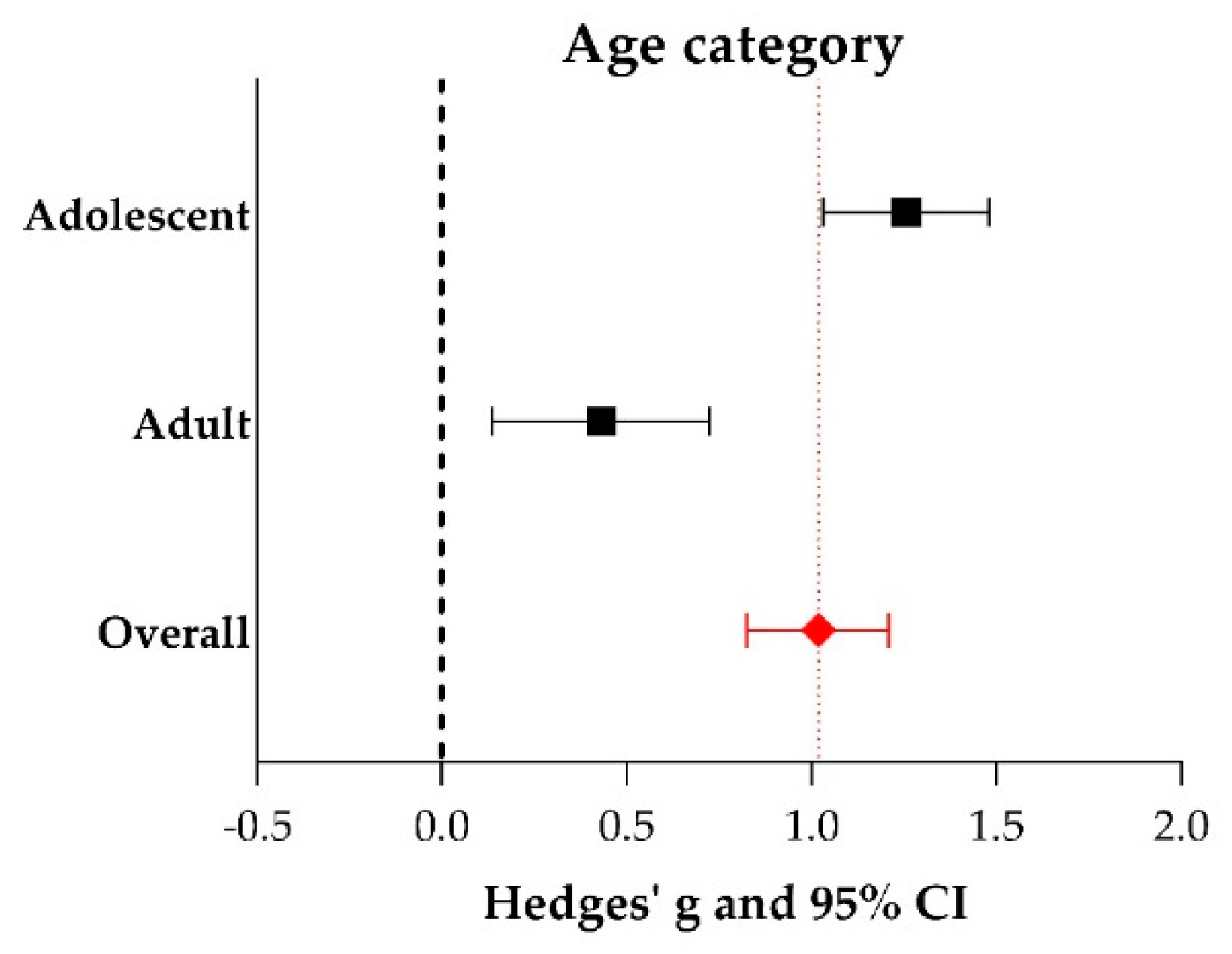
2.5. Meta-Analyses
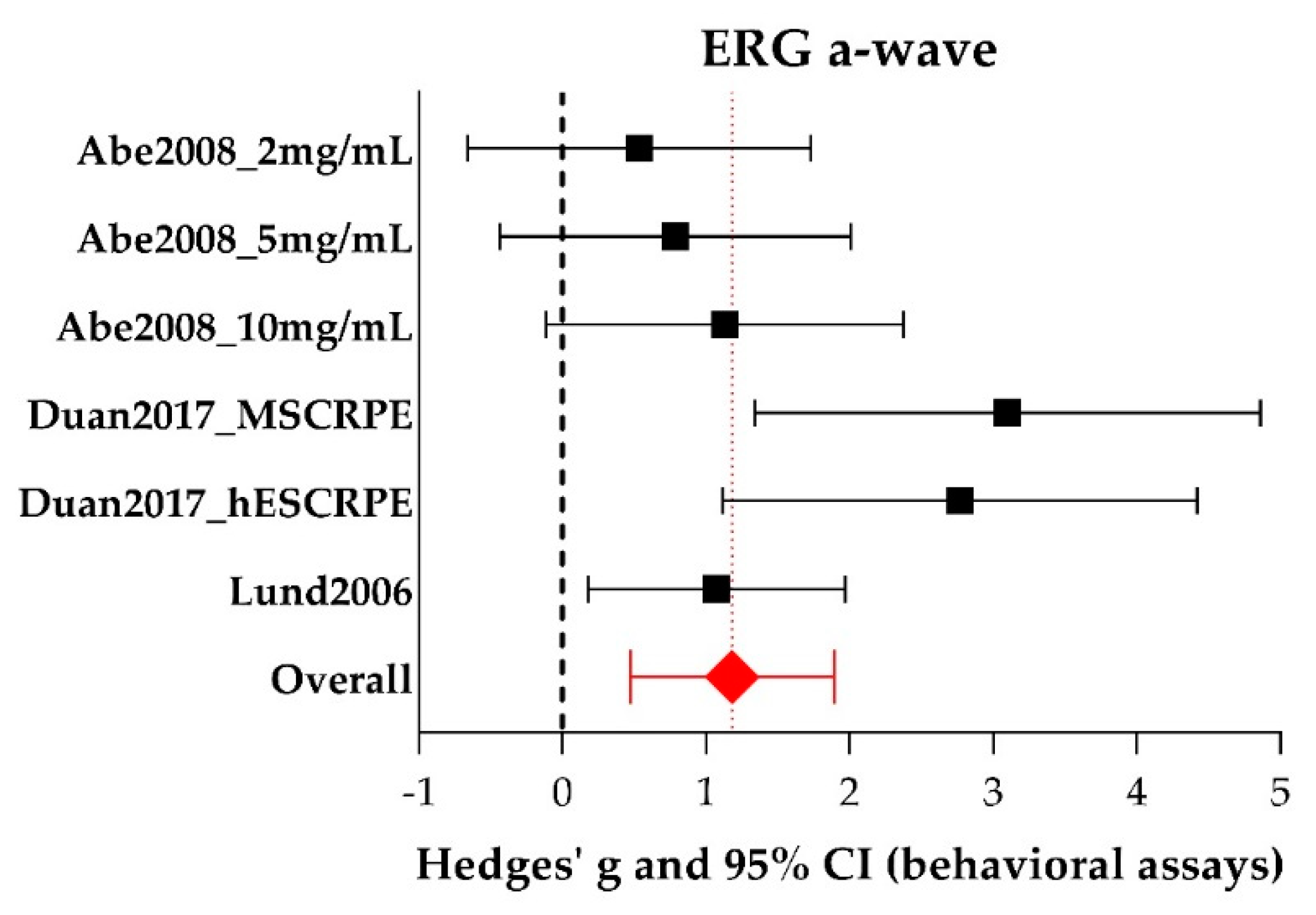
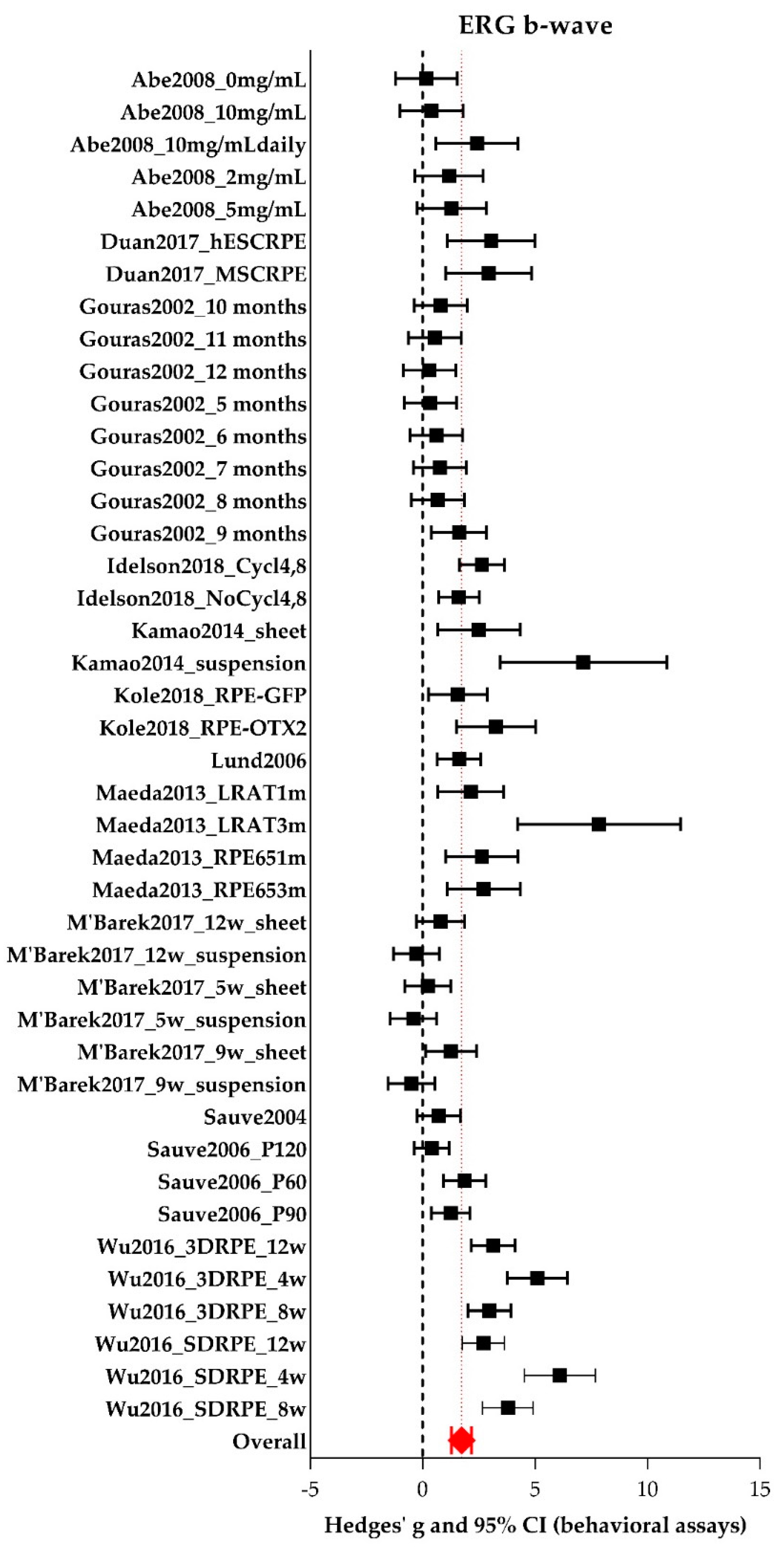
A-wave and B-wave Amplitudes Extracted from ERG Data
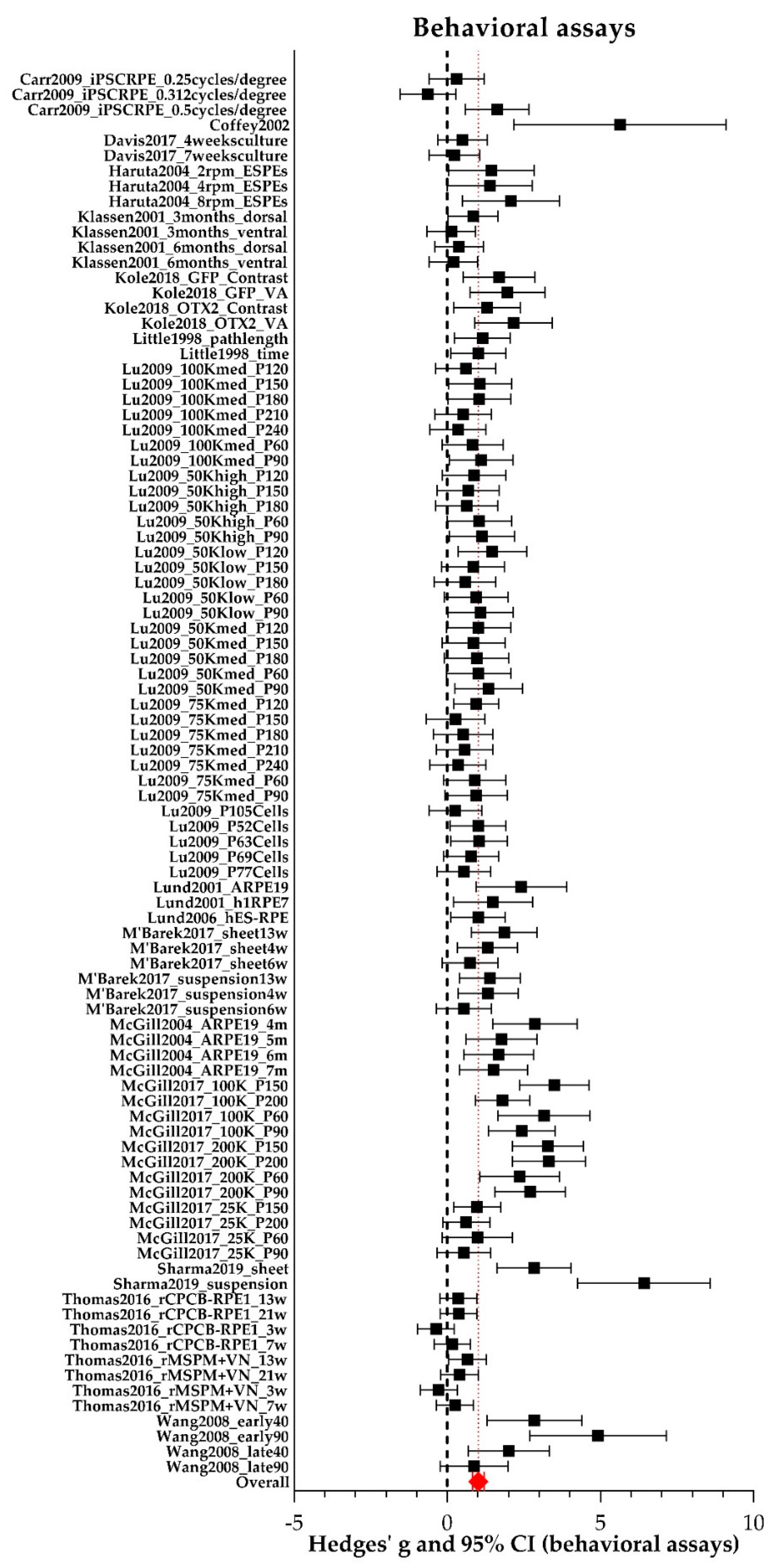
2.6. Behavioral Assays
2.7. Publication Bias
3. Discussion
3.1. Methodological Limitations
3.2. Delivery Methods: Cell Suspensions or Monolayers?
3.3. Study Variables and Characteristics
3.4. RPE Cell Source and Immune Response
3.5. Small Eyes, Big Eyes
3.6. Clinical Implications and Future Perspective
4. Materials and Methods
4.1. Adjustments to the Review Protocol
4.2. Search Strategy
4.3. Study Selection
4.4. Study Characteristics
4.5. Risk of Bias Assessment
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three Dimensions |
| AMD | Age-related Macular Degeneration |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BM | Bruch’s Membrane |
| BMSC | Bone Marrow-derived Mesenchymal Stem Cell |
| CI | Confidence Interval |
| CMA | Comprehensive Meta-analysis Software |
| CPCB | Parylene C Membrane |
| Cycl | Cyclosporine |
| ERG | Electroretinography |
| FDA | Food and Drug Administration |
| GA | Gauge |
| GFP | Green Fluorescent Protein |
| Hedges’ g | Measure of Effect Size |
| hESC | Human embryonic Stem Cell |
| hfRPE | Human Fetal Retinal Pigment Epithelium |
| HLA | Human Leukocyte Antigen |
| I2 | Measure of Heterogeneity |
| iPSC | Induced Pluripotent Stem Cell |
| LRAT | Lecithin Retinol Acyltransferase |
| MERTK | Proto-oncogene Tyrosine-protein Kinase Mer |
| MSC | Mesenchymal Stem Cell |
| MSPM | Mesh-supported Parylene C Membrane |
| NA | Not Applicable |
| NR | Not Reported |
| OCT | Optical Coherence Tomography |
| ONL | Outer Nuclear Layer |
| OTX2 | Orthodenticle Homeobox 2 |
| PLGA | Poly Lactic-co-glycolic Acid |
| PROSPERO | International Prospective Register of Systematic Reviews |
| pRPE | Primary Retinal Pigment Epithelium |
| RPE | Retinal Pigment Epithelium |
| RPE65 | Retinal Pigment Epithelium-specific Protein 65 Kilodalton |
| SD | Single Dimension |
| SD | Standard Deviation |
| SE | Standard Error |
| SLO | Scanning Laser Ophthalmoscopy |
| SMD | Standard Mean Difference |
| SYRCLE | Systematic Review Center for Laboratory Animal Experimentation |
| VN | Vitronectin |
References
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Figueroa, A.G.; McKay, B.S. GPR143 Signaling and Retinal Degeneration. Adv. Exp. Med. Biol. 2019, 1185, 15–19. [Google Scholar]
- Schatz, P.; Preising, M.; Lorenz, B.; Sander, B.; Larsen, M.; Rosenberg, T. Fundus albipunctatus associated with compound heterozygous mutations in RPE65. Ophthalmology 2011, 118, 888–894. [Google Scholar] [CrossRef]
- Talib, M.; van Schooneveld, M.J.; van Duuren, R.J.G.; Van Cauwenbergh, C.; Ten Brink, J.B.; De Baere, E.; Florijn, R.J.; Schalij-Delfos, N.E.; Leroy, B.P.; Bergen, A.A.; et al. Long-Term Follow-Up of Retinal Degenerations Associated with LRAT Mutations and Their Comparability to Phenotypes Associated with RPE65 Mutations. Transl. Vis. Sci. Technol. 2019, 8, 24. [Google Scholar] [CrossRef]
