Regeneration of Pulmonary Tissue in a Calf Model of Fibrinonecrotic Bronchopneumonia Induced by Experimental Infection with Chlamydia psittaci
Abstract
:1. Introduction
2. Results
2.1. Clinical Signs, Acute Phase Response and Pulmonary Dysfunctions
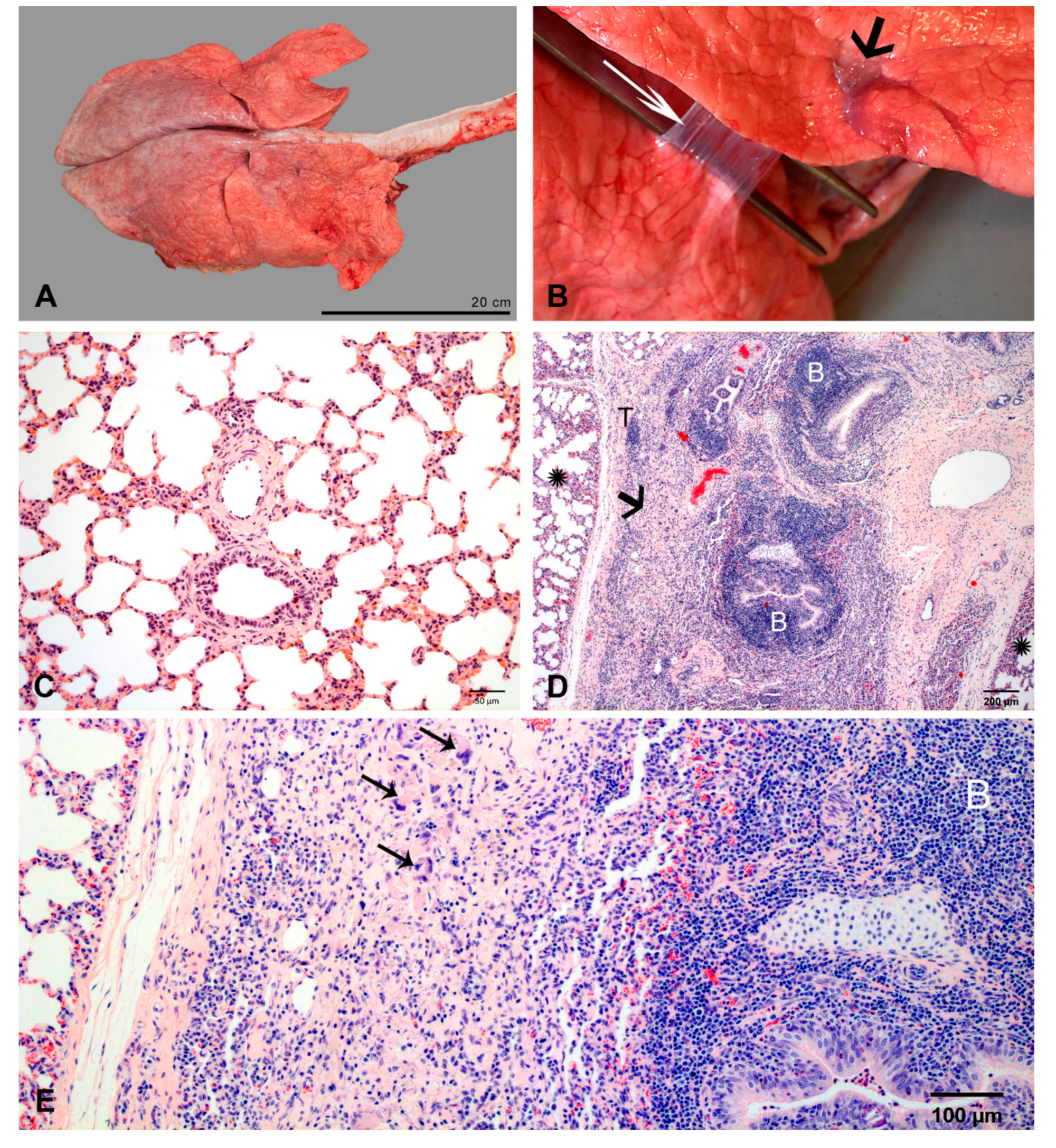
2.2. Pulmonary Lesions
2.2.1. At 2 dpi
2.2.2. At 3 dpi
2.2.3. At 4 dpi
2.2.4. At 7 dpi
2.2.5. At 10 dpi
2.2.6. At 14 dpi
2.2.7. At 35/37 dpi
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Inoculum and Inoculation
4.4. Clinical Scoring and Pulmonary Function Testing
4.5. Necropsy, Gross Pathology and Tissue Samples
4.6. Histopathology
4.7. Immunohistochemistry
4.8. Exclusion of Co-Infections
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEC1 | type 1 alveolar epithelial cells |
| AEC2 | type 2 alveolar epithelial cells |
| BALT | bronchus-associated lymphoid tissue |
| C. | chlamydia |
| CAP | community acquired pneumonia |
| dpi | days after inoculation |
| FFPE | formalin-fixed paraffin embedded tissue |
| ifu | inclusion-forming units |
| IL | interleukin |
References
- Marshall, D.C.; Goodson, R.J.; Xu, Y.; Komorowski, M.; Shalhoub, J.; Maruthappu, M.; Salciccioli, J.D. Trends in mortality from pneumonia in the Europe union: A temporal analysis of the European detailed mortality database between 2001 and 2014. Resp. Res. 2018, 19, 81. [Google Scholar] [CrossRef]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012, 67, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Cillóniz, C.; Torres, A.; Niederman, M.; van der Eerden, M.; Chalmers, J.; Welte, T.; Blasi, F. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016, 42, 1374–1386. [Google Scholar] [CrossRef]
- Torres, A.; Blasi, F.; Peetermans, W.E.; Viegi, G.; Welte, T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: A literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1065–1079. [Google Scholar] [CrossRef] [Green Version]
- Wubbel, L.; Muniz, L.; Ahmed, A.; Trujillo, M.; Carubelli, C.; Mccoig, C.; Abramo, T.; Leinonen, M.; McCracken, G.H., Jr. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 1999, 43, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Schnee, C.; Pletz, M.W.; Rupp, J.; Jacobs, E.; Sachse, K.; Rohde, G.; CAPNETZ Study Group. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg. Infect. Dis. 2015, 21, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Spoorenberg, S.M.C.; Bos, W.J.W.; van Hannen, E.J.; Dijkstra, F.; Heddema, E.R.; van Velzen-Blad, H.; Heijligenberg, R.; Grutters, J.C.; de Jongh, B.M.; Ovidius Study Group. Chlamydia psittaci: A relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth. J. Med. 2016, 75–81. [Google Scholar]
- Herriges, M.; Morrisey, E.E. Lung development: Orchestrating the generation and regeneration of a complex organ. Development 2014, 141, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Zemans, R.L.; Henson, P.M.; Henson, J.E.; Janssen, W.J. Conceptual approaches to lung injury and repair. Ann. Am. Thorac. Soc. 2015, 12, S9–S15. [Google Scholar] [CrossRef] [Green Version]
