Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses
Abstract
1. Introduction
2. Activation of Caspase-11 Non-Canonical Inflammasome
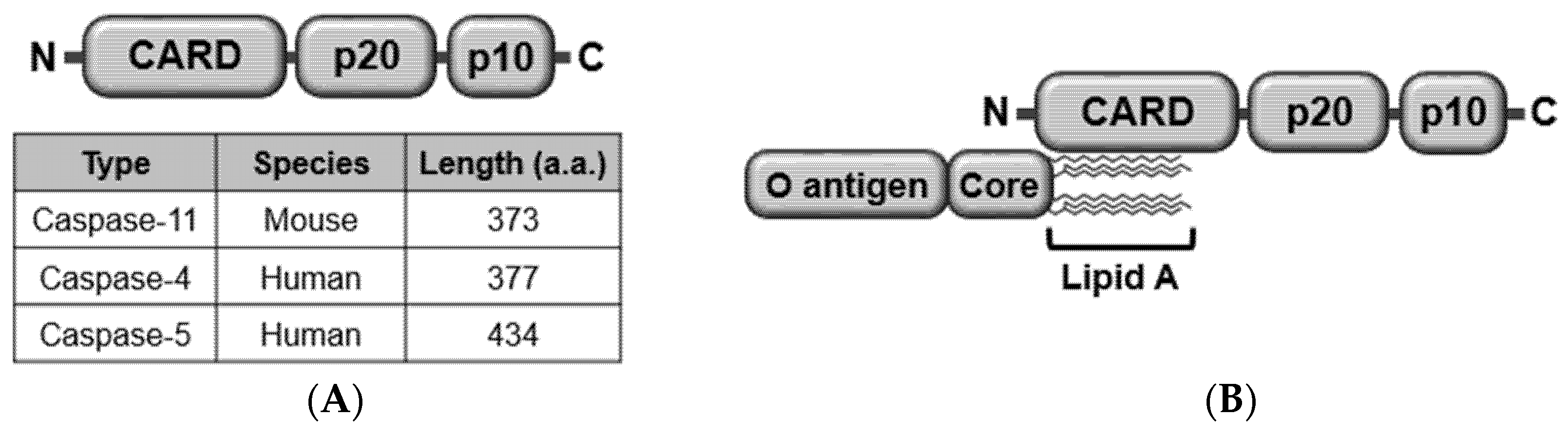
2.1. Structure
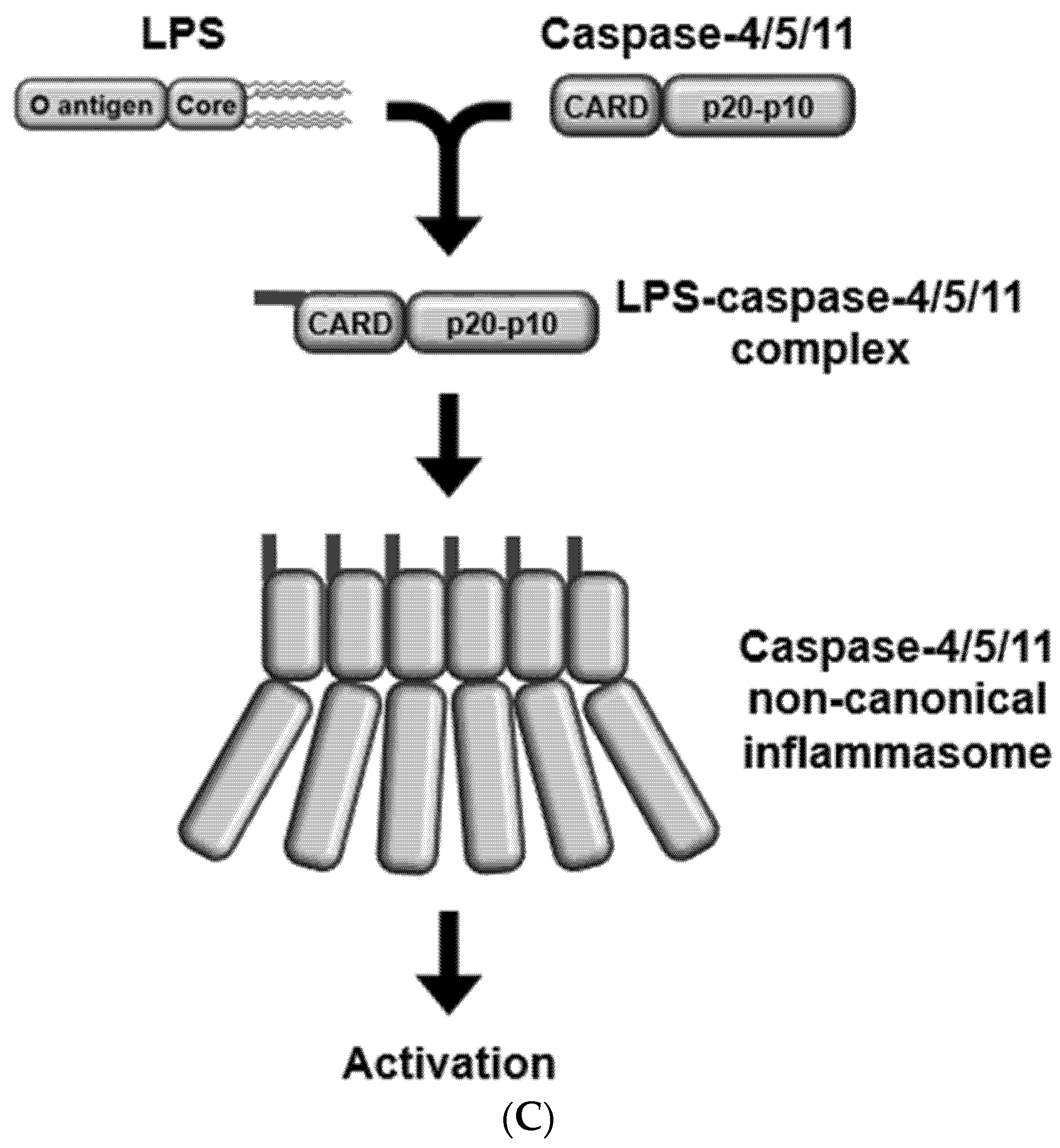
2.2. Activators
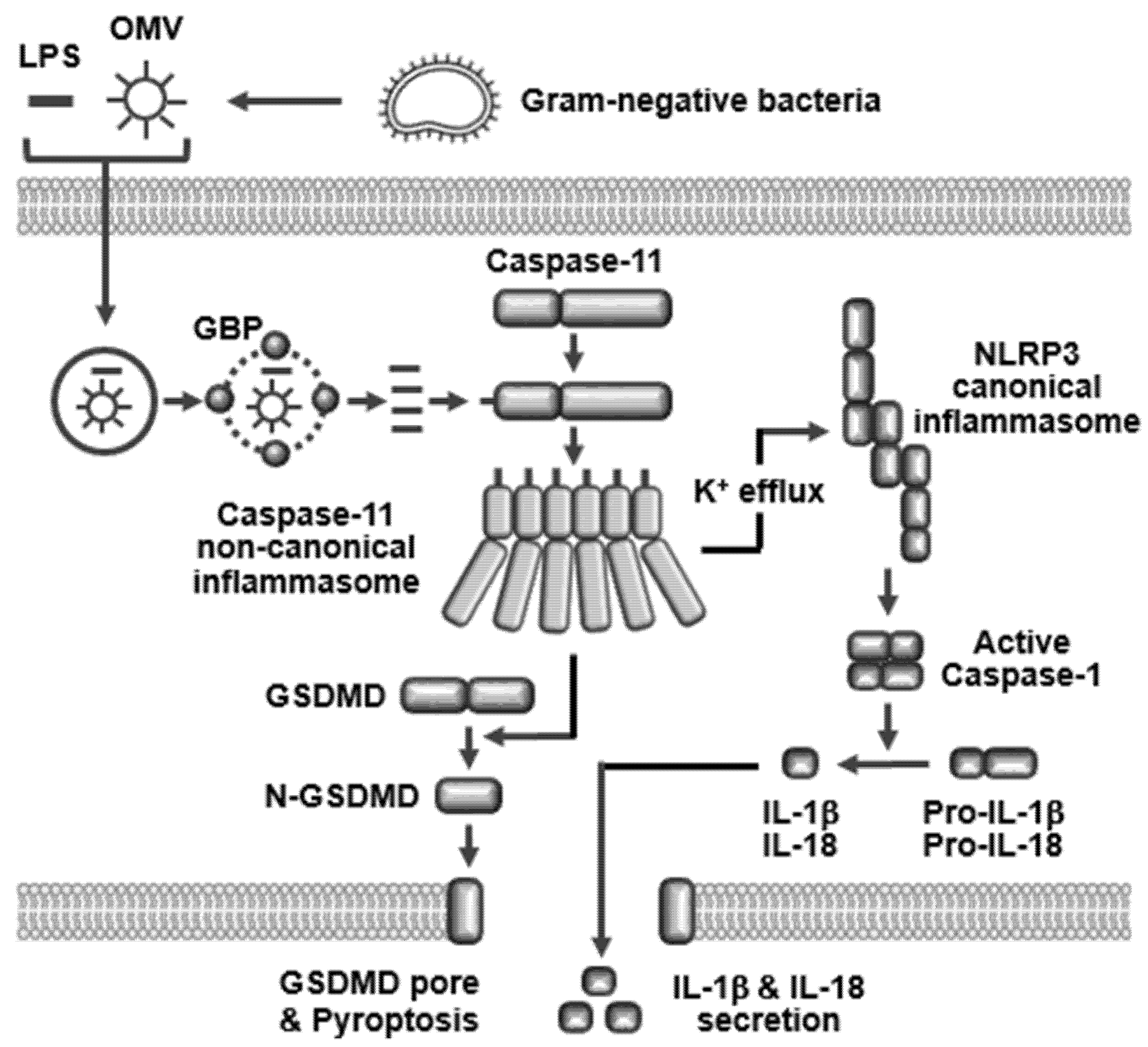
2.3. Ligand Internalization
2.4. Caspase-11 Non-Canonical Inflammasome-Activated Inflammatory Responses
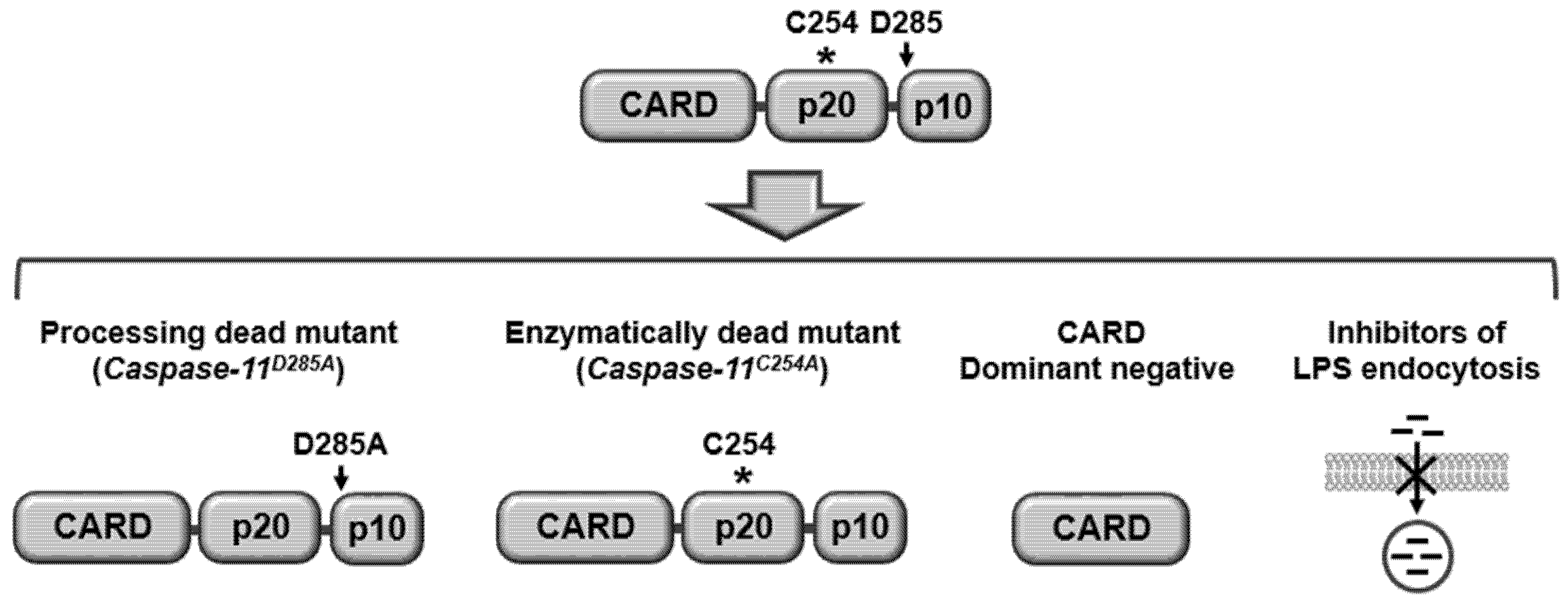
3. Caspase-11 Non-Canonical Inflammasome as a Novel Target for Immunotherapy of Infectious and Inflammatory Diseases
4. Conclusion and Perspectives
Funding
Conflicts of Interest
Abbreviations
| PRR | Pattern recognition receptor |
| PAMP | Pattern-associated molecular pattern |
| DAMP | Danger-associated molecular pattern |
| TLR | Toll-like receptor |
| NLR | NOD-like receptor |
| GSDMD | Gasdermin D |
| CARD | Caspase recruit domain |
| LPS | Lipopolysaccharide |
| TRIF | TIR-domain-containing adapter-inducing interferon β |
| HMGB1 | Hepatocyte-related high-mobility group box 1 |
| RAGE | Receptor for advanced glycan end-product |
| OMV | Outer membrane vesicle |
| GBP | Guanylate-binding proteins |
References
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Ann. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of inflammation in autoimmune disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, M.K.; Aggarwal, H. Inflammation, immunity, and cancer. Mediators Inflamm. 2017, 2017, 6027305. [Google Scholar] [CrossRef]
