Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photosensitizers and Their Optical Properties
2.2. Detection of Reactive Oxygen Species Using Fluorescent Probes
2.3. Lipophilicity of Photosensitizer (logP Determination)
2.4. Biological Studies
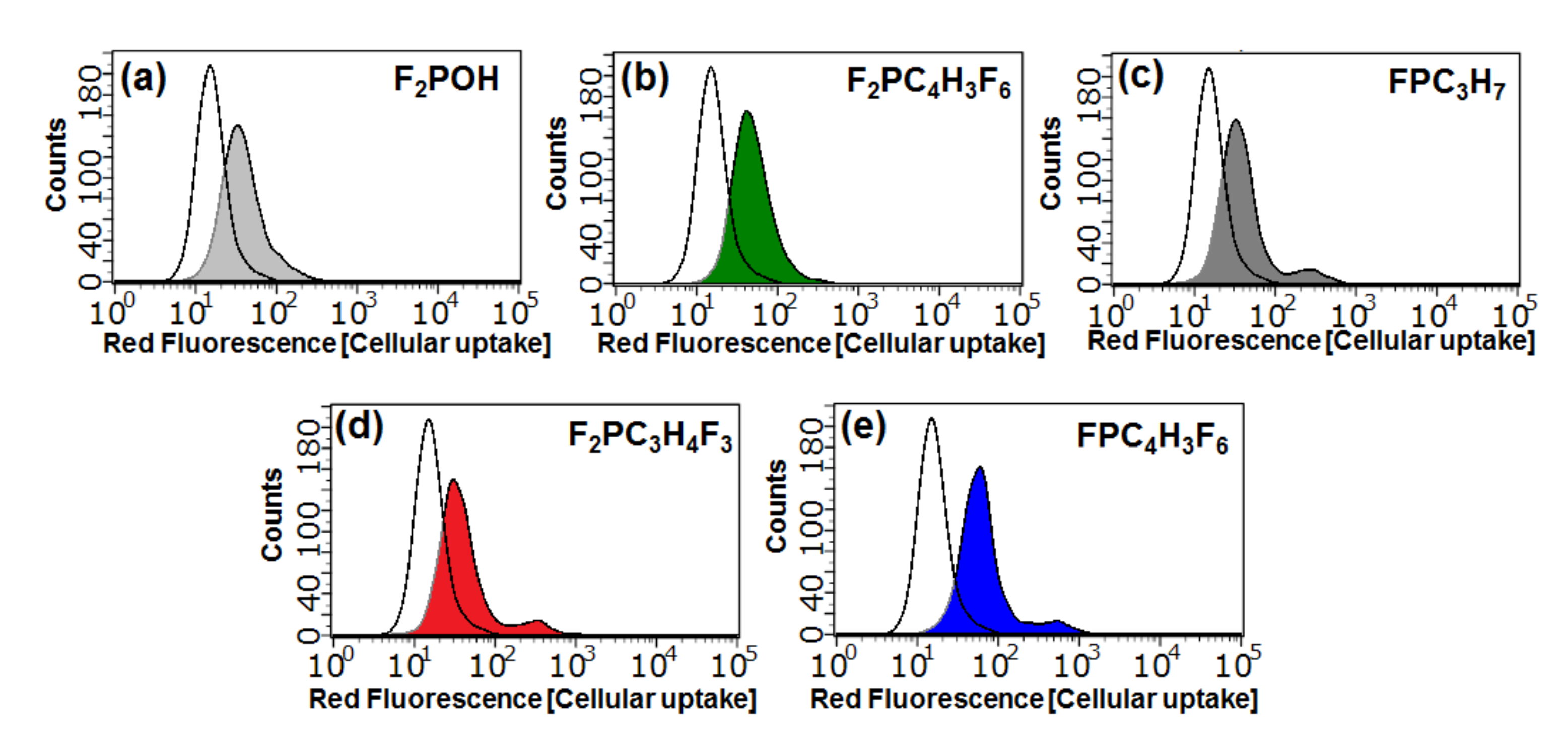
2.4.1. Cellular Uptake
2.4.2. CLSM Imaging and Subcellular Localization
2.4.3. Cytotoxicity in the Dark
2.4.4. ROS Generation In Vitro
2.4.5. Photodynamic Effect
3. Materials and Methods
3.1. Chemicals
3.2. UV/VIS/NIR Electronic Absorption and Emission Spectra Measurements
3.3. Detection of Reactive Oxygen Species
3.4. n-Octanol/Water Partition Coefficients
3.5. Cells Culture
3.6. Cellular Uptake
3.7. Confocal Laser Scanning Microscopy (CLSM) Imaging
3.8. Dark Cytotoxicity and Cells Viability Assay
3.9. ROS Generation In Vitro
3.10. In Vitro Phototoxicity of Sulfonate Ester Porphyrins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Rocha, L.B.; Gomes-da-Silva, L.C.; Dąbrowski, J.M.; Arnaut, L.G. Elimination of primary tumours and control of metastasis with rationally designed bacteriochlorin photodynamic therapy regimens. Eur. J. Cancer 2015, 51, 1822–1830. [Google Scholar] [CrossRef]
- Lobo, S.; Catarina, A.; Gomes-da-Silva, L.C.; Rodrigues-Santos, P.; Cabrita, A.; Santos-Rosa, M.; Arnaut, L.G. Immune Responses after Vascular Photodynamic Therapy with Redaporfin. J. Clin. Med. 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucelik, B.; Arnaut, L.G.; Dąbrowski, J.M. Lipophilicity of bacteriochlorin-based photosensitizers as a determinant for PDT optimization through the modulation of the inflammatory mediators. J. Clin. Med. 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Monteiro, C.J.; Simões, A.V.; Pinto, S.M.; Abreu, A.R.; Sá, G.F.; Silva, E.F.; Rocha, L.B.; Dąbrowski, J.M.; Formosinho, S.J. Synthesis and photophysical characterization of a library of photostable halogenated bacteriochlorins: An access to near infrared chemistry. Tetrahedron 2010, 66, 9545–9551. [Google Scholar] [CrossRef]
- Ogura, S.-i.; Fujita, Y.; Kamachi, T.; Okura, I. Preparation of chlorin e6–monoclonal antibody conjugate and its effect for photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 486–489. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5, 10, 15, 20-tetra (m-hydroxyphenyl) chlorin)—A second-generation photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmuş, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn (II) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar] [CrossRef]
- Tekdaş, D.A.; Kumru, U.; Gürek, A.G.; Durmuş, M.; Ahsen, V.; Dumoulin, F. Towards near-infrared photosensitisation: A photosensitising hydrophilic non-peripherally octasulfanyl-substituted Zn phthalocyanine. Tetrahedron Lett. 2012, 53, 5227–5230. [Google Scholar] [CrossRef]
- Ghazal, B.; Kaya, E.N.; Husain, A.; Ganesan, A.; Durmuş, M.; Makhseed, S. Biotinylated-cationic zinc (II) phthalocyanine towards photodynamic therapy. J. Porphyr. Phthalocyanines 2019, 23, 46–55. [Google Scholar] [CrossRef]
- Kielmann, M.; Prior, C.; Senge, M.O. Porphyrins in troubled times: A spotlight on porphyrins and their metal complexes for explosives testing and CBRN defense. N. J. Chem. 2018, 42, 7529–7550. [Google Scholar] [CrossRef]
