Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats
Abstract
:1. Introduction
2. Results
2.1. Study Design
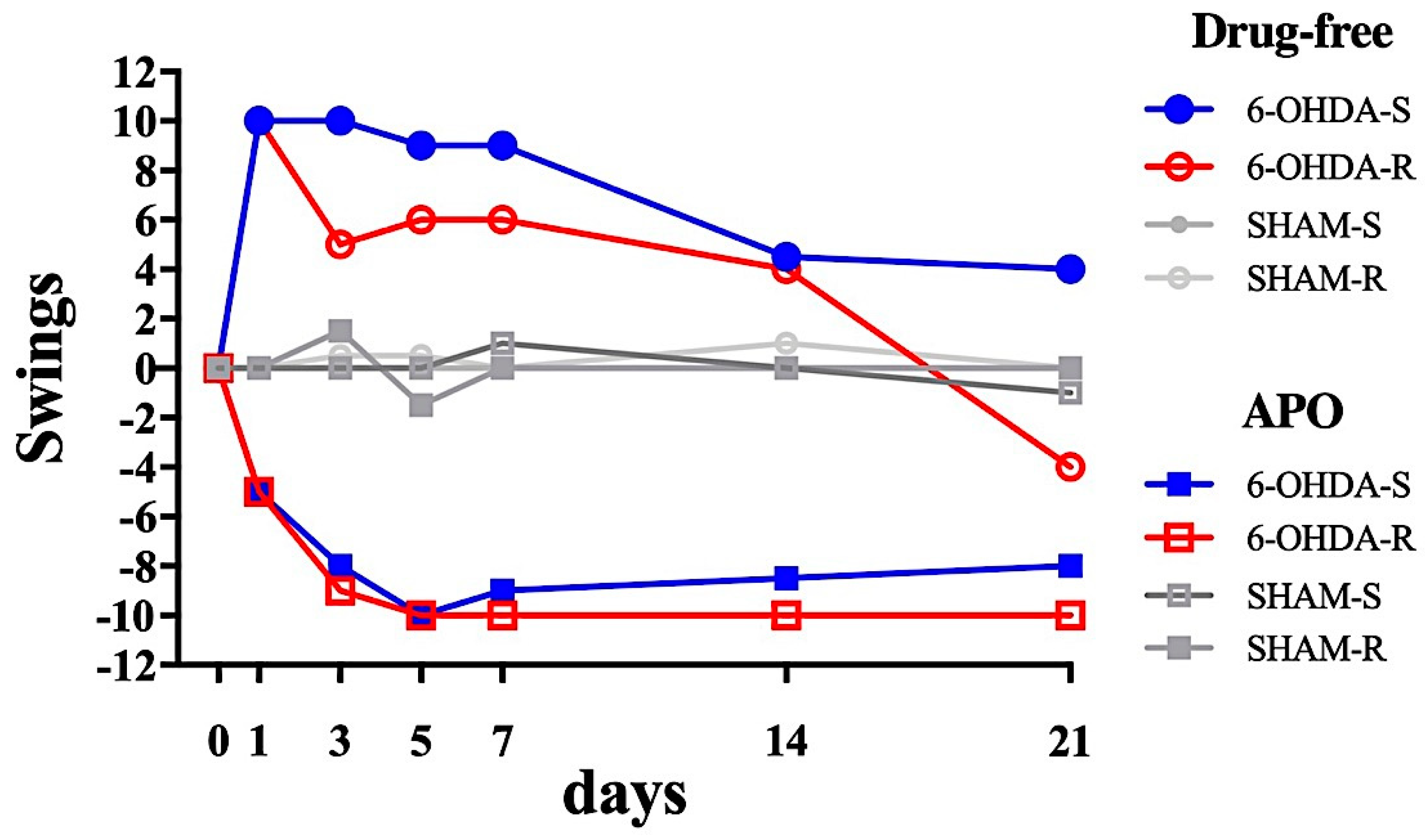
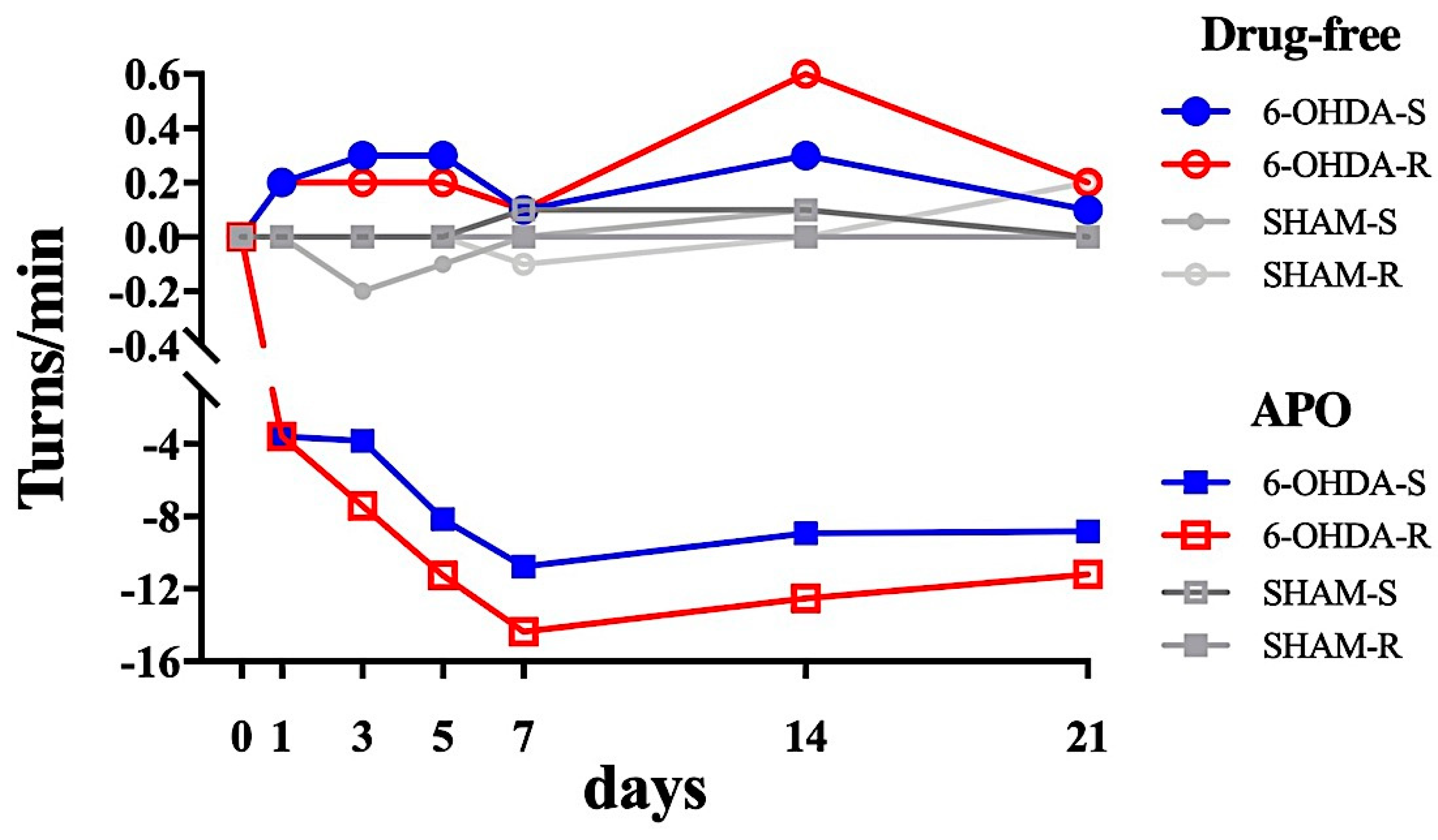
2.2. Behavioral Results
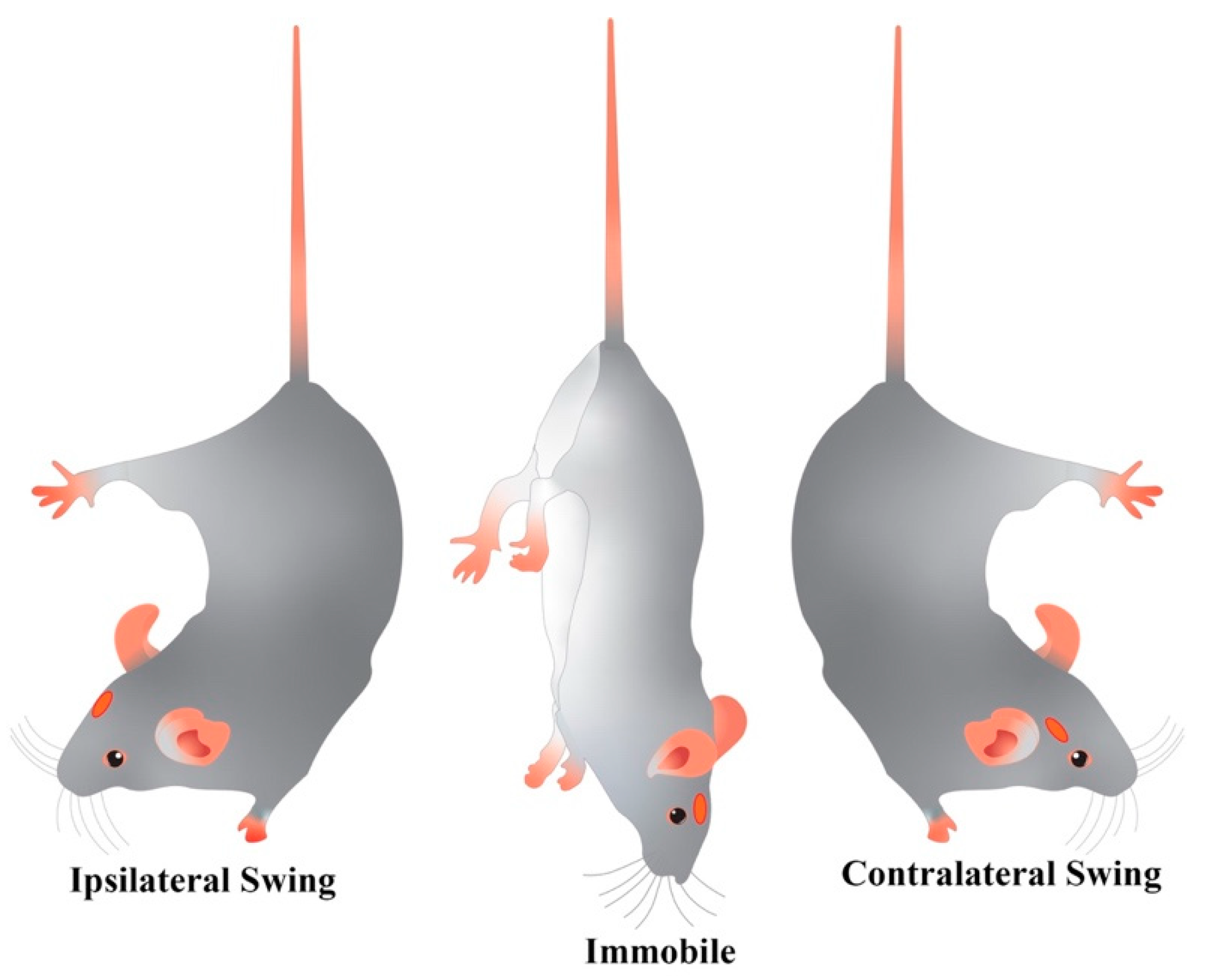
2.2.1. Tail Suspension Swing Test
2.2.2. Rotational Test
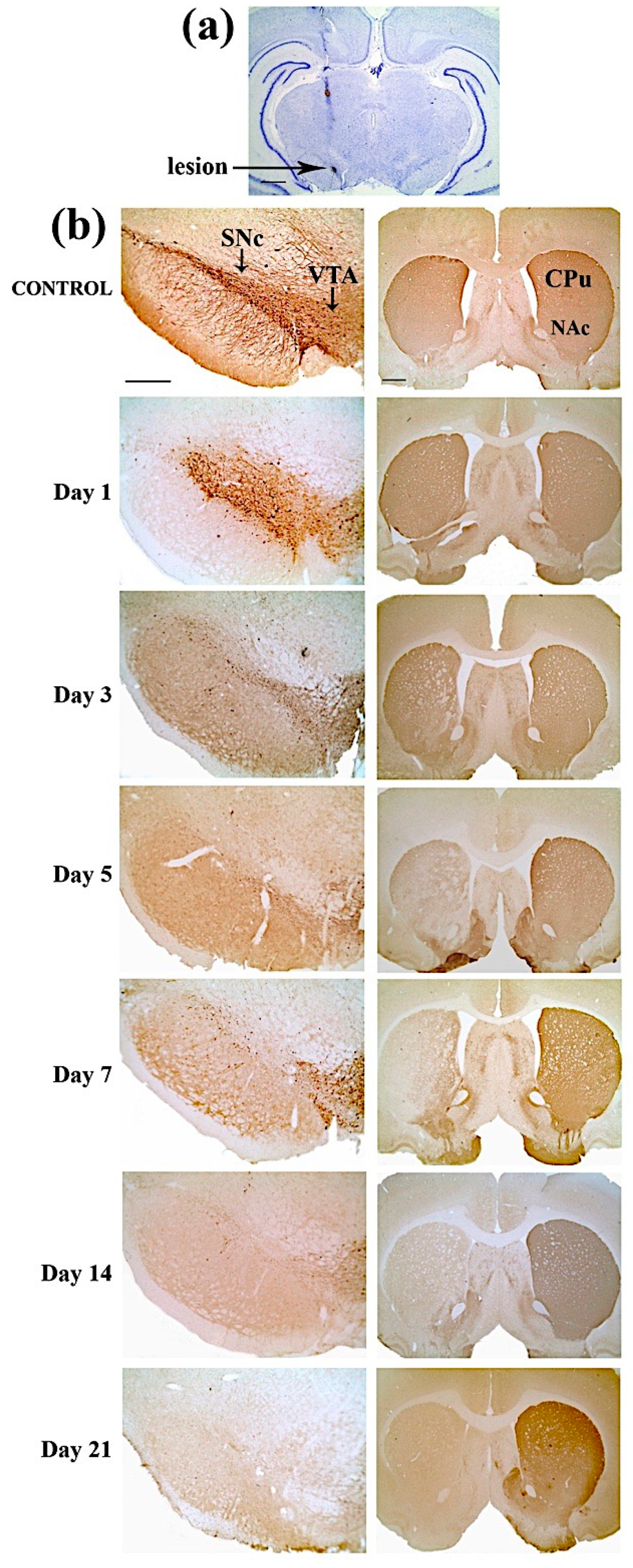
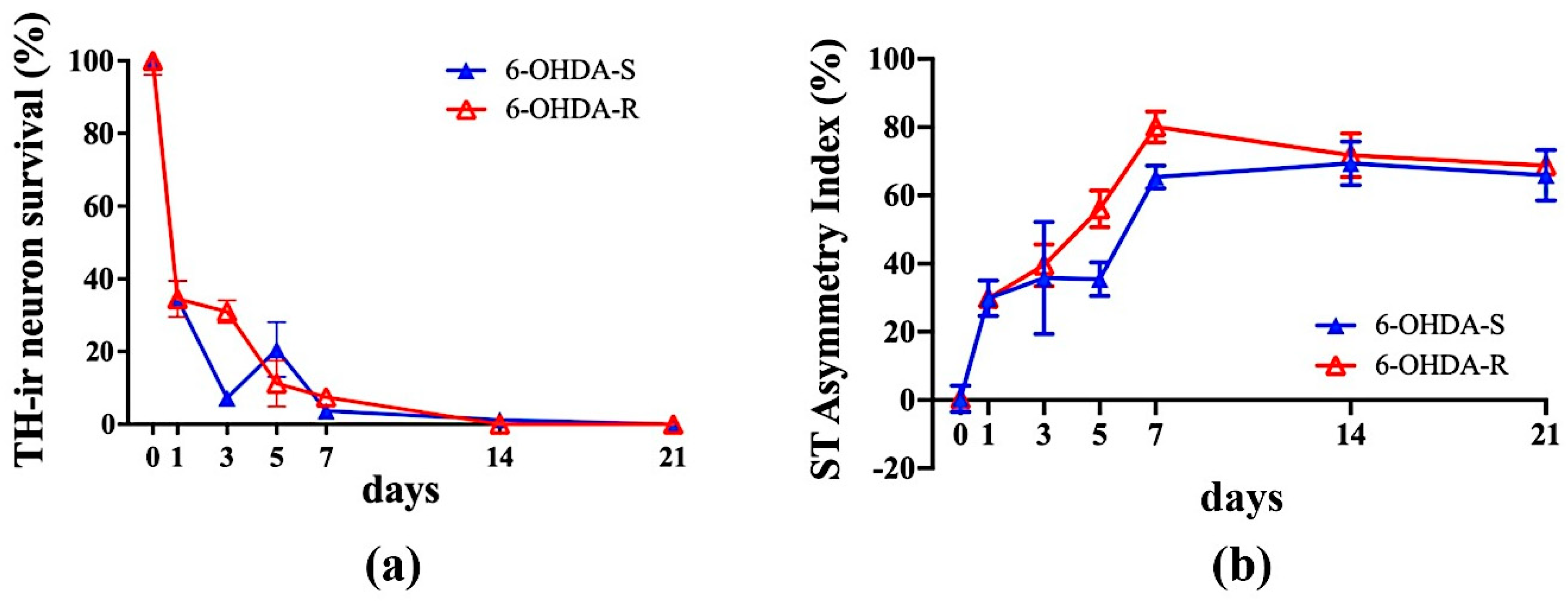
2.3. Immunohistochemical Results
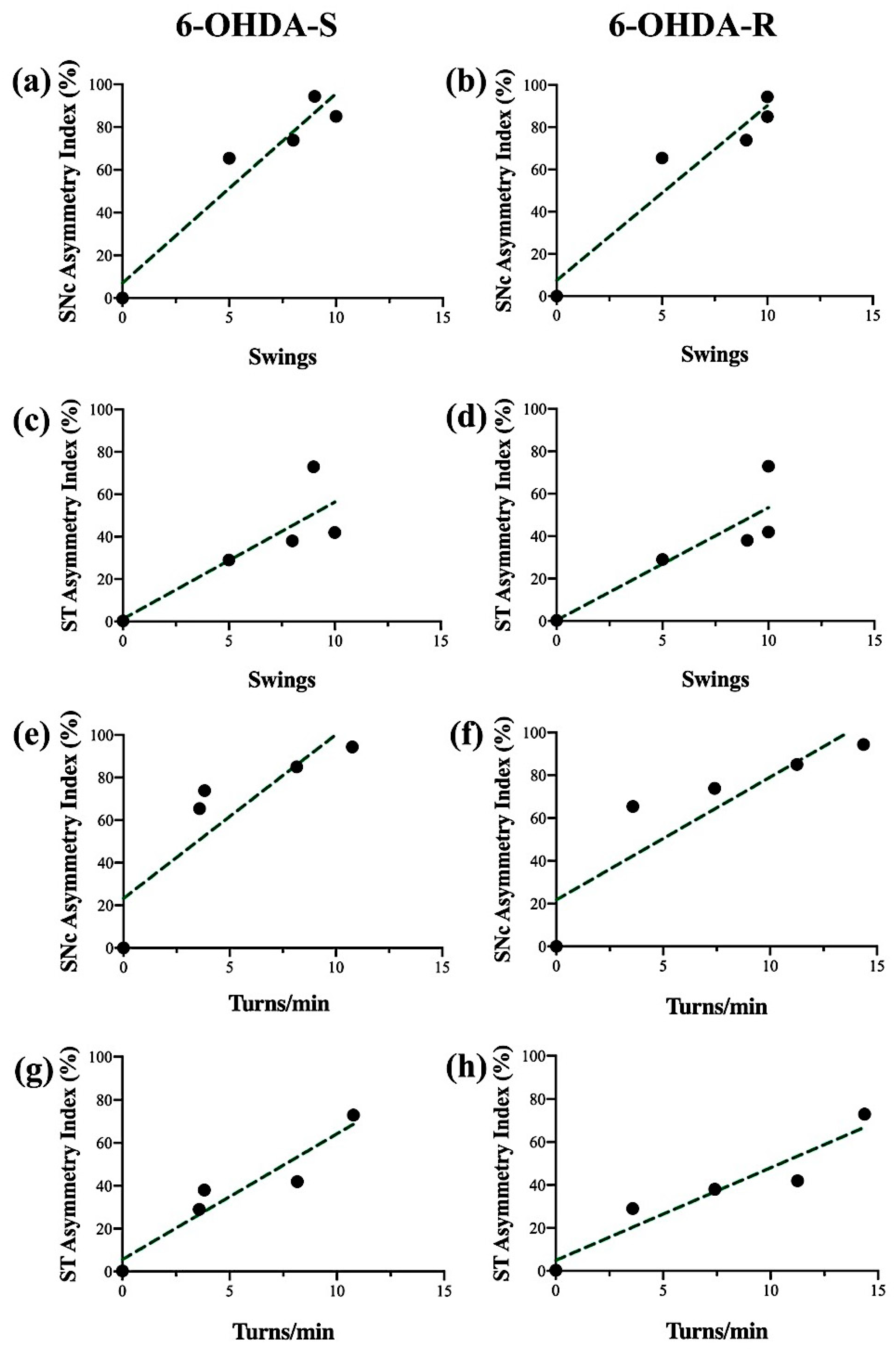
2.4. Regression Analysis
3. Discussion
4. Material and Methods
4.1. Animals and Surgical Procedure
4.2. Behavioral Tests
4.2.1. Tail Suspension Swing Test (TSST)
4.2.2. Rotation Test (RT)
4.3. Tissue Processing
4.4. Immunohistochemistry
4.5. Quantitative Analysis
4.6. Statistical Comparisons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Esposito, E.; Di Matteo, V.; Di Giovanni, G. Death in the substantia nigra: A motor tragedy. Expert Rev. Neurother. 2007, 7, 677–697. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Di Matteo, V.; Esposito, E. Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra. J. Neural Transm. Suppl. 2009. [Google Scholar] [CrossRef]
- Kalia, L.V.; E Lang, A. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.-T. Animal models of Parkinson’s disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grealish, S.; Xie, L.; Kelly, M.; Dowd, E. Unilateral axonal or terminal injection of 6-hydroxydopamine causes rapid-onset nigrostriatal degeneration and contralateral motor impairments in the rat. Brain Res. Bull. 2008, 77, 312–319. [Google Scholar] [CrossRef]
- Grealish, S.; Mattsson, B.; Dräxler, P.; Björklund, A. Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. Eur. J. Neurosci. 2010, 31, 2266–2278. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Dopamine autoreceptor stimulation: Clinical significance. Pharmacol. Biochem. Behav. 1982, 17, 1–10. [Google Scholar] [CrossRef]
- Su, R.; Zhen, J.; Wang, W.; Zhang, J.; Zheng, Y.; Wang, X.-M. Time-course behavioral features are correlated with Parkinson’s disease-associated pathology in a 6-hydroxydopamine hemiparkinsonian rat model. Mol. Med. Rep. 2017, 17, 3356–3363. [Google Scholar] [CrossRef] [Green Version]
