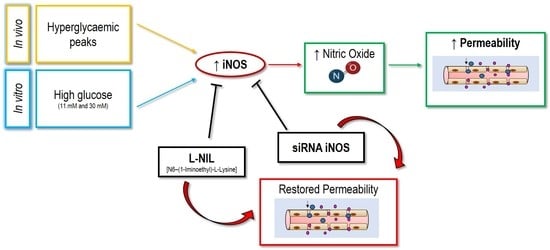
Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation
Abstract
1. Introduction
2. Results
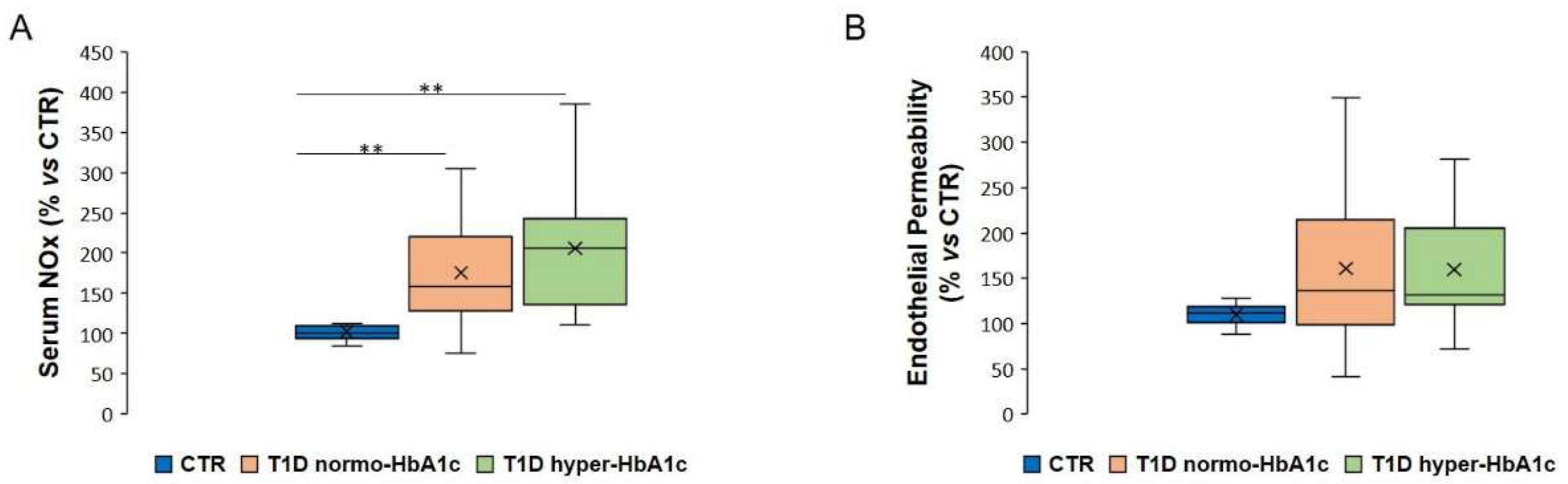
2.1. Serum NOx Levels and Endothelial Permeability Are Not Associated with Increased Glycated Haemoglobin in T1D Subjects
2.2. Serum NOx Levels and Endothelial Permeability Are Associated with Hyperglycaemia
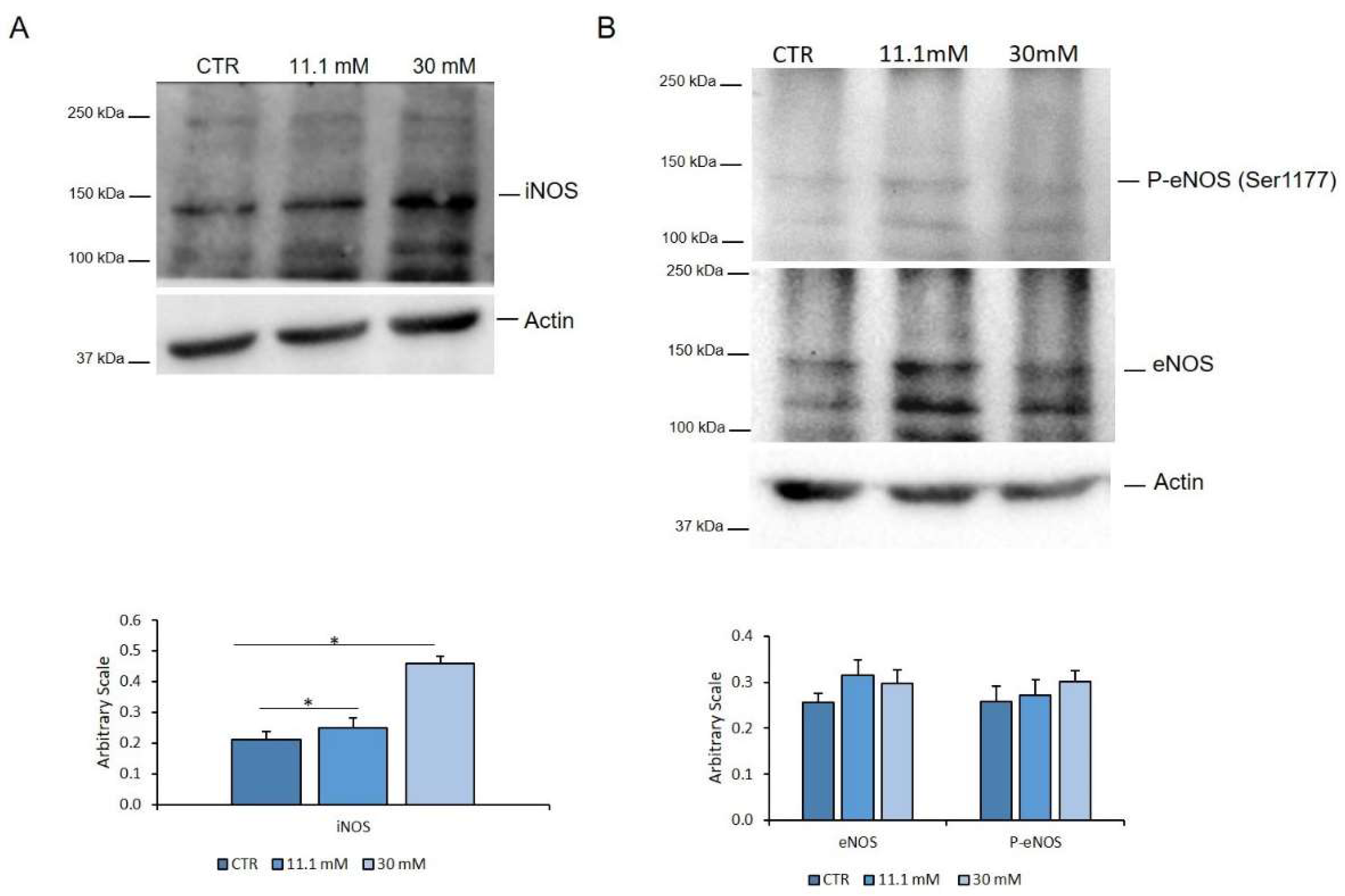
2.3. High Concentrations of Extracellular Glucose Increase Endothelial NOx Release and Permeability in Endothelial Cells
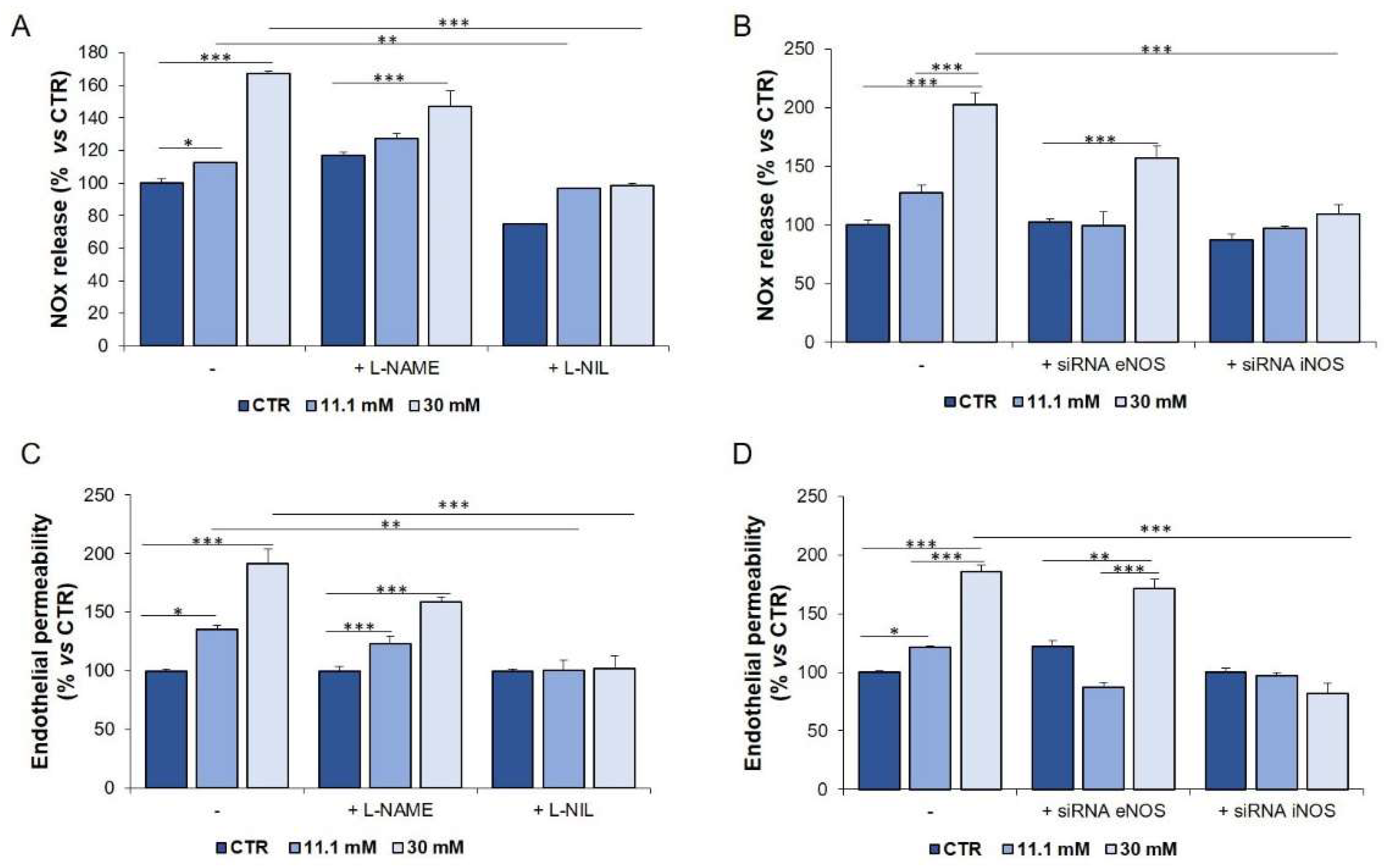
2.4. The Upregulation of iNOS is Responsible for the Increase of NOx in HUVEC Exposed to High Glucose
2.5. Genetic and Pharmacological Inhibition of iNOS Restores Endothelial Permeability in Cells Exposed to Sera from Hyperglycaemic T1D Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cell Culture
4.3. Transwell Permeability Assay
4.4. NOS Activity
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T1D | Type 1 diabetes |
| NO | Nitric Oxide |
| ROS | Reactive Oxygen Species |
| eNOS | endothelial Nitric Oxide Synthase |
| eNOSSer1177 | eNOS phosphorylated on Ser1177 |
| iNOS | inducible Nitric Oxide Synthase |
| NOx | nitrate and nitrite |
| Hb1Ac | glycated haemoglobin |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| FBS | Fetal Bovine Serum |
| L-NAME | L-Nω–Nitroarginine-Methyl-Ester |
| L-NIL | N6–(1-Iminoethyl)-L-Lysine |
| LPS | lipopolysaccharide |
References
- Thomas, C.C.; Philipson, L.H. Update on Diabetes Classification. Med Clin. N. Am. 2015, 99, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Mohn, A.; Chiarelli, F.; Kelnar, C.J.H. Macrovascular angiopathy in children and adolescents with type 1 diabetes. Diabetes/Metab. Res. Rev. 2011, 27, 436–460. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.P. Vascular endothelial dysfunction and nutritional compounds in early type 1 diabetes. Curr. Diabetes Rev. 2014, 10, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.; Rabelink, T.J. Endothelial Function and Dysfunction. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; Van Hinsbergh, V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2008, 335, 165–189. [Google Scholar] [CrossRef]
