Abstract
Rheumatoid arthritis (RA) is an inflammatory joint disorder characterized by synovial proliferation and inflammation, with eventual joint destruction if inadequately treated. Modern therapies approved for RA target the proinflammatory cytokines or Janus kinases that mediate the initiation and progression of the disease. However, these agents fail to benefit all patients with RA, and many lose therapeutic responsiveness over time. More effective or adjuvant treatments are needed. Melatonin has shown beneficial activity in several animal models and clinical trials of inflammatory autoimmune diseases, but the role of melatonin is controversial in RA. Some research suggests that melatonin enhances proinflammatory activities and thus promotes disease activity in RA, while other work has documented substantial anti-inflammatory and immunoregulatory properties of melatonin in preclinical models of arthritis. In addition, disturbance of the circadian rhythm is associated with RA development and melatonin has been found to affect clock gene expression in joints of RA. This review summarizes current understanding about the immunopathogenic characteristics of melatonin in RA disease. Comprehensive consideration is required by clinical rheumatologists to balance the contradictory effects.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that is characterized by synovial proliferation and inflammatory responses, the presence of autoantibodies including rheumatoid factor and anti-citrullinated protein antibodies (ACPA) in sera, cartilage, and bone erosion with deformity, and co-occurring health conditions such as cardiovascular disease events, pulmonary, psychological, and metabolic bone disorders [1]. Proinflammatory cytokines that mediate the progression of RA disease include tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and IL-6. Current international guidelines for patients with early RA recommend starting disease-modifying antirheumatic drugs (DMARDs) as soon as possible, with methotrexate being the preferred choice [2]. Methotrexate is usually supplemented with short-term, low-dose oral or intra-articular glucocorticoids (GCs) for fast relief of pain and swelling and for arresting the inflammatory process. GCs must be carefully managed to prevent their inappropriate use and tapered as soon as possible to avoid long-term adverse effects [2]. The highly efficacious biologic DMARDs targeting the proinflammatory cytokines and Janus kinase inhibitors are intended for patients with persistently active disease after initial methotrexate failure and, in some cases, another conventional DMARD [2]. However, although these novel medications help to control RA disease activity, they are not universally effective in all RA patients [3,4] and many will lose therapeutic responsiveness after a period of time [5]. More potent or complementary treatments are needed.
The anti-inflammatory and antioxidant effects of melatonin have proven beneficial in several inflammatory autoimmune diseases [6]. Melatonin is also capable of regulating responses of T cell subsets, such as CD4+ T helper (Th)1, Th17, and regulatory T cells (Tregs) [7]. However, numerous studies indicate that melatonin, instead of being beneficial, could exacerbate RA disease-related activities. Nevertheless, in recent years, some conflicting results show that melatonin is capable of alleviating RA through anti-inflammatory and immunoregulatory mechanisms. The aim of this review is to elucidate the complex reactions of melatonin in RA and determine whether melatonin could serve as a potential therapeutic agent.
2. Mechanisms of Melatonin
Endocrine circadian rhythms are regulated by the endogenous hormone melatonin (N-acetyl-5-methoxytryptamine), which activates specific high-affinity melatonin receptors expressed on several different types of cells, including immunocompetent cells [8]. The pineal gland is the primary source of melatonin, which is also synthesized by tissues and organs of multicellular organisms including the retina, gastrointestinal tract, skin, and leukoctyes (in peripheral blood and in bone marrow) [9]. In the skin, melatonin can regulate cutaneous pigmentation and perform photoprotective and anticancer activities [10,11]. N-acetylserotonin, a precursor to melatonin, enters the systemic circulation from different peripheral organs for transformation to melatonin [12]. Furthermore, the effects of melatonin can be mediated by its metabolites because of the rapid metabolism of melatonin at the peripheral sites [13,14]. As the production of melatonin by the non-endocrine organs responds to signals other than circadian cycles, the endocrine, autocrine, and paracrine effects of melatonin mean that this substance is extensively involved in the regulation of the human immune system [9].
