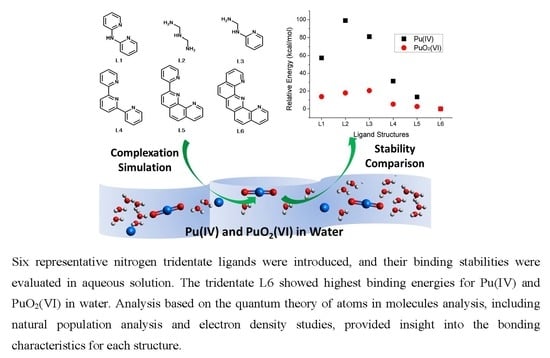
Prediction of Binding Stability of Pu(IV) and PuO2(VI) by Nitrogen Tridentate Ligands in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
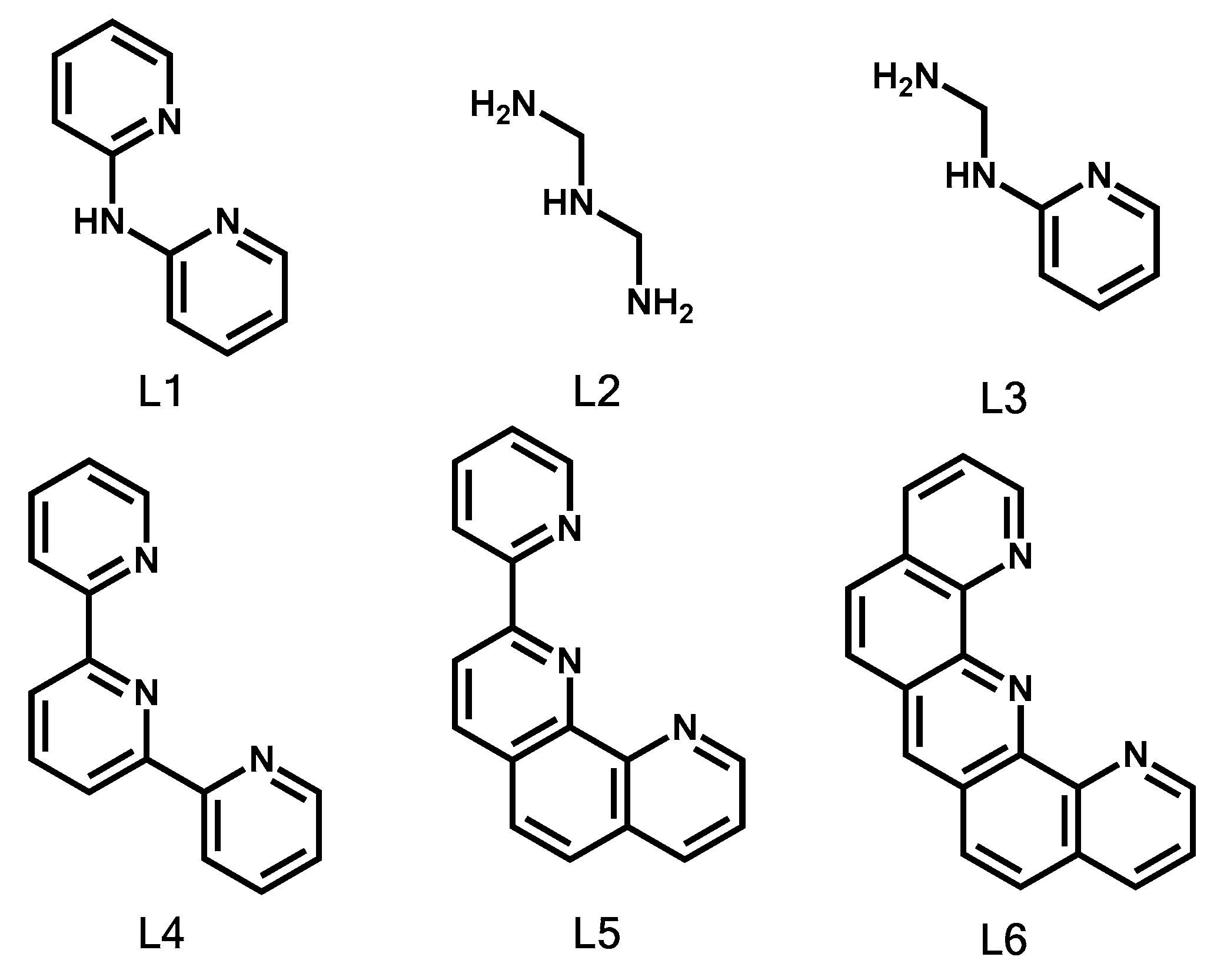
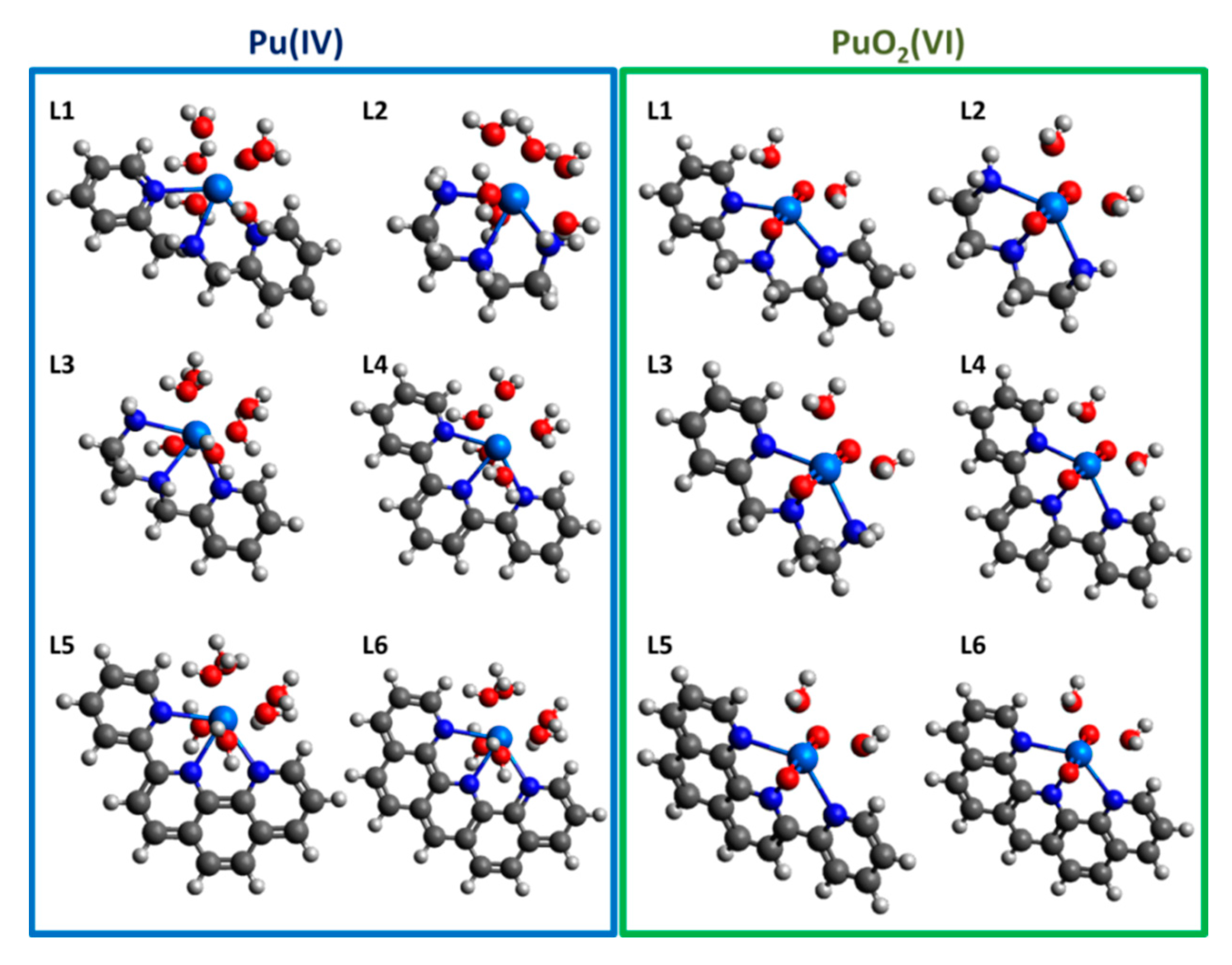
2.1. Complexation and Bond Formation
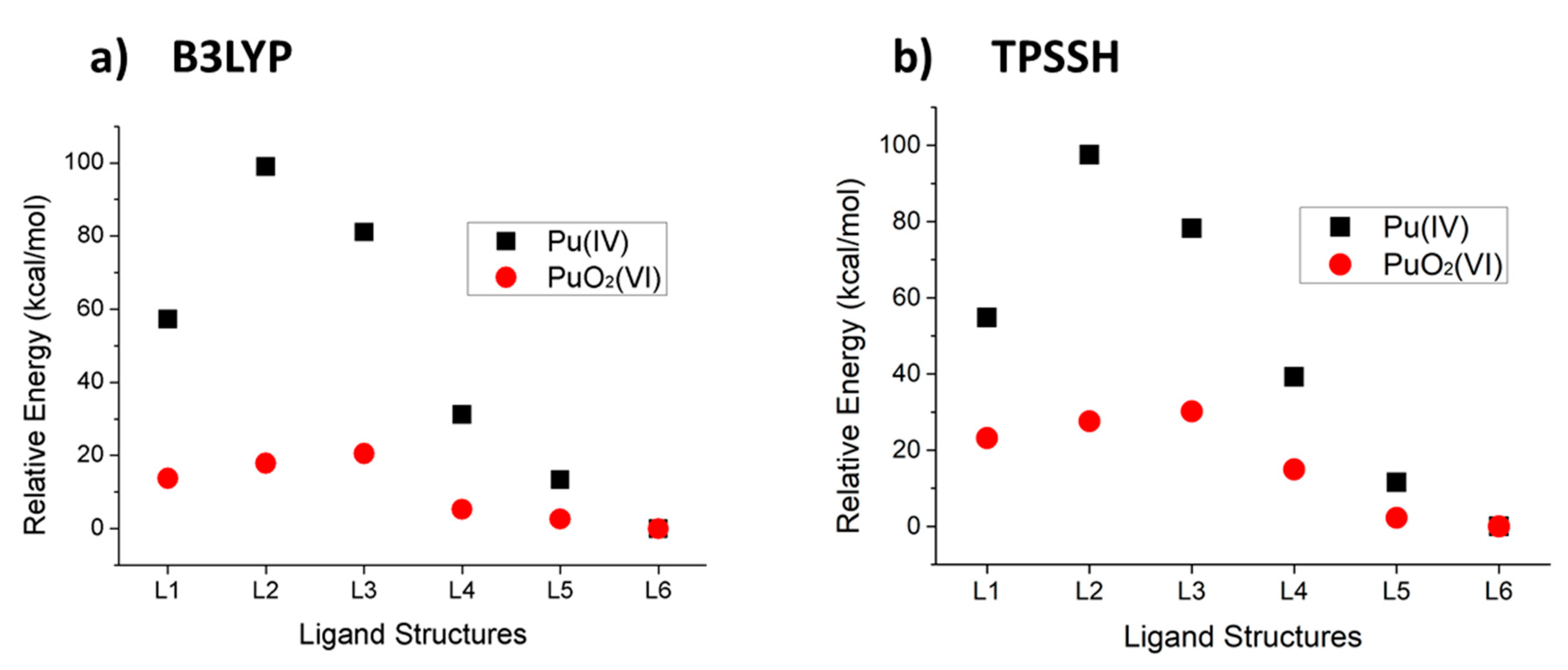
2.2. Comparison of Stability
2.3. Electronic Structure Analysis
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bratt, D. Re-igniting the Atom: The Political Consequences of the Global Nuclear Revival. Whitehead J. Dipl. Int’l Rel. 2010, 11, 59. [Google Scholar]
- Hajima, R.; Hayakawa, T.; Kikuzawa, N.; Minehara, E. Proposal of nondestructive radionuclide assay using a high-flux gamma-ray source and nuclear resonance fluorescence. J. Nucl. Sci. Technol. 2008, 45, 441–451. [Google Scholar] [CrossRef]
- Mueller, M. 5 Fast Facts About Nuclear Energy | Department of Energy. Available online: https://www.energy.gov/ne/articles/5-fast-facts-about-spent-nuclear-fuel (accessed on 12 April 2020).