- Scaramuzza, A.E.; Redaelli, F.; Giani, E.; Macedoni, M.; Giudici, V.; Gazzarri, A.; Bosetti, A.; De Angelis, L.; Zuccotti, G. V Adolescents and young adults with type 1 diabetes display a high prevalence of endothelial dysfunction. Acta Paediatr. 2015, 104, 192–197. [Google Scholar] [CrossRef]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef]
- Popov, D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes. Int. J. Diabetes Mellit. 2010, 2, 189–195. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Caimi, G.; Hopps, E.; Montana, M.; Noto, D.; Canino, B.; Presti, R.L.; Averna, M. Evaluation of nitric oxide metabolites in a group of subjects with metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2012, 6, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Lin, M.I.; Murata, T.; Landskroner-Eiger, S.; Schleicher, M.; Kothiya, M.; Iwakiri, Y.; Yu, J.; Huang, P.; Sessa, W.C. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 2013, 126, 5541–5552. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Páli, T.; Bencsik, P.; Gorbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in biological samples. Br. J. Pharmacol. 2014, 172, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Cipollone, F.; Romano, F.; Tumini, S.; Costantini, F.; Di Ricco, L.; Pomilio, M.; Pierdomenico, S.D.; Marini, M.; Cuccurullo, F.; et al. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: Relation to glomerular hyperfiltration. Diabetes 2000, 49, 1258–1263. [Google Scholar] [CrossRef]
- González, M.; Rojas, S.; Avila, P.; Cabrera, L.; Villalobos, R.; Palma, C.; Aguayo, C.; Peña, E.; Gallardo, V.; Guzmán-Gutiérrez, E.; et al. Insulin Reverses D-Glucose–Increased Nitric Oxide and Reactive Oxygen Species Generation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2015, 10, e0122398. [Google Scholar] [CrossRef]