The biological effects of melatonin involve three pathways: (i) G-protein-coupled membrane receptor signaling; (ii) nuclear signaling; and (iii) receptor-independent signaling that accounts for radical scavenging activities of melatonin [7,15]. The binding of melatonin to the G protein-coupled melatonin receptors (MT1 and MT2) on the plasma membrane of the target cells enables melatonin to stimulate signaling pathways and reduce cell proliferation [16]. MT1 receptors are expressed throughout the body but mainly in the central nervous system, in the thymus and the spleen, B cells, CD4, and CD8 cells [7]. Neuroanatomical mapping of melatonin receptors has revealed marked differences in distribution patterns of MT1 and MT2 proteins in the adult rat brain, with for instance MT2 receptors identified in the reticular thalamic nucleus, mediating neuronal firing and burst activity related to nonrapid eye movement (NREM) sleep, whereas no MT1 receptors are found in this area, confirming the highly specific functioning of melatonin receptors in sleep neurophysiology [17]. MT1 and MT2 affect gene transcription activities through extracellular signal-regulated kinase (ERK) pathways and CREP phosphorylation [7]. Melatonin receptors have also been identified in RA synovial macrophages [18]. A positive correlation has been observed between the polymorphism of the melatonin receptor type 1B (MTNR1B) and levels of rheumatoid factor in Korean patients with RA [19]. Other researchers have reported significantly lower levels of MT1 expression in RA synovial tissue compared with normal healthy tissue, and the finding that siRNAs against MT1 reverse melatonin-mediated inhibition of TNF-α and IL-1β production, confirming that melatonin suppresses TNF-α and IL-1β via the MT1 receptor [20]. Thus, the activity of membrane melatonin receptors and their specific agonists is implicated in circadian rhythmicity [7]. Retinoic acid-related orphan receptor alpha (RORα) is an important member of the ROR subfamily of nuclear receptors, which mediate several physiological functions, such as metabolic, immunologic, and circadian actions [21]. The identified endogenous ligands for RORα consist of sterols and their derivatives [22]. It appears that RORα mediates the indirect effects of melatonin in the periphery, such as immunomodulation, cellular growth, and bone differentiation [23]. Moreover, while RORα is not a receptor for melatonin or its metabolites, the constitutive activity of RORα may be modulated by membrane melatonin receptors [21,23].
Melatonin also displays antioxidant and anti-inflammatory activity, depending on the cellular state [24,25]. Evidence suggests that melatonin serves as a link between circadian rhythms and joint diseases, including RA and osteoarthritis (OA) [26,27]. For instance, oxidative stress induced by RA is reduced by melatonin and/or its metabolites, which not only neutralize the reactive oxygen (ROS) and reactive nitrogen species (RNS), but also upregulate levels of glutathione and antioxidant enzyme expression and activity [28,29]. In RA and OA, melatonin and its metabolites modulate several molecular signaling pathways including those governing inflammation, proliferation, and apoptosis [28,29].
3. A Role for Melatonin in Rheumatoid Arthritis Therapy?
In investigations involving melatonin in animal models of inflammatory autoimmune diseases (multiple sclerosis, systemic lupus erythematosus, inflammatory bowel disease, and type 1 diabetes), melatonin has demonstrated beneficial effects in these diseases, including prophylactic and therapeutic effects in rats with adjuvant-induced arthritis (AA), in which melatonin dose-dependently repressed the inflammatory response and enhanced proliferation of thymocytes and secretion of IL-2 [30]. In addition, melatonin decreased the elevated level of cyclic 3′,5′-AMP (cAMP) induced by forskolin. The drop in thymocyte proliferation induced by injection of Freund’s complete adjuvant was highly correlated with a decrease in the levels of Met-enkephalin (Met-Enk) in the thymocytes, which were strikingly augmented by melatonin; this effect was blocked by the Ca2+ channel antagonist, nifedipine. The anti-inflammatory and immunoregulatory actions of melatonin involved a G protein-adenyl cyclase-cAMP transmembrane signal and Met-Enk release by thymocytes [30].
