Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options
Abstract
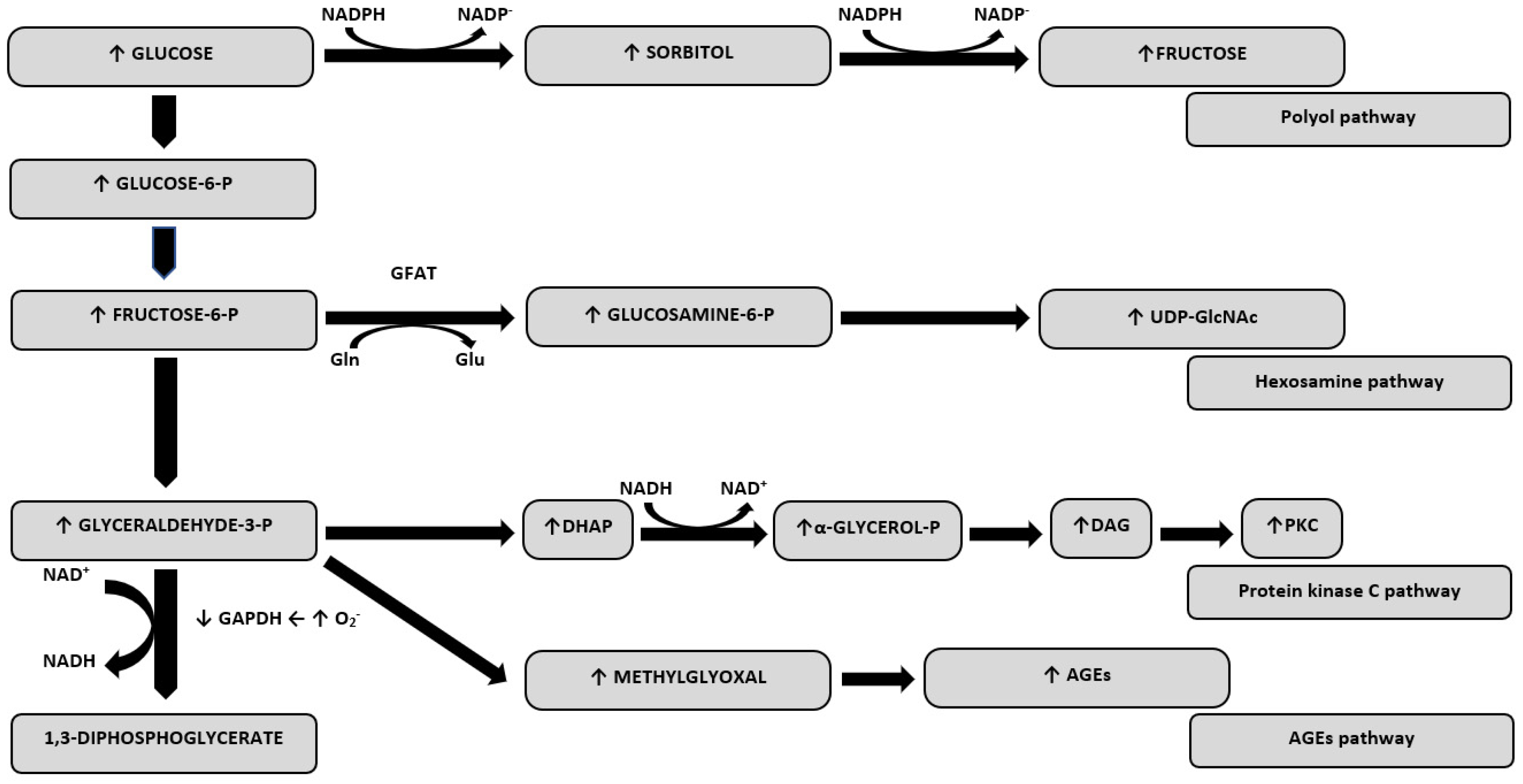
1. Introduction
2. The Mitochondrial Oxidative Phosphorylation (OXPHOS) System and Respiratory States
3. Oxidative Stress in T1DM Hearts
4. Interfibrillar Mitochondria (IFM) vs. Subsarcolemmal Mitochondria (SSM) in T1DM Hearts
5. Protection of Mitochondria in T1DM Hearts
5.1. Role of Insulin and Combined Therapy
5.2. Role of Antioxidants
5.3. Role of Mitofilin
5.4. Non-Pharmacological Options of Heart Protection
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Hebert, S.L.; Nair, K.S. Protein and energy metabolism in type 1 diabetes. Clin. Nutr. 2010, 29, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, A.I.; Smeitink, J.A.; Rodenburg, R.J. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic cardiomyopathy: A hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia 2018, 61, 21–28. [Google Scholar] [CrossRef]
- Marciniak, C.; Marechal, X.; Montaigne, D.; Neviere, R.; Lancel, S. Cardiac contractile function and mitochondrial respiration in diabetes-related mouse models. Cardiovasc. Diabetol. 2014, 13, 118. [Google Scholar] [CrossRef]
- Marinari, U.M.; Monacelli, R.; Cottalasso, D.; Novelli, A. Effects of Alloxan Diabetes and Insulin on Morphology and Certain Functional Activities of Mitochondria of the Rat Liver and Heart. Acta Diabetol. Lat. 1974, 11, 296–314. [Google Scholar] [CrossRef]
- Rendon, D.A. The Bioenergetics of Isolated Mitochondria from Different Animal Models for Diabetes. Curr. Diabetes Rev. 2016, 12, 66–80. [Google Scholar] [CrossRef]
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23. [Google Scholar] [CrossRef]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- Ferreira, R.; Guerra, G.; Padrão, A.I.; Melo, T.; Vitorino, R.; Duarte, J.A.; Remião, F.; Domingues, P.; Amado, F.; Domingues, M.R. Lipidomic characterization of streptozotocin-induced heart mitochondrial dysfunction. Mitochondrion 2013, 13, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Stanley, B.A.; Sivakumaran, V.; Bedja, D.; O’Rourke, B.; Paolocci, N.; Cortassa, S.; Aon, M.A. Impaired mitochondrial energy supply coupled to increased H2O2 emission under energy/redox stress leads to myocardial dysfunction during Type I diabetes. Clin. Sci. 2015, 129, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, E.J.; Berthiaume, J.M.; Kamath, V.; Achike, O.; Buchanan, E.; Montano, M.M.; Chandler, M.P.; Miyagi, M.; Rosca, M.G. Mitochondrial complex I defect and increased fatty acid oxidation enhance protein lysine acetylation in the diabetic heart. Cardiovasc. Res. 2015, 107, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Vadvalkar, S.S.; Matsuzaki, S.; Eyster, C.A.; Giorgione, J.R.; Bockus, L.B.; Kinter, C.S.; Kinter, M.; Humphries, K.M. Decreased Mitochondrial Pyruvate Transport Activity in the Diabetic Heart: Role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J. Biol. Chem. 2017, 292, 4423–4433. [Google Scholar] [CrossRef]
- Pham, T.; Loiselle, D.; Power, A.; Hickey, A.J. Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am. J. Physiol. Cell Physiol. 2014, 307, C499–C507. [Google Scholar] [CrossRef]
- Bugger, H.; Chen, D.; Riehle, C.; Soto, J.; Theobald, H.A.; Hu, X.X.; Ganesan, B.; Weimer, B.C.; Abel, E.D. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes 2009, 58, 1986–1997. [Google Scholar] [CrossRef]