- Slauson, D.O.; Hahn, F.F. Criteria for development of animal models of diseases of the respiratory system. Am. J. Pathol. 1980, 101, 103–122. [Google Scholar]
- Bonniaud, P.; Fabre, A.; Frossard, N.; Guignabert, C.; Inman, M.; Kuebler, W.M.; Maes, T.; Shi, W.; Stampfli, M.; Uhlig, S.; et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur. Respir. J. 2018, 51, 1702133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireland, J.J.; Roberts, R.M.; Palmer, G.H.; Bauman, D.E.; Bazer, F.W. A commentary on domestic animals as dual-purpose models that benefit agricultural and biomedical research. J. Anim. Sci. 2008, 86, 2797–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsaia, Y.-F.; Kub, Y.-H. Necrotizing pneumonia: A rare complication of pneumonia requiring special consideration. Curr. Opin. Pulm. Med. 2012, 1, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Krutikov, M.; Rahman, A.; Tiberi, S. Necrotizing pneumonia (aetiology, clinical features and management). Curr. Opin. Pulm. Med. 2019, 25, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hilton, B.; Tavare, A.N.; Creer, D. Necrotising pneumonia caused by non-PVL Staphylococcus aureus with 2-year follow-up. BMJ Case Rep. 2017. Published Online First: 28.11.2017, bcr-2017-221779. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Lu, F.L.; Valim, C.; Cleveland, R.H.; Colin, A.A. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur. Respir. J. 2008, 31, 1285–1291. [Google Scholar] [CrossRef]
- Alifano, M.; Lorut, C.; Lefebvre, A.; Khattar, L.; Damotte, D.; Huchon, G.; Regnard, J.-F.; Rabbat, A. Necrotizing pneumonia in adults: Multidisciplinary management. Intens. Care Med. 2011, 37, 1888–1889. [Google Scholar] [CrossRef]
- Koivula, I.; Stén, M.; Mäkelä, P.H. Prognosis after community-acquired pneumonia in the elderly: A population-based 12-year follow-up study. Arch. Intern. Med. 1999, 159, 1550–1555. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, P.; Ostermann, C.; Liebler-Tenorio, E.; Berndt, A.; Vogel, A.; Lambertz, J.; Rothe, M.; Rüttger, A.; Schubert, E.; Sachse, K. A bovine model of respiratory Chlamydia psittaci infection: Challenge dose titration. PLoS ONE 2012, 7, e30125. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, C.; Schroedl, W.; Schubert, E.; Sachse, K.; Reinhold, P. Dose-dependent effects of Chlamydia psittaci infection on pulmonary gas exchange, innate immunity and acute-phase reaction in a bovine respiratory model. Vet. J. 2013, 196, 351–359. [Google Scholar] [CrossRef]
- Ostermann, C.; Rüttger, A.; Schubert, E.; Schrödl, W.; Sachse, K.; Reinhold, P. Infection, disease, and transmission dynamics in calves after experimental and natural challenge with a bovine Chlamydia psittaci isolate. PLoS ONE 2013, 8, e64066. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, C.; Linde, S.; Siegling-Vlitakis, C.; Reinhold, P. Evaluation of pulmonary dysfunctions and acid–base imbalances induced by Chlamydia psittaci in a bovine model of respiratory infection. Multidiscip. Respir. Med. 2014, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prohl, A.; Lohr, M.; Ostermann, C.; Liebler-Tenorio, E.; Berndt, A.; Schroedl, W.; Rothe, M.; Schubert, E.; Sachse, K.; Reinhold, P. Enrofloxacin and macrolides alone or in combination with rifampicin as antimicrobial treatment in a bovine model of acute Chlamydia psittaci infection. PLoS ONE 2015, 10, e0119736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prohl, A.; Lohr, M.; Ostermann, C.; Liebler-Tenorio, E.; Berndt, A.; Schroedl, W.; Rothe, M.; Schubert, E.; Sachse, K.; Reinhold, P. Evaluation of antimicrobial treatment in a bovine model of acute Chlamydia psittaci infection: Tetracycline versus tetracycline plus rifampicin. FEMS Pathog. Dis. 2015, 73, 1–12. [Google Scholar]
- Panciera, R.J.; Confer, A.W. Pathogenesis and pathology of bovine pneumonia. Vet. Clin. North. Am. Food Anim. Pract. 2010, 26, 191–214. [Google Scholar] [CrossRef]
- Beers, M.F.; Morrisey, E.E. The three R’s of lung health and disease: Repair, remodeling, and regeneration. J. Clin. Investig. 2011, 121, 2065–2073. [Google Scholar] [CrossRef] [Green Version]
- Virok, D.P.; Nelson, D.E.; Whitmire, W.M.; Crane, D.D.; Goheen, M.M.; Caldwell, H.D. Chlamydial infection induces pathobiotype-specific protein tyrosine phosphorylation in epithelial cells. Infect. Immun. 2005, 73, 1939–1946. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.J.; Eckmann, L.; Quayle, A.J.; Shen, L.; Zhang, Y.-X.; Anderson, D.J.; Fierer, J.; Stephens, R.S.; Kagnoff, M.F. Secretion of proinflammatory cytokines by epithelial cells in response to chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 1997, 99, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Jahn, H.-U.; Krüll, M.; Wuppermann, F.N.; Klucken, A.C.; Rosseau, S.; Seybold, J.; Hegemann, J.H.; Jantos, C.A.; Suttorp, N. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J. Infect. Dis. 2000, 182, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Vanella, K.M.; Wynn, T.A. Mechanism of organ injury and repair by macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Kovtun, A.; Messerer, D.A.C.; Scharffetter-Kochanek, K.; Huber-Lang, M.; Ignatius, A. Neutrophils in tissue trauma of the Skin, bone, and lung: Two sides of the same coin. J. Immunol. Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef] [PubMed]
- Carden, D.; Xiao, F.; Moak, C.; Willis, B.H.; Robinson-Jackson, S.; Alexander, S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 1998, 275, H385–H392. [Google Scholar] [CrossRef] [PubMed]
- Ginzberg, H.H.; Cherapanov, V.; Dong, Q.; Cantin, A.; McCulloch, C.A.G.; Shannon, P.T.; Downey, G.P. Neutrophil-mediated epithelial injury during transmigration: Role of elastase. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G705–G717. [Google Scholar] [CrossRef] [Green Version]
- Car, B.D.; Suyemoto, M.M.; Neilsen, N.R.; Slauson, D.O. The role of leukocytes in the pathogenesis of fibrin deposition in bovine acute lung injury. Am. J. Pathol. 1991, 138, 1191–1198. [Google Scholar] [PubMed]