3.1. Modulation of the Circadian Clock by Melatonin in RA
Importantly, circadian rhythms exist in almost all cells of the body, and are regulated by circadian clock gene expression [7,15]. Any disruption in these circadian clocks is associated with the onset of inflammatory-related disease states and joint diseases, including RA [7,15]. Patients with RA exhibit abnormal clock gene expression, with disturbances in the hypothalamic-pituitary-adrenal axis influencing changes in circadian rhythms of circulating serum levels of melatonin, IL-6, cortisol and in chronic fatigue [15]. Melatonin exerts its effects in RA by modulating clock gene expression, including the Cry1 gene [7,15]. By attenuating the expression of the Cry1 gene, melatonin upregulates levels of cAMP production and increases activation of protein kinase A (PKA) and nuclear factor kappa B (NF-κB), which increases CIA severity in rats [31,32]. As detailed earlier, the diurnal secretion of melatonin is also closely related to the production of IL-12 and NO among RA synovial macrophages and human monocytic myeloid THP-1 cells [33].
A positive correlation has been reported between elevated early morning serum melatonin concentrations and disease activity scores as well as erythrocyte sedimentation rate (ESR) levels in patients with juvenile rheumatoid arthritis, although higher melatonin concentrations did not correlate with disease severity [34], echoing the findings of Forrest and colleagues reported earlier, who noted that elevated ESR and neopterin concentrations following melatonin treatment did not worsen the severity of RA disease [35]. El-Awady and colleagues suggested that melatonin may promote the activity of RA disease, rather than its severity [34]. However, as reported above, Akfhamizadeh and colleagues found no link between elevated morning serum melatonin concentrations and RA disease activity or other disease characteristics, despite also observing significantly higher melatonin values in newly diagnosed RA patients compared with those who had established RA disease [36].
There also appears to be a relationship between melatonin and the Bmal1 and ROR clock genes [15]. It is speculated that high melatonin concentrations in RA patients may modulate ROR activation [15]. ROR acts as a negative regulator of inflammation via the NF-κB signaling pathway and is essential in the activity of both melatonin and the clock gene Bmal1, which helps to maintain 24-h rhythms and regulate immune responses [15,37]. Moreover, ROR proteins bind into the promoter region and drive Bmal1 gene expression [38]. This activity at the binding site is inhibited by reverse-eritroblastosis viruses (REV-ERBs), which may contribute to Bmal1 suppression and exacerbation of RA [15].
3.2. Adverse Effects of Melatonin in RA
Evidence suggests that melatonin is not beneficial in RA. For instance, the development of collagen-induced arthritis (CIA) in DBA/1 mice is exacerbated by constant darkness [39] and by daily exogenous administration of melatonin 1 mg/kg [40]. Hansson and colleagues then investigated the effects of surgical pinealectomy in DBA/1 and NFR/N mice with collagen-induced arthritis (CIA) [41]. Serum melatonin levels were reduced in the pinealectomized mice to around 30% of levels in normal or sham-operated controls [41]. In both mouse strains, pinealectomy was associated with a delay in onset of arthritic disease, less severe arthritis (lower clinical scores), and lower serum anti-CII levels compared with sham-operated animals [41]. The researchers interpreted these findings as showing that high physiological levels of melatonin stimulate the immune system and worsen CIA, while inhibiting the release of melatonin is beneficial [41]. Their speculation was supported by observations from mice subjected to 30 days of Bacillus Calmette-Guérin (BCG) inoculations into the left hind paw, inducing chronic granulomatous inflammation [42]. Higher vascular permeability was seen around the granulomatous lesions at midnight than at midday; this rhythmic variation was eliminated by pinealectomy and restored by nocturnal replacement of melatonin [42].
This ability of melatonin to modulate immune response was further illustrated by experiments in which the production of IL-12 and nitric oxide (NO) was significantly increased in the media of melatonin-stimulated RA synovial macrophages and cultured THP-1 cells compared with RPMI-treated synovial macrophage controls [33]. Unexpectedly, the opposite effects in IL-12 and NO levels were seen when RA synovial macrophages were pretreated with lipopolysaccharide (LPS) prior to melatonin, as compared with synovial macrophages treated with LPS alone [33]. This study explained the possible mechanism of joint morning stiffness in relation to diurnal rhythmicity of neuroendocrine pathways [33].