- Yi, Y.S. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw. 2016, 16, 337–343. [Google Scholar] [CrossRef]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Yu, T.; Yi, Y.S.; Yang, Y.; Oh, J.; Jeong, D.; Cho, J.Y. The pivotal role of tbk1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012, 2012, 979105. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kang, M.; Choi, E.Y. Tlr/myd88-mediated innate immunity in intestinal graft-versus-host disease. Immune Netw. 2017, 17, 144–151. [Google Scholar] [CrossRef]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef]
- Xia, S.; Hollingsworth, L.R.t.; Wu, H. Mechanism and regulation of gasdermin-mediated cell death. Cold Spring Harb. Perspect. Biol. 2020. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory roles of the caspase-11 non-canonical inflammasome in inflammatory diseases. Immune Netw. 2018, 18, e41. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.P.; Cassel, S.L. Inflammasome-mediated autoinflammatory disorders. Postgrad. Med. 2010, 122, 125–133. [Google Scholar] [CrossRef]
- Yi, Y.S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular lps. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Turco, S.J.; Descoteaux, A. The lipophosphoglycan of leishmania parasites. Ann. Rev. Microbiol. 1992, 46, 65–94. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.V.H.; Andrade, W.A.; Lima-Junior, D.S.; Dilucca, M.; de Oliveira, C.V.; Wang, K.; Nogueira, P.M.; Rugani, J.N.; Soares, R.P.; Beverley, S.M.; et al. Leishmania lipophosphoglycan triggers caspase-11 and the non-canonical activation of the nlrp3 inflammasome. Cell Rep. 2019, 26, 429–437 e425. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxpapc protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Vanaja, S.K.; Waggoner, L.; Sokolovska, A.; Becker, C.; Stuart, L.M.; Leong, J.M.; Fitzgerald, K.A. Trif licenses caspase-11-dependent nlrp3 inflammasome activation by gram-negative bacteria. Cell 2012, 150, 606–619. [Google Scholar] [CrossRef]