- Hussain, R.M.; Ciulla, T.A.; Berrocal, A.M.; Gregori, N.Z.; Flynn, H.W., Jr.; Lam, B.L. Stargardt macular dystrophy and evolving therapies. Expert Opin. Biol. Ther. 2018, 18, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Inborn Errors of Metabolism: Gyrate Atrophy. Adv. Exp. Med. Biol. 2018, 1085, 183–185. [Google Scholar] [PubMed]
- Guziewicz, K.E.; Sinha, D.; Gomez, N.M.; Zorych, K.; Dutrow, E.V.; Dhingra, A.; Mullins, R.F.; Stone, E.M.; Gamm, D.M.; Boesze-Battaglia, K.; et al. Bestrophinopathy: An RPE-photoreceptor interface disease. Prog. Retin. Eye Res. 2017, 58, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Chen, Y. Application of stem cell-derived retinal pigmented epithelium in retinal degenerative diseases: Present and future. Int. J. Ophthalmol. 2018, 11, 150–159. [Google Scholar] [PubMed]
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Khanna, S.; Komati, R.; Eichenbaum, D.A.; Hariprasad, I.; Ciulla, T.A.; Hariprasad, S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019, 4, e000398. [Google Scholar] [CrossRef]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.C.M.; Lai, T.Y.Y.; Gomi, F.; Ruamviboonsuk, P.; Koh, A.; Lee, W.K. Anti-VEGF Therapy for Neovascular AMD and Polypoidal Choroidal Vasculopathy. Asia Pac. J. Ophthalmol. 2017, 6, 527–534. [Google Scholar]
- Dang, Y.; Zhang, C.; Zhu, Y. Stem cell therapies for age-related macular degeneration: The past, present, and future. Clin. Interv. Aging 2015, 10, 255–264. [Google Scholar] [CrossRef] [PubMed]
- van Meurs, J.C.; Van Den Biesen, P.R. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: Short-term follow-up. Am. J. Ophthalmol. 2003, 136, 688–695. [Google Scholar] [CrossRef]
- van Meurs, J.C.; Kirchhof, B.; MacLaren, R.E. Retinal Pigment Epithelium and Choroid Translocation in Patients with Age-Related Macular Degeneration. In Ryan’s Retina, 6th ed.; Schachat, A., Ed.; Elsevier: Edinburgh, UK, 2018; pp. 2254–2265. [Google Scholar]
- van Meurs, J.C.; ter Averst, E.; Hofland, L.J.; van Hagen, P.M.; Mooy, C.M.; Baarsma, G.S.; Kuijpers, R.W.; Boks, T.; Stalmans, P. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br. J. Ophthalmol. 2004, 88, 110–113. [Google Scholar] [CrossRef]
- Lee, E.; MacLaren, R.E. Sources of retinal pigment epithelium (RPE) for replacement therapy. Br. J. Ophthalmol. 2011, 95, 445–449. [Google Scholar] [CrossRef]
- Pauleikhoff, D.; Harper, C.A.; Marshall, J.; Bird, A.C. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology 1990, 97, 171–178. [Google Scholar] [CrossRef]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.A.; Held, K.S.; Viswanath, V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef]
- Petrus-Reurer, S.; Bartuma, H.; Aronsson, M.; Westman, S.; Lanner, F.; Kvanta, A. Subretinal Transplantation of Human Embryonic Stem Cell Derived-retinal Pigment Epithelial Cells into a Large-eyed Model of Geographic Atrophy. JoVE 2018, 131, e56702. [Google Scholar] [CrossRef]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.; Bergen, A.A. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Sabrosa, A.S.; Duker, J.S.; Waheed, N.K. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: A review. Eye Vis. 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Girman, S.V.; Wang, S.; Lund, R.D. Cortical visual functions can be preserved by subretinal RPE cell grafting in RCS rats. Vis. Res. 2003, 43, 1817–1827. [Google Scholar] [CrossRef]