These conclusions are supported by later evidence from in vitro and in vivo studies, as well as clinical investigations, showing how melatonin stimulates the production of NO, T helper type 1 (Th1)-type and other inflammatory cytokines besides IL-12 (IL-1, IL-2, IL-4, IL-5, IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor [GM-CSF], and transforming growth factor [TGF]-β, interferon [IFN]-γ), and enhances both cell-mediated and humoral responses [43,44,45]. In the early morning, patients with RA exhibit high serum levels of proinflammatory cytokines, especially TNF-α and IL-6, when melatonin serum concentrations are also higher [6,43]. The effects of these circadian rhythms are thought to promote the joint pain and morning stiffness that characterizes RA [6]. Animal studies have shown that melatonin treatment (10 mg/kg) dysregulates circadian clock genes, which may promote the progression of RA [31]. Intriguingly, a dual effect of melatonin as a proinflammatory agent and antioxidant has been observed in CIA rats [32]. In that study, a lower dosage of melatonin (30 µg/kg) increased anti-collagen antibodies, IL-1β, and IL-6 levels in the serum and joints of arthritic rats, worsening the severity of joint damage, while simultaneously lowering oxidative markers nitrite/nitrate and lipid peroxidation in serum, but not in joints [32].
3.3. Neutral or Beneficial Effects of Melatonin in RA
Notably, a cross-sectional study from Iran has reported finding significantly higher morning serum levels of melatonin in patients with RA compared with healthy controls, but no correlation between melatonin and RA disease activity score or other disease characteristics, including age, disease duration, medications, gender, or season of sampling [36]. The study also reported finding higher serum melatonin values in newly diagnosed patients compared with patients with established RA, which needs further investigation [36].
Matrix metalloproteinases (MMPs) are a family of endopeptidases primarily responsible for catalyzing the degradation of the extracellular matrix (ECM) [46]. MMPs play important roles in RA. Elevated levels of circulating MMP-3, MMP-8 and MMP-9 are associated with disease progression in RA [47]. In particular, MMP-2 and MMP-9 are expressed in synoviocytes, CD34+ endothelial cells, monocytes and macrophages of rheumatoid synovium, indicating that both molecules are critical to pannus formation and invasion in RA progression [47]. Interestingly, melatonin reportedly directly inhibits secreted MMP-9 by binding to the active site and significantly reducing the catalytic activity of MMP-9 in both in vitro and cultured cells, in a dose- and time-dependent manner [46]. Thus, melatonin could have an important role in the prevention of joint destruction in RA.
Research demonstrating that melatonin dose-dependently inhibits the proliferation of RA fibroblast-like synoviocytes (FLS) through the activation of the ERK/P21/P27 pathway suggests that inhibiting the invasion of RA FLS through cartilage and into bone may have important implications in the treatment of RA [48]. Blocking NF-κB signaling appears to be the way in which melatonin protects cells from oxidative stress [28] and largely explains how melatonin suppresses proinflammatory cytokines such as IL-1β and TNF-α [20]. Other pathways and molecules associated with inflammation that are modulated by melatonin include the mitogen-activated protein kinase (MAPK) and nuclear erythroid 2-related factor 2 (Nrf2) pathways, as well as Toll-like receptors [15].
In a clinical trial involving RA patients, six months of melatonin treatment (10 mg/day) was associated with a general decrease from baseline in concentrations of peroxidation markers [35]. Conversely, ESR and neopterin concentrations were increased from baseline with melatonin and significantly higher at six months than concentrations in the placebo-treated cohort, which experienced a significant downward trend in these inflammatory indicators during the trial [35]. Paradoxically, neither the elevations in ESR and neopterin concentrations nor the decrease in tissue peroxidation associated with melatonin translated into significant differences from the placebo group in terms of patients’ symptoms, or in the concentrations of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) [35]. The study researchers concluded that melatonin does not appear to be beneficial in RA [35].
The findings of Forrest et al. are discussed by Maestroni et al., who argue that because melatonin enhances the production of Th1-type and inflammatory cytokines in RA, upregulates cell-mediated and humoral responses, and also exacerbates CIA in mice, melatonin likely promotes RA disease and is inappropriate for therapeutic use [49]. Maestroni et al. also emphasized the high blood melatonin concentrations (280 pg/mL) observed 12 h after dosing in the RA cohort [49], in relation to the notion that higher blood melatonin concentrations, especially in the early morning, may be responsible for morning stiffness and joint swelling experienced by patients with arthritis [6,43]. Maestroni et al. conjecture that autoreactive T cells in RA patients synthesize and release melatonin, thereby worsening the disease process [49].