- Gurung, P.; Malireddi, R.K.; Anand, P.K.; Demon, D.; Vande Walle, L.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Toll or interleukin-1 receptor (tir) domain-containing adaptor inducing interferon-beta (trif)-mediated caspase-11 protease production integrates toll-like receptor 4 (tlr4) protein- and nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 2012, 287, 34474–34483. [Google Scholar] [CrossRef]
- Lacey, C.A.; Mitchell, W.J.; Dadelahi, A.S.; Skyberg, J.A. Caspase-1 and caspase-11 mediate pyroptosis, inflammation, and control of brucella joint infection. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Gabrielli, E.; Pericolini, E.; Luciano, E.; Sabbatini, S.; Roselletti, E.; Perito, S.; Kasper, L.; Hube, B.; Vecchiarelli, A. Induction of caspase-11 by aspartyl proteinases of candida albicans and implication in promoting inflammatory response. Infect. Immun. 2015, 83, 1940–1948. [Google Scholar] [CrossRef]
- Gegner, J.A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide (lps) signal transduction and clearance. Dual roles for lps binding protein and membrane cd14. J. Biol. Chem. 1995, 270, 5320–5325. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The endotoxin delivery protein hmgb1 mediates caspase-11-dependent lethality in sepsis. Immunity 2018, 49, 740–753 e747. [Google Scholar] [CrossRef]