- Abe, T.; Wakusawa, R.; Seto, H.; Asai, N.; Saito, T.; Nishida, K. Topical doxycycline can induce expression of BDNF in transduced retinal pigment epithelial cells transplanted into the subretinal space. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3631–3639. [Google Scholar] [CrossRef]
- Carr, A.J.; Vugler, A.A.; Hikita, S.T.; Lawrence, J.M.; Gias, C.; Chen, L.L.; Buchholz, D.E.; Ahmado, A.; Semo, M.; Smart, M.J.; et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE 2009, 4, e8152. [Google Scholar] [CrossRef]
- Coffey, P.J.; Girman, S.; Wang, S.M.; Hetherington, L.; Keegan, D.J.; Adamson, P.; Greenwood, J.; Lund, R.D. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat. Neurosci. 2002, 5, 53–56. [Google Scholar] [CrossRef]
- Davis, R.J.; Alam, N.M.; Zhao, C.; Muller, C.; Saini, J.S.; Blenkinsop, T.A.; Mazzoni, F.; Campbell, M.; Borden, S.M.; Charniga, C.J.; et al. The Developmental Stage of Adult Human Stem Cell-Derived Retinal Pigment Epithelium Cells Influences Transplant Efficacy for Vision Rescue. Stem Cell Rep. 2017, 9, 42–49. [Google Scholar] [CrossRef]
- Duan, P.; Zeng, Y.; Liu, Y.; Wang, Y.; Xu, H.; Yin, Z. Comparison of protective effects of hESCs-derived and hBMSCs-derived RPE cells on sodium iodate-injuried rat retina. Int. J. Clin. Exp. Pathol. 2017, 10, 5274–5284. [Google Scholar]
- Gouras, P.; Kong, J.; Tsang, S.H. Retinal degeneration and RPE transplantation in Rpe65(-/-) mice. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3307–3311. [Google Scholar]
- Haruta, M.; Sasai, Y.; Kawasaki, H.; Amemiya, K.; Ooto, S.; Kitada, M.; Suemori, H.; Nakatsuji, N.; Ide, C.; Honda, Y.; et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1020–1025. [Google Scholar] [CrossRef]
- Idelson, M.; Alper, R.; Obolensky, A.; Yachimovich-Cohen, N.; Rachmilewitz, J.; Ejzenberg, A.; Beider, E.; Banin, E.; Reubinoff, B. Immunological Properties of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Stem Cell Rep. 2018, 11, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Okamoto, S.; Sakai, N.; Suga, A.; Sugita, S.; Kiryu, J.; Takahashi, M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.; Whiteley, S.J.; Young, M.J.; Lund, R.D. Graft location affects functional rescue following RPE cell transplantation in the RCS rat. Exp. Neurol. 2001, 169, 114–121. [Google Scholar] [CrossRef]
- Kole, C.; Klipfel, L.; Yang, Y.; Ferracane, V.; Blond, F.; Reichman, S.; Millet-Puel, G.; Clerin, E.; Ait-Ali, N.; Pagan, D.; et al. Otx2-Genetically Modified Retinal Pigment Epithelial Cells Rescue Photoreceptors after Transplantation. Mol. Ther. 2018, 26, 219–237. [Google Scholar] [CrossRef]