- Gauthier-Lafaye, F.; Holliger, P.; Blanc, P.L. Natural fission reactors in the Franceville basin, Gabon: A review of the conditions and results of a “critical event” in a geologic system. Geochim. Cosmochim. Acta 1996, 60, 4831–4852. [Google Scholar] [CrossRef]
- Series, P. The Actinide and Transactinide Elements; Springer: Dordrecht, The Netherlands, 1940; ISBN 9781402035555. [Google Scholar]
- Shirai, O.; Iizuka, M.; Iwai, T.; Suzuki, Y.; Arai, Y. Electrode reaction of plutonium at liquid cadmium in LiCl-KCl eutectic melts. J. Electroanal. Chem. 2000, 490, 31–36. [Google Scholar] [CrossRef]
- Serp, J.; Konings, R.J.M.; Malmbeck, R.; Rebizant, J.; Scheppler, C.; Glatz, J.P. Electrochemical behaviour of plutonium ion in LiCl—KCl eutectic melts. J. Electroanal. Chem. 2004, 561, 143–148. [Google Scholar] [CrossRef]
- Wolf, S.F.; Bates, J.K.; Buck, E.C.; Dietz, N.L.; Fortner, J.A.; Brown, N.R. Physical and chemical characterization of actinides in soil from Johnston Atoll. Environ. Sci. Technol. 1997, 31, 467–471. [Google Scholar] [CrossRef]
- Sengupta, A.; Mohapatra, P.K.; Iqbal, M.; Huskens, J.; Verboom, W. A highly efficient solvent system containing functionalized diglycolamides and an ionic liquid for americium recovery from radioactive wastes. Dalton Trans. 2012, 41, 6970. [Google Scholar] [CrossRef]
- Slater, J. Nuclear Wastelands: A Global Guide to Nuclear Weapons Production and its Health and Environmental Effects. BMJ 1995, 311, 1100–1101. [Google Scholar] [CrossRef]
- Wen, J.; Dong, L.; Tian, J.; Jiang, T.; Yang, Y.Q.; Huang, Z.; Yu, X.Q.; Hu, C.W.; Hu, S.; Yang, T.Z.; et al. Fluorescent BINOL-based sensor for thorium recognition and a density functional theory investigation. J. Hazard. Mater. 2013, 263, 638–642. [Google Scholar] [CrossRef]
- Sessler, J.L.; Melfi, P.J.; Seidel, D.; Gorden, A.E.V.; Ford, D.K.; Palmer, P.D.; Tait, C.D. Hexaphyrin(1.0.1.0.0.0). A new colorimetric actinide sensor. Tetrahedron 2004, 60, 11089–11097. [Google Scholar] [CrossRef]
- Reilly, S.D.; Gaunt, A.J.; Scott, B.L.; Modolo, G.; Iqbal, M.; Verboom, W.; Sarsfield, M.J. Plutonium(iv) complexation by diglycolamide ligands—Coordination chemistry insight into TODGA-based actinide separations. Chem. Commun. 2012, 48, 9732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelis, A.V.; Lumetta, G.J. Actinide lanthanide separation process—ALSEP. Ind. Eng. Chem. Res. 2014, 53, 1624–1631. [Google Scholar] [CrossRef]
- Brown, J.; McLachlan, F.; Sarsfield, M.; Taylor, R.; Modolo, G.; Wilden, A. Plutonium Loading of Prospective Grouped Actinide Extraction (GANEX) Solvent Systems based on Diglycolamide Extractants. Solvent Extr. Ion Exch. 2012, 30, 127–141. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pathak, P.; Mohapatra, P.K.; Manchanda, V.K. Chemistry of diglycolamides: Promising extractants for actinide partitioning. Chem. Rev. 2012, 112, 1751–1772. [Google Scholar] [CrossRef]
- Serrano-Purroy, D.; Baron, P.; Christiansen, B.; Glatz, J.P.; Madic, C.; Malmbeck, R.; Modolo, G. First demonstration of a centrifugal solvent extraction process for minor actinides from a concentrated spent fuel solution. Sep. Purif. Technol. 2005, 45, 157–162. [Google Scholar] [CrossRef]