- Wygrecka, M.; Jablonska, E.; Guenther, A.; Preissner, K.T.; Markart, P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb. Haemost. 2008, 99, 494–501. [Google Scholar] [PubMed] [Green Version]
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell. Biol. 2019, 97, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.A.; Fitch, P.M.; Simpson, A.J.; Howie, S.E. Inflammation-associated remodelling and fibrosis in the lung - a process and an end point. Int. J. Exp. Pathol. 2007, 88, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A. Respiratory System. In Pathologic Basis of Veterinary Disease, 4th ed.; McGavin, M.D., Zachary, J.F., Eds.; Mosby Elsevier: St. Louis, MO, USA, 2007; pp. 509–513. [Google Scholar]
- Roan, N.R.; Starnbach, M.N. Immune-mediated control of chlamydia infection. Cell. Microbiol. 2008, 10, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.J.; Cabral, L.J.; Stephens, R.J.; Freeman, G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp. Mol. Pathol. 1975, 22, 142–150. [Google Scholar] [CrossRef]
- Chen, F.; Fine, A. Stem cells in lung injury and repair. Am. J. Pathol. 2016, 186, 2544–2550. [Google Scholar] [CrossRef] [Green Version]
- Tata, P.R.; Rajagopal, J. Plasticity in the lung: Making and breaking cell identity. Development 2017, 144, 755–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.C. Mesenchymal regulation of alveolar repair in pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Vracko, R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am. J. Pathol. 1974, 77, 314–346. [Google Scholar] [PubMed]
- Jain, R.; Barkauskas, C.E.; Takeda, N.; Bowie, E.J.; Aghajanian, H.; Wang, Q.; Padmanabhan, A.; Manderfield, L.J.; Gupta, M.; Li, D.; et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015, 6, 6727. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castillo, J.A.; Pérez, D.B.; Ntokou, A.; Seeger, W.; Morty, R.E.; Ahlbrecht, K. Understanding alveolarization to induce lung regeneration. BMC Respir. Res. 2018, 19, 148. [Google Scholar] [CrossRef] [Green Version]
- De Smet, F.; Segura, I.; De Bock, K.; Hohensinner, P.J.; Carmeliet, P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Chua, F.; Gauldie, J.; Laurent, G.J. Pulmonary fibrosis: Searching for model answers. Am. J. Respir. Cell. Mol. Biol. 2005, 33, 9–13. [Google Scholar] [CrossRef]
- Izbicki, G.; Segel, M.J.; Christensen, T.G.; Conner, M.W.; Breuer, R. Time course of bleomycin-induced lung fibrosis. Int. J. Exp. Pathol. 2002, 83, 111–119. [Google Scholar] [CrossRef]
- Veit, H.P.; Farrell, R.L. The anatomy and physiology of the bovine respiratory system relating to pulmonary disease. Cornell Vet. 1978, 68, 555–581. [Google Scholar]