- Little, C.W.; Cox, C.; Wyatt, J.; del Cerro, C.; del Cerro, M. Correlates of photoreceptor rescue by transplantation of human fetal RPE in the RCS rat. Exp. Neurol. 1998, 149, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Malcuit, C.; Wang, S.; Girman, S.; Francis, P.; Lemieux, L.; Lanza, R.; Lund, R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009, 27, 2126–2135. [Google Scholar] [CrossRef]
- Lund, R.D.; Adamson, P.; Sauve, Y.; Keegan, D.J.; Girman, S.V.; Wang, S.; Winton, H.; Kanuga, N.; Kwan, A.S.; Beauchene, L.; et al. Subretinal transplantation of genetically modified human cell lines attenuates loss of visual function in dystrophic rats. Proc. Natl. Acad. Sci. USA 2001, 98, 9942–9947. [Google Scholar] [CrossRef]
- Lund, R.D.; Wang, S.; Klimanskaya, I.; Holmes, T.; Ramos-Kelsey, R.; Lu, B.; Girman, S.; Bischoff, N.; Sauve, Y.; Lanza, R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 2006, 8, 189–199. [Google Scholar] [CrossRef]
- Maeda, T.; Lee, M.J.; Palczewska, G.; Marsili, S.; Tesar, P.J.; Palczewski, K.; Takahashi, M.; Maeda, A. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J. Biol. Chem. 2013, 288, 34484–34493. [Google Scholar] [CrossRef]
- Ben M’Barek, K.; Habeler, W.; Plancheron, A.; Jarraya, M.; Regent, F.; Terray, A.; Yang, Y.; Chatrousse, L.; Domingues, S.; Masson, Y.; et al. Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci. Transl. Med. 2017, 9, eaai7471. [Google Scholar] [CrossRef]
- McGill, T.J.; Lund, R.D.; Douglas, R.M.; Wang, S.; Lu, B.; Prusky, G.T. Preservation of vision following cell-based therapies in a model of retinal degenerative disease. Vis. Res. 2004, 44, 2559–2566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGill, T.J.; Bohana-Kashtan, O.; Stoddard, J.W.; Andrews, M.D.; Pandit, N.; Rosenberg-Belmaker, L.R.; Wiser, O.; Matzrafi, L.; Banin, E.; Reubinoff, B.; et al. Long-Term Efficacy of GMP Grade Xeno-Free hESC-Derived RPE Cells Following Transplantation. Transl. Vis. Sci. Technol. 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Sauve, Y.; Lu, B.; Lund, R.D. The relationship between full field electroretinogram and perimetry-like visual thresholds in RCS rats during photoreceptor degeneration and rescue by cell transplants. Vis. Res. 2004, 44, 9–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sauve, Y.; Pinilla, I.; Lund, R.D. Partial preservation of rod and cone ERG function following subretinal injection of ARPE-19 cells in RCS rats. Vis. Res. 2006, 46, 1459–1472. [Google Scholar] [CrossRef][Green Version]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Thomas, B.B.; Zhu, D.; Zhang, L.; Thomas, P.B.; Hu, Y.; Nazari, H.; Stefanini, F.; Falabella, P.; Clegg, D.O.; Hinton, D.R.; et al. Survival and Functionality of hESC-Derived Retinal Pigment Epithelium Cells Cultured as a Monolayer on Polymer Substrates Transplanted in RCS Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2877–2887. [Google Scholar] [CrossRef]