- Blandini, F.; Armentero, M.-T.; Martignoni, E. The 6-hydroxydopamine model: News from the past. Park. Relat. Disord. 2008, 14, S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Stanic, D.; Finkelstein, D.I.; Bourke, D.W.; Drago, J.; Horne, M. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur. J. Neurosci. 2003, 18, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.; Liu, L.; Wang, X. Time course study of fractional anisotropy in the substantia nigra of a parkinsonian rat model induced by 6-OHDA. Behav. Brain Res. 2017, 328, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Boix-I-Coll, J.; Padel, T.; Paul, G. A partial lesion model of Parkinson’s disease in mice – Characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Allbutt, H.; Kassiou, M.; Henderson, J. Developing a preclinical model of Parkinson’s disease: A study of behaviour in rats with graded 6-OHDA lesions. Behav. Brain Res. 2006, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sarre, S.; Ebinger, G.; Michotte, Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J. Neurosci. Methods 2005, 144, 35–45. [Google Scholar] [CrossRef]
- Jeon, B.S.; Jackson-Lewis, V.; Burke, R.E. 6-Hydroxydopamine Lesion of the Rat Substantia Nigra: Time Course and Morphology of Cell Death. Neurodegeneration 1995, 4, 131–137. [Google Scholar] [CrossRef]
- Dunnett, S. Chapter V Motor function(s) of the nigrostriatal dopamine system: Studies of lesions and behavior. Handb. Chem. Neuroanat. 2005, 21, 237–301. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Del Rey, N.L.-G.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef]
- Fitzsimmons, D.F.; Moloney, T.C.; Dowd, E. Further validation of the corridor task for assessing deficit and recovery in the hemi-Parkinsonian rat: Restoration of bilateral food retrieval by dopamine receptor agonism. Behav. Brain Res. 2006, 169, 352–355. [Google Scholar] [CrossRef]