- Madic, C.; Ouvrier, N. EUROPART: EUROpean research program for the PARTitioning of minor actinides from high active wastes arising from the reprocessing of spent nuclear fuels. Radiochimica Acta 2008, 96, 183–185. [Google Scholar] [CrossRef]
- Musikas, C. Solvent extraction for the chemical separations of the 5f elements. Inorg. Chim. Acta 1987, 140, 197–206. [Google Scholar] [CrossRef]
- Ozawa, M.; Koma, Y.; Nomura, K.; Tanaka, Y. Separation of actinides and fission products in high-level liquid wastes by the improved TRUEX process. J. Alloys Compd. 1998, 271–273, 538–543. [Google Scholar] [CrossRef]
- Pahan, S.; Boda, A.; Ali, S.M. Density functional theoretical analysis of structure, bonding, interaction and thermodynamic selectivity of hexavalent uranium (UO22+) and tetravalent plutonium (Pu4+) ion complexes of tetramethyl diglycolamide (TMDGA). Theor. Chem. Acc. 2015, 134, 41. [Google Scholar] [CrossRef]
- Rai, D.; Moore, D.A.; Rosso, K.M.; Felmy, A.R.; Bolton, H. Environmental mobility of Pu(IV) in the presence of ethylenediaminetetraacetic acid: Myth or reality? J. Solut. Chem. 2008, 37, 957–986. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, X.; Liao, J.; Liu, N.; Yang, Y.; Chai, Z.; Wang, D. A computational study on the complexation of Np(V) with N,N,N′,N′-tetramethyl-3-oxa-glutaramide (TMOGA) and its carboxylate analogs. Phys. Chem. Chem. Phys. 2014, 16, 16536–16546. [Google Scholar] [CrossRef]
- Brown, J.L.; Gaunt, A.; King, D.M.; Liddle, S.; Reilly, S.D.; Scott, B.; Wooles, A. Neptunium and plutonium complexes with a sterically encumbered triamidoamine (TREN) scaffold. Chem. Commun. 2016, 52, 5428–5431. [Google Scholar] [CrossRef] [Green Version]
- Šulka, M.; Cantrel, L.; Vallet, V. Theoretical study of plutonium(IV) complexes formed within the PUREX process: A proposal of a plutonium surrogate in fire conditions. J. Phys. Chem. A 2014, 118, 10073–10080. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Mayer, I. Bond order and valence indices: A personal account. J. Comput. Chem. 2007, 28, 204–221. [Google Scholar] [CrossRef]
- Wang, D.; van Gunsteren, W.F.; Chai, Z. Recent advances in computational actinoid chemistry. Chem. Soc. Rev. 2012, 41, 5836. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wang, C.Z.; Lan, J.H.; Xiao, C.L.; Wang, X.K.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Theoretical investigation on multiple bonds in terminal actinide nitride complexes. Inorg. Chem. 2014, 53, 9607–9614. [Google Scholar] [CrossRef]
- Gendron, F.; Páez-Hernández, D.; Notter, F.P.; Pritchard, B.; Bolvin, H.; Autschbach, J. Magnetic properties and electronic structure of neptunyl(VI) complexes: Wavefunctions, orbitals, and crystal-field models. Chem. A Eur. J. 2014, 20, 7994–8011. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lan, J.; Yuan, L.; Xu, C.; Chai, Z.; Shi, W. Highly selective extraction of Pu (IV)and Am (III)by N,N′′-diethyl-N,N′′-ditolyl-2,9-diamide-1,10-phenanthroline ligand: An experimental and theoretical study. Sep. Purif. Technol. 2019, 223, 274–281. [Google Scholar] [CrossRef]