- Wang, S.; Lu, B.; Girman, S.; Holmes, T.; Bischoff, N.; Lund, R.D. Morphological and functional rescue in RCS rats after RPE cell line transplantation at a later stage of degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 416–421. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Y.; Li, Z.; Li, Q.; Xu, H.; Yin, Z.Q. Features specific to retinal pigment epithelium cells derived from three-dimensional human embryonic stem cell cultures—A new donor for cell therapy. Oncotarget 2016, 7, 22819–22833. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. Syrcle’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab. Anim. 2010, 44, 170–175. [Google Scholar] [CrossRef]
- Garcia Delgado, A.B.; De La Cerda, B.; Alba Amador, J.; Valdes Sanchez, M.L.; Fernandez-Munoz, B.; Relimpio Lopez, I.; Rodriguez De La Rua, E.; Diez Lloret, A.; Calado, S.M.; Sanchez Pernaute, R.; et al. Subretinal Transplant of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium on Nanostructured Fibrin-Agarose. Tissue Eng. Part A 2019, 25, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.B.; Zhu, D.; Lin, T.C.; Kim, Y.C.; Seiler, M.J.; Martinez-Camarillo, J.C.; Lin, B.; Shad, Y.; Hinton, D.R.; Humayun, M.S. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 56, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, J.; Mao, X.; Li, G.; Xie, L.; You, Z. Transplantation of rat embryonic stem cell-derived retinal cells restores visual function in the Royal College of Surgeons rats. Doc. Ophthalmol. 2018, 137, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.S.; Newsome, D.A.; Fenech, T.; Hessburg, T.P.; Diamond, J.G.; Miceli, M.V.; Kratz, K.E.; Oliver, P.D. Experimental transplantation of human retinal pigment epithelial cells on collagen substrates. Am. J. Ophthalmol. 1994, 117, 214–221. [Google Scholar] [CrossRef]
- Carido, M.; Zhu, Y.; Postel, K.; Benkner, B.; Cimalla, P.; Karl, M.O.; Kurth, T.; Paquet-Durand, F.; Koch, E.; Munch, T.A.; et al. Characterization of a mouse model with complete RPE loss and its use for RPE cell transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5431–5444. [Google Scholar] [CrossRef]
- Engelhardt, M.; Tosha, C.; Lopes, V.S.; Chen, B.; Nguyen, L.; Nusinowitz, S.; Williams, D.S. Functional and morphological analysis of the subretinal injection of retinal pigment epithelium cells. Vis. Neurosci. 2012, 29, 83–93. [Google Scholar] [CrossRef]
- Ilmarinen, T.; Hiidenmaa, H.; Koobi, P.; Nymark, S.; Sorkio, A.; Wang, J.H.; Stanzel, B.V.; Thieltges, F.; Alajuuma, P.; Oksala, O.; et al. Ultrathin Polyimide Membrane as Cell Carrier for Subretinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigment Epithelium. PLoS ONE 2015, 10, e0143669. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Hamasaki, D. Corneal electroretinographic function rescued by normal retinal pigment epithelial grafts in retinal degenerative Royal College of Surgeons rats. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4300–4309. [Google Scholar]
- Li, Y.; Tsai, Y.T.; Hsu, C.W.; Erol, D.; Yang, J.; Wu, W.H.; Davis, R.J.; Egli, D.; Tsang, S.H. Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol. Med. 2012, 18, 1312–1319. [Google Scholar] [CrossRef]