- Seo, D.Y.; Ko, J.R.; Jang, J.E.; Kim, T.N.; Youm, J.B.; Kwak, H.B.; Bae, J.H.; Kim, A.H.; Ko, K.S.; Rhee, B.D.; et al. Exercise as A Potential Therapeutic Target for Diabetic Cardiomyopathy: Insight into the Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 6284. [Google Scholar] [CrossRef]
- Grieco, G.E.; Brusco, N.; Licata, G.; Nigi, L.; Formichi, C.; Dotta, F.; Sebastiani, G. Targeting microRNAs as a Therapeutic Strategy to Reduce Oxidative Stress in Diabetes. Int. J. Mol. Sci. 2019, 20, 6358. [Google Scholar] [CrossRef]
- Ye, G.; Metreveli, N.S.; Donthi, R.V.; Xia, S.; Xu, M.; Carlson, E.C.; Epstein, P.N. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 2004, 53, 1336–1343. [Google Scholar] [CrossRef]
- Makino, A.; Scott, B.T.; Dillmann, W.H. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010, 53, 1783–1794. [Google Scholar] [CrossRef]
- Patterson, C.; Portbury, A.L.; Schisler, J.C.; Willis, M.S. Tear me down: Role of calpain in the development of cardiac ventricular hypertrophy. Circ. Res. 2011, 109, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Zheng, D.; Xiong, S.; Hill, D.J.; Sun, T.; Gardiner, R.B.; Fan, G.C.; Lu, Y.; Abel, E.D.; Greer, P.A.; et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes 2016, 65, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Lin, C.J.; Chua, S.; Chung, S.Y.; Chen, S.M.; Lee, C.H.; Hang, C.L. Deletion of RasGRF1 Attenuated Interstitial Fibrosis in Streptozotocin-Induced Diabetic Cardiomyopathy in Mice through Affecting Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 3094. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.M.; Thapa, D.; Shepherd, D.L. Physiological and structural differences in spatially distinct subpopulations of cardiac mitochondria: Influence of cardiac pathologies. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1–H14. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Nichols, C.E.; Lewis, S.E.; Shepherd, D.L.; Jagannathan, R.; Croston, T.L.; Tveter, K.J.; Holden, A.A.; Baseler, W.A.; Hollander, J.M. Transgenic overexpression of mitofilin attenuates diabetes mellitus-associated cardiac and mitochondria dysfunction. J. Mol. Cell Cardiol. 2015, 79, 212–223. [Google Scholar] [CrossRef]
- Baseler, W.A.; Dabkowski, E.R.; Williamson, C.L.; Croston, T.L.; Thapa, D.; Powell, M.J.; Razunguzwa, T.T.; Hollander, J.M. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: Contribution of protein import dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R186–R200. [Google Scholar] [CrossRef]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef]
- Croston, T.L.; Shepherd, D.L.; Thapa, D.; Nichols, C.E.; Lewis, S.E.; Dabkowski, E.R.; Jagannathan, R.; Baseler, W.A.; Hollander, J.M. Evaluation of the cardiolipin biosynthetic pathway and its interactions in the diabetic heart. Life Sci. 2013, 93, 313–322. [Google Scholar] [CrossRef]
- Ardehali, H.; O’Rourke, B. Mitochondrial K(ATP) channels in cell survival and death. J. Mol. Cell Cardiol. 2005, 39, 7–16. [Google Scholar] [CrossRef]
- Fancher, I.S.; Dick, G.M.; Hollander, J.M. Diabetes mellitus reduces the function and expression of ATP-dependent K⁺ channels in cardiac mitochondria. Life Sci. 2013, 92, 664–668. [Google Scholar] [CrossRef]