- Jian, T.; Yu, X.; Dan, D.; Albrecht-Schmitt, T.E.; Autschbach, J.; Gibson, J.K. Gas-Phase Complexes of Americium and Lanthanides with a Bis-Triazinyl Pyridine: Reactivity and Bonding of Archetypes for F-Element Separations. J. Phys. Chem. A 2020. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Q.-Y.; Wang, C.-Z.; Lan, J.-H.; Nie, C.-M.; Chai, Z.-F.; Shi, W.-Q. Theoretical insights into selective separation of trivalent actinide and lanthanide by ester and amide ligands based on phenanthroline skeleton. Dalton Trans. 2020, 49, 4093–4099. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Lan, J.H.; Wang, C.Z.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Understanding the interactions of neptunium and plutonium ions with graphene oxide: Scalar-relativistic DFT investigations. J. Phys. Chem. A 2014, 118, 10273–10280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009; pp. 2–3. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
| Ligand | B3LYP | TPSSH | ||||||
|---|---|---|---|---|---|---|---|---|
| Pu(IV) | PuO2(VI) | Pu(IV) | PuO2(VI) | |||||
| Pu–N | Pu–O | Pu–N | Pu–O | Pu–N | Pu–O | Pu–N | Pu–O | |
| L1 | 2.488 | 2.509 | 2.536 | 2.482 | 2.501 | 2.529 | 2.522 | 2.473 |
| L2 | 2.544 | 2.504 | 2.558 | 2.476 | 2.561 | 2.509 | 2.551 | 2.467 |
| L3 | 2.509 | 2.513 | 2.5473 | 2.483 | 2.574 | 2.530 | 2.536 | 2.478 |
| L4 | 2.457 | 2.511 | 2.516 | 2.498 | 2.560 | 2.562 | 2.502 | 2.489 |
| L5 | 2.498 | 2.529 | 2.517 | 2.512 | 2.570 | 2.571 | 2.499 | 2.533 |
| L6 | 2.518 | 2.535 | 2.524 | 2.508 | 2.600 | 2.581 | 2.506 | 2.535 |
| Ligand | B3LYP | TPSSH | ||
|---|---|---|---|---|
| Pu(IV) (Ediff) | PuO2(VI) (Ediff’) | Pu(IV) (Ediff) | PuO2(VI) (Ediff’) | |
| L1 | 57.251 | 13.724 | 54.775 | 23.182 |
| L2 | 98.955 | 17.835 | 97.511 | 27.587 |
| L3 | 81.101 | 20.506 | 78.194 | 30.178 |
| L4 | 31.228 | 5.288 | 39.219 | 14.959 |
| L5 | 13.362 | 2.564 | 11.546 | 2.240 |
| L6 | 0 | 0 | 0 | 0 |
| Ligand | Pu(IV) | PuO2(VI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Wiberg Index | −V(r)/G(r) | Wiberg Index | −V(r)/G(r) | |||||
| Pu–N | Pu–O | Pu–N | Pu–O | Pu–N | Pu–O | Pu–N | Pu–O | |
| L1 | 0.414 | 0.278 | 1.121 | 0.919 | 0.369 | 0.317 | 0.929 | 0.894 |
| L2 | 0.794 | 0.727 | 1.170 | 0.985 | 0.716 | 0.608 | 1.053 | 0.958 |
| L3 | 0.774 | 0.574 | 1.048 | 0.950 | 0.355 | 0.317 | 0.928 | 1.275 |
| L4 | 0.420 | 0.275 | 1.143 | 0.929 | 0.349 | 0.313 | 0.921 | 0.898 |
| L5 | 0.734 | 0.559 | 1.003 | 0.947 | 0.348 | 0.307 | 0.923 | 0.897 |
| L6 | 0.389 | 0.279 | 1.049 | 0.917 | 0.345 | 0.311 | 0.921 | 1.650 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, K.; Jeong, H.J.; Woo, S.M.; Bae, S. Prediction of Binding Stability of Pu(IV) and PuO2(VI) by Nitrogen Tridentate Ligands in Aqueous Solution. Int. J. Mol. Sci. 2020, 21, 2791. https://doi.org/10.3390/ijms21082791
Jeong K, Jeong HJ, Woo SM, Bae S. Prediction of Binding Stability of Pu(IV) and PuO2(VI) by Nitrogen Tridentate Ligands in Aqueous Solution. International Journal of Molecular Sciences. 2020; 21(8):2791. https://doi.org/10.3390/ijms21082791
Chicago/Turabian StyleJeong, Keunhong, Hye Jin Jeong, Seung Min Woo, and Sungchul Bae. 2020. "Prediction of Binding Stability of Pu(IV) and PuO2(VI) by Nitrogen Tridentate Ligands in Aqueous Solution" International Journal of Molecular Sciences 21, no. 8: 2791. https://doi.org/10.3390/ijms21082791