- Yokoyama, S.; Cai, Y.; Murata, M.; Tomita, T.; Yoneda, M.; Xu, L.; Pilon, A.L.; Cachau, R.E.; Kimura, S. A novel pathway of lps uptake through syndecan-1 leading to pyroptotic cell death. Elife 2018, 7. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial outer membrane vesicles mediate cytosolic localization of lps and caspase-11 activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell. Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Tripal, P.; Bauer, M.; Naschberger, E.; Mortinger, T.; Hohenadl, C.; Cornali, E.; Thurau, M.; Sturzl, M. Unique features of different members of the human guanylate-binding protein family. J. Interferon Cytokine Res. 2007, 27, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Meunier, E.; Dick, M.S.; Dreier, R.F.; Schurmann, N.; Kenzelmann Broz, D.; Warming, S.; Roose-Girma, M.; Bumann, D.; Kayagaki, N.; Takeda, K.; et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by ifn-induced gtpases. Nature 2014, 509, 366–370. [Google Scholar] [CrossRef]
- Pilla, D.M.; Hagar, J.A.; Haldar, A.K.; Mason, A.K.; Degrandi, D.; Pfeffer, K.; Ernst, R.K.; Yamamoto, M.; Miao, E.A.; Coers, J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic lps. Proc. Natl. Acad. Sci. USA 2014, 111, 6046–6051. [Google Scholar] [CrossRef]
- Ding, J.; Shao, F. Snapshot: The noncanonical inflammasome. Cell 2017, 168, 544–544.e1. [Google Scholar] [CrossRef]
- Lee, B.L.; Stowe, I.B.; Gupta, A.; Kornfeld, O.S.; Roose-Girma, M.; Anderson, K.; Warming, S.; Zhang, J.; Lee, W.P.; Kayagaki, N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 2018, 215, 2279–2288. [Google Scholar] [CrossRef]
- Ruhl, S.; Broz, P. Caspase-11 activates a canonical nlrp3 inflammasome by promoting k(+) efflux. Eur. J. Immunol. 2015, 45, 2927–2936. [Google Scholar] [CrossRef]