- Blandini, F.; Levandis, G.; Bazzini, E.; Nappi, G.; Armentero, M.-T. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: New clues from an old model. Eur. J. Neurosci. 2007, 25, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Westphal, R.; Simmons, C.; Mesquita, M.; Wood, T.C.; Williams, S.C.; Vernon, A.C.; Cash, D. Characterization of the resting-state brain network topology in the 6-hydroxydopamine rat model of Parkinson’s disease. PLoS ONE 2017, 12, e0172394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornaguera, J.; Schwarting, R.K. Early behavioral changes after nigro-striatal system damage can serve as predictors of striatal dopamine depletion. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 1999, 23, 1353–1368. [Google Scholar] [CrossRef]
- Van Camp, N.; Vreys, R.; Van Laere, K.; Lauwers, E.; Beque, D.; Verhoye, M.; Casteels, C.; Verbruggen, A.; Debyser, Z.; Mortelmans, L.; et al. Morphologic and functional changes in the unilateral 6-hydroxydopamine lesion rat model for Parkinson’s disease discerned with μSPECT and quantitative MRI. Magn. Reson. Mater. Physics Boil. Med. 2010, 23, 65–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galati, S.; Di Giovanni, G. Neuroprotection in Parkinson’s Disease: A Realistic Goal? CNS Neurosci. Ther. 2010, 16, 327–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giovanni, G. Will it Ever Become Possible to Prevent Dopaminergic Neuronal Degeneration? CNS Neurol. Disord.-Drug Targets 2008, 7, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Pagan, F.L. Improving outcomes through early diagnosis of Parkinson’s disease. Am. J. Manag. Care 2012, 18 (Suppl. 7), 176–182. [Google Scholar]
- Borlongan, C.V.; Sanberg, P. Elevated body swing test: A new behavioral parameter for rats with 6- hydroxydopamine-induced hemiparkinsonism. J. Neurosci. 1995, 15 Pt 2, 5372–5378. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Ungerstedt, U. Postsynaptic Supersensitivity after 6-Hydroxy-dopamine Induced Degeneration of the Nigro-striatal Dopamine System. Acta Physiol. Scand. 1971, 82 (Suppl. 367), 69–93. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, Y.; Halliday, G.M.; Rusznák, Z.; Watson, C.; Paxinos, G. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct. Funct. 2011, 217, 591–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornaguera, J.; Schwarting, R.K.W. Time course of deficits in open field behavior after unilateral neostriatal 6-hydroxydopamine lesions. Neurotox. Res. 2002, 4, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Grandi, L.C.; Di Giovanni, G.; Galati, S. Animal models of early-stage Parkinson’s disease and acute dopamine deficiency to study compensatory neurodegenerative mechanisms. J. Neurosci. Methods 2018, 308, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.M.; Campos, F.L.; Coimbra, B.; Pêgo, J.M.; Rodrigues, C.; Lima, R.A.R.; Rodrigues, A.J.; Sousa, N.; Salgado, A.J. Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol. Neurodegener. 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iancu, R.; Mohapel, P.; Brundin, P.; Paul, G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav. Brain Res. 2005, 162, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decressac, M.; Mattsson, B.; Björklund, A. Comparison of the behavioural and histological characteristics of the 6-OHDA and α-synuclein rat models of Parkinson’s disease. Exp. Neurol. 2012, 235, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, P.; Stayte, S.; Morris, G.P.; Vissel, B. Time dependent degeneration of the nigrostriatal tract in mice with 6-OHDA lesioned medial forebrain bundle and the effect of activin A on l-Dopa induced dyskinesia. BMC Neurosci. 2019, 20, 5. [Google Scholar] [CrossRef]
- Kordys, E.; Apetz, N.; Schneider, K.; Duncan, E.; Bueschbell, B.; Rohleder, C.; Sué, M.; Drzezga, A.; Neumaier, B.; Timmermann, L.; et al. Motor impairment and compensation in a hemiparkinsonian rat model: Correlation between dopamine depletion severity, cerebral metabolism and gait patterns. EJNMMI Res. 2017, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Baluchnejadmojarad, T.; Roghani, M. Evaluation of functional asymmetry in rats with dose-dependent lesions of dopaminergic nigrostriatal system using elevated body swing test. Physiol. Behav. 2004, 82, 369–373. [Google Scholar] [CrossRef]