- Peng, C.H.; Chuang, J.H.; Wang, M.L.; Jhan, Y.Y.; Chien, K.H.; Chung, Y.C.; Hung, K.H.; Chang, C.C.; Lee, C.K.; Tseng, W.L.; et al. Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget 2016, 7, 64631–64648. [Google Scholar] [CrossRef]
- Pinilla, I.; Cuenca, N.; Sauve, Y.; Wang, S.; Lund, R.D. Preservation of outer retina and its synaptic connectivity following subretinal injections of human RPE cells in the Royal College of Surgeons rat. Exp. Eye Res. 2007, 85, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.K.; Tosi, J.; Kasanuki, J.M.; Chou, C.L.; Kong, J.; Parmalee, N.; Wert, K.J.; Allikmets, R.; Lai, C.C.; Chien, C.L.; et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation 2010, 89, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Du, J.; Gouras, P.; Kjeldbye, H. Retinal pigment epithelial transplants and retinal function in RCS rats. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3068–3075. [Google Scholar]
- Muller-Jensen, K.; Mandelcorn, M.S. Membrane formation by autotransplanted retinal pigment epithelium (RPE). Mod. Probl. Ophthalmol. 1975, 15, 228–234. [Google Scholar]
- Little, C.W.; Castillo, B.; DiLoreto, D.A.; Cox, C.; Wyatt, J.; del Cerro, C.; del Cerro, M. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Investig. Ophthalmol. Vis. Sci. 1996, 37, 204–211. [Google Scholar]
- Wang, S.; Girman, S.; Lu, B.; Bischoff, N.; Holmes, T.; Shearer, R.; Wright, L.S.; Svendsen, C.N.; Gamm, D.M.; Lund, R.D. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3201–3206. [Google Scholar] [CrossRef]
- Phillips, S.J.; Sadda, S.R.; Tso, M.O.; Humayan, M.S.; de Juan, E., Jr.; Binder, S. Autologous transplantation of retinal pigment epithelium after mechanical debridement of Bruch’s membrane. Curr. Eye Res. 2003, 26, 81–88. [Google Scholar] [CrossRef]
- Stanzel, B.; Ader, M.; Liu, Z.; Amaral, J.; Aguirre, L.I.R.; Rickmann, A.; Barathi, V.A.; Tan, G.S.W.; Degreif, A.; Al-Nawaiseh, S.; et al. Surgical Approaches for Cell Therapeutics Delivery to the Retinal Pigment Epithelium and Retina. Adv. Exp. Med. Biol. 2019, 1186, 141–170. [Google Scholar]
- Binder, S.; Stanzel, B.V.; Krebs, I.; Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007, 26, 516–554. [Google Scholar] [CrossRef]
- Enzmann, V.; Faude, F.; Wiedemann, P.; Kohen, L. Immunological problems of transplantation into the subretinal space. Cells Tissues Organs 1998, 162, 178–183. [Google Scholar] [CrossRef]
- Li, L.X.; Turner, J.E. Inherited retinal dystrophy in the RCS rat: Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp. Eye Res. 1988, 47, 911–917. [Google Scholar] [CrossRef]
- Li, L.; Turner, J.E. Optimal conditions for long-term photoreceptor cell rescue in RCS rats: The necessity for healthy RPE transplants. Exp. Eye Res. 1991, 52, 669–679. [Google Scholar] [CrossRef]
- Gabrielian, K.; Oganesian, A.; Patel, S.C.; Verp, M.S.; Ernest, J.T. Cellular response in rabbit eyes after human fetal RPE cell transplantation. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Y.; Wang, Y.; Zhang, D.; Shen, B.; Luo, M.; Gu, P. Progress of stem/progenitor cell-based therapy for retinal degeneration. J. Transl. Med. 2017, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- M’Barek, K.B.; Habeler, W.; Monville, C. Stem Cell-Based RPE Therapy for Retinal Diseases: Engineering 3D Tissues Amenable for Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1074, 625–632. [Google Scholar]