- Pollesello, P.; Mebazaa, A. ATP-dependent potassium channels as a key target for the treatment of myocardial and vascular dysfunction. Curr. Opin. Crit. Care. 2004, 10, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.F.; Natali, A.J.; da Silva, E.; Gomes, G.J.; Teodoro, B.G.; Cunha, D.N.; Drummond, L.R.; Drummond, F.R.; Moura, A.G.; Belfort, F.G.; et al. Attenuation of Ca2+ homeostasis, oxidative stress, and mitochondrial dysfunctions in diabetic rat heart: Insulin therapy or aerobic exercise? J. Appl. Physiol. 2015, 119, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Remor, A.P.; de Matos, F.J.; Ghisoni, K.; da Silva, T.L.; Eidt, G.; Búrigo, M.; de Bem, A.F.; Silveira, P.C.; de León, A.; Sanchez, M.C.; et al. Differential effects of insulin on peripheral diabetes-related changes in mitochondrial bioenergetics: Involvement of advanced glycosylated end products. Biochim. Biophys. Acta 2011, 1812, 1460–1471. [Google Scholar] [CrossRef]
- Bai, J.; Cederbaum, A.I. Mitochondrial catalase and oxidative injury. Neurosignals 2001, 10, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Lismont, C.; Walton, P. The Peroxisome-Mitochondria Connection: How and Why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Baseler, W.A.; Dabkowski, E.R.; Jagannathan, R.; Thapa, D.; Nichols, C.E.; Shepherd, D.L.; Croston, T.L.; Powell, M.; Razunguzwa, T.T.; Lewis, S.E.; et al. Reversal of mitochondrial proteomic loss in Type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R553–R565. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, W.; Sun, D.; Wang, J.; Li, C.; Zhang, R.; Babcock, S.A.; Li, Y.; Liu, M.; Ma, M.; et al. A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetes-induced cardiac dysfunction: Role of AMPK-regulated autophagy. Biochim. Biophys. Acta 2015, 1852, 319–331. [Google Scholar] [CrossRef]
- Harner, M.; Körner, C.; Walther, D.; Mokranjac, D.; Kaesmacher, J.; Welsch, U.; Griffith, J.; Mann, M.; Reggiori, F.; Neupert, W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011, 30, 4356–4370. [Google Scholar] [CrossRef]
- Von der Malsburg, K.; Müller, J.M.; Bohnert, M.; Oeljeklaus, S.; Kwiatkowska, P.; Becker, T.; Loniewska-Lwowska, A.; Wiese, S.; Rao, S.; Milenkovic, D.; et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell 2011, 21, 694–707. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, X.; Yu, L.; Li, W.; Qian, D.; Cheng, P.; He, L.; Yang, H.; Zhang, C. Low-dose radiation prevents type 1 diabetes-induced cardiomyopathy via activation of AKT mediated anti-apoptotic and anti-oxidant effects. J. Cell. Mol. Med. 2016, 20, 1352–1366. [Google Scholar] [CrossRef]
| Author | Year | Animal Model of T1DM | Changes in Mitochondrial Function |
|---|---|---|---|
| Ferreira et al. [11] | 2013 | rats |
|
| Tocchetti et al. [12] | 2015 | guinea pigs |
|
| Vazquez et al. [13] | 2015 | rats |
|
| Vadvalkar et al. [14] | 2017 | Akita mice |
|
| Pham et al. [15] | 2014 | rats |
|
| Bugger et al. [16] | 2009 | Akita mice |
|
| Author | Year | Country | Test Factor | Study Group | Effect of the Examined Factor on Mitochondria |
|---|---|---|---|---|---|
| Ye et al. [19] | 2004 | USA | Catalase | mice | reduction of MDA level and excessive production of ROS, prevention of morphologic damage to mitochondria |
| Semaming et al. [9] | 2014 | Thailand | Insulin and protocatechuic acid | rats | decrease in MDA level and ROS production, attenuation of mitochondrial depolarization and mitochondrial swelling, increase in anti-apoptotic BCL2 protein expression |