- Roghani, M.; Behzadi, G.; Baluchnejadmojarad, T. Efficacy of elevated body swing test in the early model of Parkinson’s disease in rat. Physiol. Behav. 2002, 76, 507–510. [Google Scholar] [CrossRef]
- Trainor, B.C. Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Horm. Behav. 2011, 60, 457–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, J.A.; Tecuapetla, F.; Paixão, V.; Costa, R.M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 2018, 554, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, J.; Carey, R.; Huston, J.; Schwarting, R. Behavioral asymmetries and recovery in rats with different degrees of unilateral striatal dopamine depletion. Brain Res. 1994, 664, 178–188. [Google Scholar] [CrossRef]
- Abrous, N.; Rodriguez, J.J.; Montaron, M.-F.; Aurousseau, C.; Le Moal, M.; Barneoud, P. Behavioural recovery after unilateral lesion of the dopaminergic mesotelencephalic pathway: Effect of repeated testing. Neuroscience 1998, 84, 213–221. [Google Scholar] [CrossRef]
- Gancher, S.; Mayer, A. Sensitization to apomorphine-induced rotational behavior in 6-OHDA-lesioned rats: Effects of NMDA antagonists on drug response. Brain Res. 1995, 682, 63–68. [Google Scholar] [CrossRef]
- Gancher, S.; Mayer, A.; Youngman, S. Changes in Apomorphine Pharmacodynamics Following Repeated Treatment in 6-Hydroxydopamine-Lesioned Rats. Brain Res. 1996, 729, 190–196. [Google Scholar] [CrossRef]
- Kyriazis, M. Neuroprotective, Anti-Apoptotic Effects of Apomorphine. J. Anti-Aging Med. 2003, 6, 21–28. [Google Scholar] [CrossRef]
- Hara, H.; Ohta, M.; Adachi, T. Apomorphine protects against 6-hydroxydopamine-induced neuronal cell death through activation of the Nrf2-ARE pathway. J. Neurosci. Res. 2006, 84, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Grünblatt, E.; Mandel, S.; Maor, G.; Youdim, M.B. Effects of R- and S-apomorphine on MPTP-induced nigro-striatal dopamine neuronal loss. J. Neurochem. 2008, 77, 146–156. [Google Scholar] [CrossRef]