- Da GH Vicente, M.; Smith, K.M. Syntheses and functionalizations of porphyrin macrocycles. Curr. Org. Synth. 2014, 11, 3–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, R.K.; Vicente, M.G.H.; Shiau, F.-Y.; Dougherty, T.J.; Smith, K.M. Syntheses of porphyrin and chlorin dimers for photodynamic therapy. In Proceedings of the Optical Methods for Tumor Treatment and Early Diagnosis: Mechanisms and Techniques, Los Angeles, CA, USA, 1 June 1991; pp. 356–361. [Google Scholar]
- Carneiro, J.; Gonçalves, A.; Zhou, Z.; Griffin, K.E.; Kaufman, N.E.; Vicente, M.D.G.H. Synthesis and in vitro PDT evaluation of new porphyrins containing meso-epoxymethylaryl cationic groups. Lasers Surg. Med. 2018, 50, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Wiehe, A.; Simonenko, E.J.; Senge, M.O.; RÖder, B. Hydrophilicity vs. hydrophobicity—Varying the amphiphilic structure of porphyrins related to the photosensitizer m-THPC. J. Porphyr. Phthalocyanines 2001, 5, 758–761. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.-H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.-L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Rizvi, I.; Obaid, G.; Bano, S.; Hasan, T.; Kessel, D. Photodynamic therapy: Promoting in vitro efficacy of photodynamic therapy by liposomal formulations of a photosensitizing agent. Lasers Surg. Med. 2018, 50, 499–505. [Google Scholar] [CrossRef]
- Pucelik, B.; Arnaut, L.G.; Stochel, G.y.; Dabrowski, J.M. Design of Pluronic-based formulation for enhanced redaporfin-photodynamic therapy against pigmented melanoma. Acs Appl. Mater. Interfaces 2016, 8, 22039–22055. [Google Scholar] [CrossRef]
- Pucelik, B.; Gürol, I.; Ahsen, V.; Dumoulin, F.; Dąbrowski, J.M. Fluorination of phthalocyanine substituents: Improved photoproperties and enhanced photodynamic efficacy after optimal micellar formulations. Eur. J. Med. Chem. 2016, 124, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Sharma, S.K.; Dai, T.; Chung, H.; Yaroslavsky, A.; Garcia-Diaz, M.; Chang, J.; Chiang, L.Y.; Hamblin, M.R. Can nanotechnology potentiate photodynamic therapy? Nanotechnol. Rev. 2012, 1, 111–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moret, F.; Reddi, E. Strategies for optimizing the delivery to tumors of macrocyclic photosensitizers used in photodynamic therapy (PDT). J. Porphyr. Phthalocyanines 2017, 21, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Topal, S.Z.; İşci, Ü.; Kumru, U.; Atilla, D.; Gürek, A.G.; Hirel, C.; Durmuş, M.; Tommasino, J.-B.; Luneau, D.; Berber, S. Modulation of the electronic and spectroscopic properties of Zn (II) phthalocyanines by their substitution pattern. Dalton Trans. 2014, 43, 6897–6908. [Google Scholar] [CrossRef]
- Arnaut, L.G.; Pereira, M.M.; Dąbrowski, J.M.; Silva, E.F.; Schaberle, F.A.; Abreu, A.R.; Rocha, L.B.; Barsan, M.M.; Urbańska, K.; Stochel, G. Photodynamic therapy efficacy enhanced by dynamics: The role of charge transfer and photostability in the selection of photosensitizers. Chemistry 2014, 20, 5346–5357. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pereira, M.M.; Arnaut, L.G.; Monteiro, C.J.; Peixoto, A.F.; Karocki, A.; Urbańska, K.; Stochel, G. Synthesis, photophysical studies and anticancer activity of a new halogenated water-soluble porphyrin. Photochem. Photobiol. 2007, 83, 897–903. [Google Scholar] [CrossRef]