- Cunha, L.D.; Silva, A.L.N.; Ribeiro, J.M.; Mascarenhas, D.P.A.; Quirino, G.F.S.; Santos, L.L.; Flavell, R.A.; Zamboni, D.S. Aim2 engages active but unprocessed caspase-1 to induce noncanonical activation of the nlrp3 inflammasome. Cell Rep. 2017, 20, 794–805. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional crosstalk between non-canonical caspase-11 and canonical nlrp3 inflammasomes during infection-mediated inflammation. Immunology 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Zeng, Q.; Imperato, G.H.; Addorisio, M.E.; Li, J.; He, M.; Cheng, K.F.; Al-Abed, Y.; Harris, H.E.; et al. Inhibition of hmgb1/rage-mediated endocytosis by hmgb1 antagonist box a, anti-hmgb1 antibodies, and cholinergic agonists suppresses inflammation. Mol. Med. 2019, 25, 13. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.-S. Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses. Int. J. Mol. Sci. 2020, 21, 2736. https://doi.org/10.3390/ijms21082736
Yi Y-S. Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses. International Journal of Molecular Sciences. 2020; 21(8):2736. https://doi.org/10.3390/ijms21082736
Chicago/Turabian StyleYi, Young-Su. 2020. "Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses" International Journal of Molecular Sciences 21, no. 8: 2736. https://doi.org/10.3390/ijms21082736
APA StyleYi, Y.-S. (2020). Caspase-11 Non-Canonical Inflammasome: Emerging Activator and Regulator of Infection-Mediated Inflammatory Responses. International Journal of Molecular Sciences, 21(8), 2736. https://doi.org/10.3390/ijms21082736