- Castri, P.; Busceti, C.; Battaglia, G.; Girardi, F.; Cavallari, M.; Orzi, F.; Fornai, F. Protection by apomorphine in two independent models of acute inhibition of oxidative metabolism in rodents. Clin. Exp. Hypertens. 2006, 28, 387–394. [Google Scholar] [CrossRef]
- Yuan, H.; Sarre, S.; Ebinger, G.; Michotte, Y. Neuroprotective and neurotrophic effect of apomorphine in the striatal 6-OHDA-lesion rat model of Parkinson’s disease. Brain Res. 2004, 1026, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.G.; Szechtman, H. Relation between motor asymmetry and direction of rotational behaviour under amphetamine and apomorphine in rats with unilateral degeneration of the nigrostriatal dopamine system. Behav. Brain Res. 1990, 39, 123–133. [Google Scholar] [CrossRef]
- Hudson, J.L.; Fong, C.-S.; Boyson, S.J.; Hoffer, B.J. Conditioned apomorphine-induced turning in 6-OHDA-lesioned rats. Pharmacol. Biochem. Behav. 1994, 49, 147–154. [Google Scholar] [CrossRef]
- Hudson, J.L.; Van Horne, C.G.; Strömberg, I.; Brock, S.; Clayton, J.; Masserano, J.; Hoffer, B.J.; Gerhardt, G.A. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993, 626, 167–174. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Park. Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-I.; Nam, H.; Kim, J.S.; Hong, S.C.; Shin, H.J.; Park, K.; Eoh, W.; Lee, W.Y. Increased burst firing in substantia nigra pars reticulata neurons and enhanced response to selective D2 agonist in hemiparkinsonian rats after repeated administration of apomorphine. J. Korean Med Sci. 2001, 16, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Samad, N.; Sikandar, N.; Haleem, D.J. Pre- and Postsynaptic Dopamine Mechanism after the Administration of Apomorphine: Relationship with Restraint Stress. Pak. J. Biochem. Mol. Biol. 2015, 47, 149–154. [Google Scholar]
- Costall, B.; Naylor, R.J.; Pettit, J.C. Changes in dopamine agonist and antagonist activity floowing temporary inactivation of the neostriatum. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1974, 285, 103–126. [Google Scholar] [CrossRef]