- Rocha, L.B.; Schaberle, F.; Dąbrowski, J.M.; Simões, S.; Arnaut, L.G. Intravenous single-dose toxicity of redaporfin-based photodynamic therapy in rodents. Int. J. Mol. Sci. 2015, 16, 29236–29249. [Google Scholar] [CrossRef] [Green Version]
- Pucelik, B.; Paczyński, R.; Dubin, G.; Pereira, M.M.; Arnaut, L.G.; Dąbrowski, J.M. Properties of halogenated and sulfonated porphyrins relevant for the selection of photosensitizers in anticancer and antimicrobial therapies. PLoS ONE 2017, 12, e0185984. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Balasubramanian, T.; Yang, E.; Luo, D.; Diers, J.R.; Bocian, D.F.; Hamblin, M.R. Stable synthetic bacteriochlorins for photodynamic therapy: Role of dicyano peripheral groups, central metal substitution (2H, Zn, Pd), and Cremophor EL delivery. ChemMedChem 2012, 7, 2155–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, R.; Rocha, L.B.; Dąbrowski, J.M.; Arnaut, L.G. Modulation of biodistribution, pharmacokinetics, and photosensitivity with the delivery vehicle of a bacteriochlorin photosensitizer for photodynamic therapy. ChemMedChem 2014, 9, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.F.; Pucelik, B.; Pereira, M.M.; Dąbrowski, J.M.; Arnaut, L.G. Translating phototherapeutic indices from in vitro to in vivo photodynamic therapy with bacteriochlorins. Lasers Surg. Med. 2018, 50, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Barret, E.; Moore, C.M.; Villers, A.; Allen, C.; Scherz, A.; Muir, G.; de Wildt, M.; Barber, N.J.; Lebdai, S. TOOKAD® S oluble vascular-targeted photodynamic (VTP) therapy: Determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013, 112, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.L.; Oliveira, J.; Monteiro, E.; Santos, J.; Sarmento, C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep. Oncol. 2018, 11, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Sobral, A.J.; Eléouet, S.; Rousset, N.; Gonsalves, A.M.d.A.R.; Le Meur, O.; Bourré, L.; Patrice, T. New sulfonamide and sulfonic ester porphyrins as sensitizers for photodynamic therapy. J. Porphyr. Phthalocyanines 2002, 6, 456–462. [Google Scholar] [CrossRef]
- Simoes, A.V.; Adamowicz, A.; Dąbrowski, J.M.; Calvete, M.J.; Abreu, A.R.; Stochel, G.; Arnaut, L.G.; Pereira, M.M. Amphiphilic meso (sulfonate ester fluoroaryl) porphyrins: Refining the substituents of porphyrin derivatives for phototherapy and diagnostics. Tetrahedron 2012, 68, 8767–8772. [Google Scholar] [CrossRef]
- Munkelt, D.; Koehl, U.; Kloess, S.; Zimmermann, S.-Y.; El Kalaäoui, R.; Wehner, S.; Schwabe, D.; Lehrnbecher, T.; Schubert, R.; Kreuter, J. Cytotoxic effects of treosulfan and busulfan against leukemic cells of pediatric patients. Cancer Chemother. Pharmacol. 2008, 62, 821–830. [Google Scholar] [CrossRef]
- Yan, L.; Müller, C.E. Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: Toward the development of sulfonic acid prodrugs with peroral bioavailability. J. Med. Chem. 2004, 47, 1031–1043. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Arnaut, L.G. Design of porphyrin-based photosensitizers for photodynamic therapy. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 63, pp. 187–233. [Google Scholar]