- Adela, R.; Nethi, S.K.; Bagul, P.K.; Barui, A.K.; Mattapally, S.; Kuncha, M.; Patra, C.R.; Reddy, P.N.C.; Banerjee, S.K. Hyperglycaemia Enhances Nitric Oxide Production in Diabetes: A Study from South Indian Patients. PLoS ONE 2015, 10, e0125270. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; De Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A. Role of Nitric Oxide in Insulin Secretion and Glucose Metabolism. Trends Endocrinol. Metab. 2019, 31, 118–130. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Vascular complications of diabetes: Mechanisms of injury and protective factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Wang, X.-F.; Li, L.; Zhang, L.; Shen, D.-L.; Li, D.-H.; Jin, Q.-S.; Zhang, J.-Y. Effects of high glucose on human umbilical vein endothelial cell permeability and myosin light chain phosphorylation. Diabetol. Metab. Syndr. 2015, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cai, B.; Deng, P.; Wu, X.; Guan, Y.; Zhang, B.; Cai, W.; Schaper, J.; Schaper, W. Nitric Oxide Increases Arterial Endotheial Permeability through Mediating VE-Cadherin Expression during Arteriogenesis. PLoS ONE 2015, 10, e0127931. [Google Scholar] [CrossRef] [PubMed]
- Testa, I.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Schisano, B.; Tripathi, G.; McGee, K.; McTernan, P.G.; Ceriello, A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetology 2011, 54, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S.; Azizi, F. Reference values for serum nitric oxide metabolites in an adult population. Clin. Biochem. 2010, 43, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Aulich, J.; Cho, Y.H.; Januszewski, A.S.; E Craig, M.; Selvadurai, H.; Wiegand, S.; Jenkins, A.J.; Donaghue, K. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatr. Diabetes 2019, 20, 1118–1127. [Google Scholar] [CrossRef]
- Fatima, N.; Faisal, S.M.; Zubair, S.; Ajmal, M.; Siddiqui, S.S.; Moin, S.; Owais, M. Role of Pro-Inflammatory Cytokines and Biochemical Markers in the Pathogenesis of Type 1 Diabetes: Correlation with Age and Glycemic Condition in Diabetic Human Subjects. PLoS ONE 2016, 11, e0161548. [Google Scholar] [CrossRef]