- Schwarting, R.; Huston, J. UNILATERAL 6-HYDROXYDOPAMINE LESIONS OF MESO-STRIATAL DOPAMINE NEURONS AND THEIR PHYSIOLOGICAL SEQUELAE. Prog. Neurobiol. 1996, 49, 215–266. [Google Scholar] [CrossRef]
- Ungerstedt, U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef]
- Ungerstedt, U.; Arbuthnott, G.W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970, 24, 485–493. [Google Scholar] [CrossRef]
- Perese, D.; Ulman, J.; Viola, J.; Ewing, S.; Bankiewicz, K. A 6-hydroxydopamine-induced selective parkinsonian rat model. Brain Res. 1989, 494, 285–293. [Google Scholar] [CrossRef]
- Buonamici, M.; Cervini, M.A.; Rossi, A.C.; Sebastiani, L.; Raffaelli, A.; Bagnoli, P. Injections of 6-hydroxydopamine in the substantia nigra of the rat brain: Morphological and biochemical effects. Behav. Brain Res. 1990, 38, 83–95. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Hu, D.-N.; Liu, F.-X.; Chen, Z.; Luo, J.-H. Apomorphine-induced turning behavior in 6-hydroxydopamine lesioned rats is increased by histidine and decreased by histidine decarboxylase, histamine H1 and H2 receptor antagonists, and an H3 receptor agonist. Pharmacol. Biochem. Behav. 2008, 90, 325–330. [Google Scholar] [CrossRef]
- Falcone, R.; Florio, T.M.; Di Giacomo, E.; Benedetti, E.; Cristiano, L.; Antonosante, A.; Fidoamore, A.; Massimi, M.; Alecci, M.; Ippoliti, R.; et al. PPARβ/δ and γ in a Rat Model of Parkinson’s Disease: Possible Involvement in PD Symptoms. J. Cell. Biochem. 2015, 116, 844–855. [Google Scholar] [CrossRef]
- Kondoh, T.; Bannai, M.; Nishino, H.; Torii, K. 6-Hydroxydopamine-induced lesions in a rat model of hemi-Parkinson’s disease monitored by magnetic resonance imaging. Exp. Neurol. 2005, 192, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Burke, R.E.; Cadet, J.L.; Kent, J.D.; Karanas, A.L.; Jackson-Lewis, V. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: Relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. J. Neurosci. Methods 1990, 35, 63–73. [Google Scholar] [CrossRef]
- Boutelle, M.G.; Zetterström, T.; Pei, Q.; Svensson, L.; Fillenz, M. In vivo neurochemical effects of tail pinch. J. Neurosci. Methods 1990, 34, 151–157. [Google Scholar] [CrossRef]