- Dini, D.; Calvete, M.J.; Hanack, M.; Pong, R.G.; Flom, S.R.; Shirk, J.S. Nonlinear transmission of a tetrabrominated naphthalocyaninato indium chloride. J. Phys. Chem. B 2006, 110, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M. Reactive oxygen species in photodynamic therapy: Mechanisms of their generation and potentiation. In Advances in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 70, pp. 343–394. [Google Scholar]
- Silva, E.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of singlet-oxygen and superoxide-ion generation by porphyrins and bacteriochlorins and their implications in photodynamic therapy. Chemistry 2010, 16, 9273–9286. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowski, J.M.; Arnaut, L.G.; Pereira, M.M.; Urbańska, K.; Simões, S.; Stochel, G.; Cortes, L. Combined effects of singlet oxygen and hydroxyl radical in photodynamic therapy with photostable bacteriochlorins: Evidence from intracellular fluorescence and increased photodynamic efficacy in vitro. Free Radic. Biol. Med. 2012, 52, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Potter, W.R.; Camacho, S.H.; Missert, J.R.; Wang, G.; Bellnier, D.A.; Henderson, B.W.; Rodgers, M.A.; Dougherty, T.J.; Pandey, R.K. Synthesis, photophysical properties, tumor uptake, and preliminary in vivo photosensitizing efficacy of a homologous series of 3-(1 ‘-alkyloxy) ethyl-3-devinylpurpurin-18-N-alkylimides with variable lipophilicity. J. Med. Chem. 2001, 44, 1540–1559. [Google Scholar] [CrossRef] [PubMed]
- Urizzi, P.; Allen, C.M.; Langlois, R.; Ouellet, R.; La Madeleine, C.; Van Lier, J.E. Low-density lipoprotein-bound aluminum sulfophthalocyanine: Targeting tumor cells for photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 154–160. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef]
- Sun, X.; Leung, W. Photodynamic Therapy with Pyropheophorbide-a Methyl Ester in Human Lung Carcinoma Cancer Cell: Efficacy, Localization and Apoptosis. Photochem. Photobiol. 2002, 75, 644–651. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 18830. [Google Scholar] [CrossRef]










| Photosensitizer | logPOW |
|---|---|
| F2POH | −1.7 |
| F2PC3H4F3 | 1.4 |
| FPC4H3F6 | 1.6 |
| FPC3H7 | 1.6 |
| F2PC4H3F6 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucelik, B.; Sułek, A.; Drozd, A.; Stochel, G.; Pereira, M.M.; Pinto, S.M.A.; Arnaut, L.G.; Dąbrowski, J.M. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 2786. https://doi.org/10.3390/ijms21082786
Pucelik B, Sułek A, Drozd A, Stochel G, Pereira MM, Pinto SMA, Arnaut LG, Dąbrowski JM. Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. International Journal of Molecular Sciences. 2020; 21(8):2786. https://doi.org/10.3390/ijms21082786
Chicago/Turabian StylePucelik, Barbara, Adam Sułek, Agnieszka Drozd, Grażyna Stochel, Mariette M. Pereira, Sara M. A. Pinto, Luis G. Arnaut, and Janusz M. Dąbrowski. 2020. "Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species" International Journal of Molecular Sciences 21, no. 8: 2786. https://doi.org/10.3390/ijms21082786
APA StylePucelik, B., Sułek, A., Drozd, A., Stochel, G., Pereira, M. M., Pinto, S. M. A., Arnaut, L. G., & Dąbrowski, J. M. (2020). Enhanced Cellular Uptake and Photodynamic Effect with Amphiphilic Fluorinated Porphyrins: The Role of Sulfoester Groups and the Nature of Reactive Oxygen Species. International Journal of Molecular Sciences, 21(8), 2786. https://doi.org/10.3390/ijms21082786