- Weed, L.S.; Mills, J.A. Strategies for retinal cell generation from human pluripotent stem cells. Stem Cell Investig. 2017, 4, 65. [Google Scholar] [CrossRef][Green Version]
- Lane, A.; Philip, L.R.; Ruban, L.; Fynes, K.; Smart, M.; Carr, A.; Mason, C.; Coffey, P. Engineering efficient retinal pigment epithelium differentiation from human pluripotent stem cells. Stem Cells Transl. Med. 2014, 3, 1295–1304. [Google Scholar] [CrossRef]
- Drukker, M.; Katchman, H.; Katz, G.; Even-Tov Friedman, S.; Shezen, E.; Hornstein, E.; Mandelboim, O.; Reisner, Y.; Benvenisty, N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 2006, 24, 221–229. [Google Scholar] [CrossRef]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Koss, M.J.; Falabella, P.; Stefanini, F.R.; Pfister, M.; Thomas, B.B.; Kashani, A.H.; Brant, R.; Zhu, D.; Clegg, D.O.; Hinton, D.R.; et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: A feasibility and safety study in Yucatan minipigs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1553–1565. [Google Scholar] [CrossRef]
- Stanzel, B.V.; Liu, Z.; Brinken, R.; Braun, N.; Holz, F.G.; Eter, N. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; Jiao, C.; Kaalberg, E.; Cranston, C.; Mullins, R.F.; Stone, E.M.; Tucker, B.A. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: A pilot study. Sci. Rep. 2015, 5, 11791. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.B.; Hooijmans, C.R.; Tillema, A.; Leenaars, M.; Ritskes-Hoitinga, M. Updated version of the Embase search filter for animal studies. Lab. Anim. 2014, 48, 88. [Google Scholar] [CrossRef]
- Zwetsloot, P.P.; Van Der Naald, M.; Sena, E.S.; Howells, D.W.; IntHout, J.; De Groot, J.A.; Chamuleau, S.A.; MacLeod, M.R.; Wever, K.E. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife 2017, 6, e24260. [Google Scholar] [CrossRef]




| Author Year | Species | Genotype | Induction Method | Donor Species | Cell Type | Suspension or Sheet | Injection Volume (µL) | Scaffold | Immune Suppression | ERG | SLO | OCT | Behavioral |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abe2008 [25] | Rat | WT | Light | Rat | pRPE | Suspension | 2 | NA | N | Y | N | N | N |
| Carr2009 [26] | Rat | MERTK−/− | NA | Human | pRPE | Suspension | 2 | NA | Y | N | N | N | Y |
| Coffey2002 [27] | Rat | MERTK−/− | NA | Human | ARPE-19 | Suspension | 2 | NA | Y | N | N | N | Y |
| Davis2017 [28] | Rat | MERTK−/− | NA | Human | hESC-RPE | Suspension | 1.5 | NA | Y | N | N | N | Y |
| Duan2017 [29] | Rat | WT | NaIO3; i.v.; 50 mg/kg | Human | hBMSC; BMSC-RPE; hESC-RPE | Suspension | 2 | NA | Y | y | N | N | N |
| Gouras2002 [30] | Mouse | RPE65−/− | NA | Mouse | pRPE | Suspension | 10 | NA | NR | Y | N | N | N |
| Haruta2004 [31] | Rat | MERTK−/− | NA | Monkey | hESC-RPE | Suspension | 3 | NA | Y | N | N | N | Y |