- Miyanishi, K.; Choudhury, M.E.; Watanabe, M.; Kubo, M.; Nomoto, M.; Yano, H.; Tanaka, J. Behavioral tests predicting striatal dopamine level in a rat hemi-Parkinson’s disease model. Neurochem. Int. 2019, 122, 38–46. [Google Scholar] [CrossRef]
- Casarrubea, M.; Di Giovanni, G.; Crescimanno, G.; Rosa, I.; Aiello, S.; Di Censo, D.; Ranieri, B.; Santangelo, A.; Busatta, D.; Cassioli, E.; et al. Effects of Substantia Nigra pars compacta lesion on the behavioral sequencing in the 6-OHDA model of Parkinson’s disease. Behav. Brain Res. 2019, 362, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.R.; Kang, I.H.; Wheeler, D.B.; A Lindquist, R.; Papallo, A.; Sabatini, D.M.; Golland, P.; Carpenter, A. CellProfiler Analyst: Data exploration and analysis software for complex image-based screens. BMC Bioinform. 2008, 9, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, I.; Di Censo, D.; Ranieri, B.; Di Giovanni, G.; Scarnati, E.; Alecci, M.; Galante, A.; Florio, T.M. Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats. Int. J. Mol. Sci. 2020, 21, 2874. https://doi.org/10.3390/ijms21082874
Rosa I, Di Censo D, Ranieri B, Di Giovanni G, Scarnati E, Alecci M, Galante A, Florio TM. Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats. International Journal of Molecular Sciences. 2020; 21(8):2874. https://doi.org/10.3390/ijms21082874
Chicago/Turabian StyleRosa, Ilaria, Davide Di Censo, Brigida Ranieri, Giuseppe Di Giovanni, Eugenio Scarnati, Marcello Alecci, Angelo Galante, and Tiziana Marilena Florio. 2020. "Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats" International Journal of Molecular Sciences 21, no. 8: 2874. https://doi.org/10.3390/ijms21082874
APA StyleRosa, I., Di Censo, D., Ranieri, B., Di Giovanni, G., Scarnati, E., Alecci, M., Galante, A., & Florio, T. M. (2020). Comparison between Tail Suspension Swing Test and Standard Rotation Test in Revealing Early Motor Behavioral Changes and Neurodegeneration in 6-OHDA Hemiparkinsonian Rats. International Journal of Molecular Sciences, 21(8), 2874. https://doi.org/10.3390/ijms21082874