- Liu, X.-J.; Zhang, Z.-D.; Ma, X.-C. High glucose enhances LPS-stimulated human PMVEC hyperpermeability via the NO pathway. Exp. Ther. Med. 2013, 6, 361–367. [Google Scholar] [CrossRef]
- Shelton, J.L.; Wang, L.; Cepinskas, G.; Sandig, M.; Inculet, R.; McCormack, D.G.; Mehta, S. Albumin leak across human pulmonary microvascular vs. umbilical vein endothelial cells under septic conditions. Microvasc. Res. 2006, 71, 40–47. [Google Scholar] [CrossRef]
- Sartoretto, S.M.; Santos, F.F.; Costa, B.P.; Ceravolo, G.S.; Santos-Eichler, R.; Carvalho, M.H.C.; Fortes, Z.B.; Akamine, E.H. Involvement of inducible nitric oxide synthase and estrogen receptor ESR2 (ERbeta) in the vascular dysfunction in female type 1 diabetic rats. Life Sci. 2019, 216, 279–286. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, C.; Xu, G.; Tang, Z.; Zhang, Z.; Liu, Y.; Wang, Z. Effects of aerobic exercise on the expressions and activities of nitric oxide synthases in the blood vessel endothelium in prediabetes mellitus. Exp. Ther. Med. 2019, 17, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.; Croft, K.D.; Hodgson, J.M. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2015, 56, 2036–2052. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, O.; Moser, O.; Eckstein, M.L.; Bain, S.; Pitt, J.; Bracken, R.M. Supplementary Nitric Oxide Donors and Exercise as Potential Means to Improve Vascular Health in People with Type 1 Diabetes: Yes to NO? Nutrients 2019, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Locatelli, L.; Castiglioni, S.; Maier, J.A.M. The dynamic adaptation of primary human endothelial cells to simulated microgravity. FASEB J. 2019, 33, 5957–5966. [Google Scholar] [CrossRef] [PubMed]