| Tocchetti et al. [12] | 2015 | USA | Insulin and palmitate | guinea pigs | restoration of proper cardiac redox balance (both substances), protection of mitochondrial respiration (only palmitate) |
| Da Silva et al. [32] | 2015 | Brazil | Insulin and swimming training | rats | correction of such pathologies as reduced [Ca(2+)]I transient, increased uncoupling protein-2 expression, increased Ca(2+) uptake (swimming training), further normalization of Ca2+ transient amplitude, NADPH oxidase-4 expression and carbonyl protein contents in left ventricular (LV) tissue (insulin), restoration of LV tissue superoxide dismutase and mitochondrial O2 consumption, H2O2 release and permeability transition pore (MPTP) opening in heart mitochondria (combined therapy) |
| Remor et al. [33] | 2011 | Brazil | Insulin | rats | protection against the hyperglycemia-induced inhibition of mitochondrial OXPHOS enzymes activities |
| Makino et al. [20] | 2010 | USA | O(2)(-) scavenger TEMPOL | mice | decrease in mitochondrial fragmentation, oxidative stress (significant decrease in oxidative stress marker 8-iso-PGF-2α) and the oxidized level of several proteins |
| Thapa et al. [25] | 2015 | USA | Mitofilin | Mice | preservation of ETC complexes I, III, IV, V activities, state 3 respiration, mitochondrial membrane potential, damage of cristae structure and decrease in the accumulation of lipid peroxidation by-products |
| Baseler et al. [36] | 2013 | USA | Mitochondria phospholipid hydroperoxide glutathione peroxidase 4 | Mice | restoration of state 3 and state 4 respiration rates, preservation of mitochondrial respiratory chain proteins and ETC complex I, III, and IV activities, increase in ATP synthase activity, reversal of mitochondrial protein import dysfunction, decrease in H2O2 production and accumulation of lipid peroxidation by-products |
| Guo et al. [37] | 2015 | China | Aldehyde dehydrogenase 2 | Mice | promoting the AMPK-dependent autophagy |
| Zhang et al. [40] | 2016 | China | Low-dose radiation at medium or high doses (25 or 50 mGy) | Mice | inhibition of cardiac P53 activation, suppression of the increased ratio of Bax to Bcl, decrease in the contents of such classic oxidative damage markers as 3-NT, 4-HNE and MDA, inhibition of ROS production, improvement of Akt activation and increase in Nrf2 function |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieluch, A.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options. Int. J. Mol. Sci. 2020, 21, 2852. https://doi.org/10.3390/ijms21082852
Cieluch A, Uruska A, Zozulinska-Ziolkiewicz D. Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options. International Journal of Molecular Sciences. 2020; 21(8):2852. https://doi.org/10.3390/ijms21082852
Chicago/Turabian StyleCieluch, Aleksandra, Aleksandra Uruska, and Dorota Zozulinska-Ziolkiewicz. 2020. "Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options" International Journal of Molecular Sciences 21, no. 8: 2852. https://doi.org/10.3390/ijms21082852
APA StyleCieluch, A., Uruska, A., & Zozulinska-Ziolkiewicz, D. (2020). Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options. International Journal of Molecular Sciences, 21(8), 2852. https://doi.org/10.3390/ijms21082852