- Sachse, K.; Laroucau, K.; Vorimore, F.; Magnino, S.; Feige, J.; Müller, W.; Kube, S.; Hotzel, H.; Schubert, E.; Slickers, P.; et al. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet. Microbiol. 2009, 135, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Goellner, S.; Schubert, E.; Liebler-Tenorio, E.; Hotzel, H.; Saluz, H.P.; Sachse, K. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect. Immun. 2006, 74, 4801–4808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovarick, M.R.; Miller, J.C.; Snyder, J.C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J. Bacteriol. 1950, 59, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Prohl, A.; Ostermann, C.; Lohr, M.; Reinhold, P. The bovine lung in biomedical research: Visually guided bronchoscopy, intrabronchial inoculation and in vivo sampling techniques. J. Vis. Exp. 2014, 89, e51557. [Google Scholar] [CrossRef] [Green Version]







| Day of Necropsy in dpi | Absolute Change in Rectal Temperature 1 in °C, Median {min; max} | Relative Change in Respiratory Rate 2 | Relative Change in Tidal Volume per kg b.wW. 3 | Volume of Pulmonary Lesions | Histologic Characteristics of Pulmonary Lesions |
|---|---|---|---|---|---|
| in %, Median {min; max} | |||||
| 2 (n = 3) | +2.5 {+1.8; +2.6} | +150 {+133; +285} | n.a. 4 | 17 {17; 30} | purulent bronchopneumonia |
| 3 (n = 3) | + 2.4 {+1.9;+2.8} | +200 {+83; +173} | −19 {−38; −3} | 25 {11; 31} | fibrinopurulent bronchopneumonia |
| 4 (n = 3) | +1.0 {+0.5; +1.5} | +104 {+82; +127} | −27 {−31; −23} | 25 {20; 27} | fibrinopurulent to necroticzing bronchopneumonia |
| 7 (n = 3) | −0.6 {−0.7; +0.5} | +20 {0; +23} | −2 {−11; −1} | 20 {14; 22} | first signs of organization, macrophage infiltration |
| 10 (n = 3) | −0.1 {−0.7; +2.0} | 0 {−4; 0} | +13 {−3; +19} | 8 {8; 11} | cuboidal alveolar epithelial cells, new capillaries |
| 14 (n = 3) | +0.1 {−0.1; +0.2} | +23 {+13; +67} | +19 {−1; +40} | 0.5 {0.3; 3.3} | progressed organization, lymphocyte infiltration |
| 35/37 (n = 3) | -0.1 {−0.4; +0.9} | −22 {−33; −11} | +2 {−2; +6} | 0.3 {0.2; 1.5} | regeneration, remodeling (in lesions) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebler-Tenorio, E.M.; Lambertz, J.; Ostermann, C.; Sachse, K.; Reinhold, P. Regeneration of Pulmonary Tissue in a Calf Model of Fibrinonecrotic Bronchopneumonia Induced by Experimental Infection with Chlamydia psittaci. Int. J. Mol. Sci. 2020, 21, 2817. https://doi.org/10.3390/ijms21082817
Liebler-Tenorio EM, Lambertz J, Ostermann C, Sachse K, Reinhold P. Regeneration of Pulmonary Tissue in a Calf Model of Fibrinonecrotic Bronchopneumonia Induced by Experimental Infection with Chlamydia psittaci. International Journal of Molecular Sciences. 2020; 21(8):2817. https://doi.org/10.3390/ijms21082817
Chicago/Turabian StyleLiebler-Tenorio, Elisabeth M., Jacqueline Lambertz, Carola Ostermann, Konrad Sachse, and Petra Reinhold. 2020. "Regeneration of Pulmonary Tissue in a Calf Model of Fibrinonecrotic Bronchopneumonia Induced by Experimental Infection with Chlamydia psittaci" International Journal of Molecular Sciences 21, no. 8: 2817. https://doi.org/10.3390/ijms21082817
APA StyleLiebler-Tenorio, E. M., Lambertz, J., Ostermann, C., Sachse, K., & Reinhold, P. (2020). Regeneration of Pulmonary Tissue in a Calf Model of Fibrinonecrotic Bronchopneumonia Induced by Experimental Infection with Chlamydia psittaci. International Journal of Molecular Sciences, 21(8), 2817. https://doi.org/10.3390/ijms21082817