- Leidi, M.; Dellera, F.; Mariotti, M.; Banfi, G.; Crapanzano, C.; Albisetti, W.; Maier, J.A.M. Nitric oxide mediates low magnesium inhibition of osteoblast-like cell proliferation. J. Nutr. Biochem. 2012, 23, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Cazzaniga, A.; Maier, J.A.M. Magnesium and the blood-brain barrier in vitro: Effects on permeability and magnesium transport. Magnes. Res. 2019, 32, 16–24. [Google Scholar] [PubMed]
- Leidi, M.; Mariotti, M.; Maier, J.A.M. EDF-1 contributes to the regulation of nitric oxide release in VEGF-treated human endothelial cells. Eur. J. Cell Boil. 2010, 89, 654–660. [Google Scholar] [CrossRef] [PubMed]



| Healthy Subjects (n = 14) | Patients (n = 36) | |
|---|---|---|
| Sex | ||
| Male | n = 5 | n = 12 |
| Female | n = 9 | n = 24 |
| Age (years) | ||
| Mean | 12.5 | 13.8 |
| Range | 3.1–23.7 | 4.0–24.0 |
| Hb1Ac (%) | ||
| Mean | 5.7 | 7.15 |
| Range | 5.30–6.80 | 5.10–8.80 |
| PCR (mg/dL) | ||
| Mean | 0.53 | 0.71 |
| Range | 0.43–0.61 | 0.20–2.60 |
| Glycaemia (mg/dL) | ||
| Mean | 102 | 168.26 |
| Range | 89.0–117.0 | 40.0–350.0 |
| Therapy | None | MDI (n = 22) CSII (n = 14) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzaniga, A.; Scrimieri, R.; Giani, E.; Zuccotti, G.V.; Maier, J.A.M. Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation. Int. J. Mol. Sci. 2020, 21, 2798. https://doi.org/10.3390/ijms21082798
Cazzaniga A, Scrimieri R, Giani E, Zuccotti GV, Maier JAM. Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation. International Journal of Molecular Sciences. 2020; 21(8):2798. https://doi.org/10.3390/ijms21082798
Chicago/Turabian StyleCazzaniga, Alessandra, Roberta Scrimieri, Elisa Giani, Gian Vincenzo Zuccotti, and Jeanette A. M. Maier. 2020. "Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation" International Journal of Molecular Sciences 21, no. 8: 2798. https://doi.org/10.3390/ijms21082798
APA StyleCazzaniga, A., Scrimieri, R., Giani, E., Zuccotti, G. V., & Maier, J. A. M. (2020). Endothelial Hyper-Permeability Induced by T1D Sera Can be Reversed by iNOS Inactivation. International Journal of Molecular Sciences, 21(8), 2798. https://doi.org/10.3390/ijms21082798