| Idelson2018 [32] | Rat | MERTK−/− | NA | Human | hESC-RPE | Suspension | 2–3 | NA | Y | Y | Y | N | N |
| Kamao2014 [33] | Rat; Macaca fascilaris | MERTK−/−; NR | NA; NR | Human; Monkey | pRPE | Suspension; Sheet | 2; 100 | none | Y; NR | Y; N | Y | N; Y | N |
| Klassen2001 [34] | Rat | MERTK−/−; WT | NA | Rat | pRPE | Suspension | 2 | NA | NR | N | N | N | Y |
| Kole2018 [35] | Rat | MERTK−/− | NA | Pig | pRPE | Suspension | 2 | NA | Y | Y | Y | Y | Y |
| Little1998 [36] | Rat | MERTK−/− | NA | Human | pRPE | Sheet | 3–5 | NA | Y | N | N | N | Y |
| Lu2009 [37] | Rat; Mouse | MERTK−/−; ELOVL4−/− | NA | Human | pRPE | Suspension | 2 | NA | Y | N | N | N | Y |
| Lund2001 [38] | Rat | MERTK−/− | NA | Human | ARPE-19; h1RPE7 | Suspension | 2 | NA | Y | N | N | N | Y |
| Lund2006 [39] | Rat | MERTK−/− | NA | Human | pRPE | Suspension | NR | NA | Y | Y | N | N | Y |
| Maeda2013 [40] | Mouse | LRAT−/−; RPE65−/− Tyrc-2J/J | NA | Mouse; Human | pRPE; iPSC-RPE | Suspension | 1.5 | NA | Y | Y | Y | Y | N |
| M’Barek2017 [41] | Rat | MERTK−/− | NA | Human | hESC-RPE | Sheet; Suspension | NA; 4 | Gelatin; NA | Y | Y | N | Y | Y |
| McGill2004 [42] | Rat | MERTK−/− | NA | Human | ARPE-19 | Suspension | 2 | NA | Y | N | N | N | Y |
| McGill2017 [43] | Rat | MERTK−/− | NA | Human | RPE (OpRegen) | Suspension | 2 | NA | Y | N | N | N | Y |
| Sauve2004 [44] | Rat | MERTK−/− | NA | Human | ARPE-19 | Suspension | 2 | NA | Y | Y | N | N | N |
| Sauve2006 [45] | Rat | MERTK−/− | NA | Human | ARPE-19 | Suspension | 2 | NA | Y | Y | N | N | N |
| Sharma2019 [46] | Rat; Pig | MERTK−/− | Laser | Human | iPSC-RPE | Sheet; Suspension | 2 | PLGA | Y | Y | Y | Y | Y |
| Thomas2016 [47] | Rat | MERTK−/− | NA | Human | pRPE | Sheet | NA | CPCB-VN | Y | N | N | N | Y |
| Wang2008 [48] | Rat | MERTK−/− | NA | Human | ARPE-19 | Suspension | 3 | NA | Y | N | N | N | Y |
| Wu2016 [49] | Rat | MERTK−/− | NA | Human | 3DRPE; SDRPE | Suspension | 1–2 | NA | Y | Y | N | N | N |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koster, C.; Wever, K.E.; Wagstaff, E.L.; van den Hurk, K.T.; Hooijmans, C.R.; Bergen, A.A. A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. Int. J. Mol. Sci. 2020, 21, 2719. https://doi.org/10.3390/ijms21082719
Koster C, Wever KE, Wagstaff EL, van den Hurk KT, Hooijmans CR, Bergen AA. A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. International Journal of Molecular Sciences. 2020; 21(8):2719. https://doi.org/10.3390/ijms21082719
Chicago/Turabian StyleKoster, Céline, Kimberley E. Wever, Ellie L. Wagstaff, Koen T. van den Hurk, Carlijn R. Hooijmans, and Arthur A. Bergen. 2020. "A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models" International Journal of Molecular Sciences 21, no. 8: 2719. https://doi.org/10.3390/ijms21082719
APA StyleKoster, C., Wever, K. E., Wagstaff, E. L., van den Hurk, K. T., Hooijmans, C. R., & Bergen, A. A. (2020). A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. International Journal of Molecular Sciences, 21(8), 2719. https://doi.org/10.3390/ijms21082719