Nevertheless, Korkmaz [50] has defended Forrest et al., pointing out that melatonin has shown strong anti-inflammatory activity in studies using known inflammatory agents, such as zymosan [51], lipopolysaccharide [52,53], and carrageenan [54]. Korkmaz speculated that the high blood melatonin concentrations in the RA cohort may have been a compensatory response to RA inflammation and were comparable to the high levels of melatonin in cerebrospinal fluid and its metabolites in meningitis populations and in pediatric patients with epilepsy [50]. Korkmaz maintained that melatonin is an appropriate adjunctive therapy for RA [50].
Melatonin appears to play an important role in microRNA (miRNA) expression in RA. miRNAs are small, non-coding RNAs that post-transcriptionally mediate protein expression by targeting protein-coding genes implicated in cancer cell proliferation, differentiation, apoptosis, and migration [55]. A recent study found that melatonin appears to inhibit miR-590-3p expression and induce apoptosis in human osteoblasts [55]. In another study, melatonin treatment effectively downregulated TNF-α and IL-1β production in human RA synovial fibroblasts (the MH7A cell line) by suppressing PI3K/AKT, ERK, and NF-κB signaling and upregulating miR-3150a-3p expression [20]. Those investigations confirmed that the MT1 receptor mediates the anti-inflammatory effects of melatonin and that melatonin not only inhibits inflammatory cytokine release in mice with CIA-induced arthritis, but also attenuates CIA-induced cartilage degradation and bone erosion [20]. This evidence suggests that melatonin targets miRNAs, which could be explored in clinical trials examining the efficacy of melatonin in the treatment of RA. The following figure (Figure 1) and table (Table 1) illustrate the processes through which melatonin exerts its therapeutic effects.
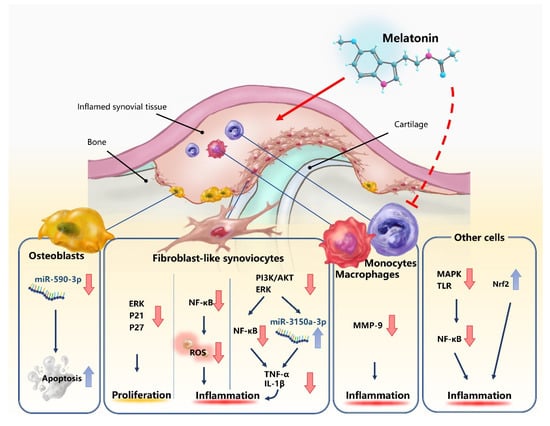
Figure 1.
Neutral or beneficial effects of melatonin in rheumatoid arthritis (RA). The dotted line represents the uncertainty over the effects of melatonin upon macrophages and monocytes.

Table 1.
Summary of the adverse, neutral, and beneficial effects of melatonin in rheumatoid arthritis.
4. Summary
Various research suggests that melatonin has disease-promoting effects in RA and that it could increase the severity of RA, in contradiction to the beneficial effects of melatonin in other autoimmune inflammatory diseases. However, in the past decade, some studies have demonstrated that melatonin can alleviate RA through the inhibition of RA synovial fibroblast proliferation, TNF-α and IL-1β expression, as well as MMP-9 activity. The anti-inflammatory character of melatonin in RA is associated with regulation of microRNAs (such as miR-3150a-3p). More investigations are therefore warranted to explore the possible double-edged effects of melatonin in RA. The use of melatonin in patients with RA needs thorough consideration by clinical physicians.
Author Contributions
Conceptualization, C.-H.T.; literature collection and preparation, I.J.M., C.-C.H.; writing—original draft preparation, I.J.M., S.-C.L.; writing—review and editing, I.J.M., C.-C.H.; supervision, C.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Science Council of Taiwan (MOST108-2320-B-039-065; MOST 108-2320-B-039-064-); China Medical University Hospital (DMR-109-208); and China Medical University, Taichung, Taiwan (CMU108-MF-114).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | Adjuvant-induced arthritis |
| ACPA | Anti-citrullinated protein antibodies |
| BCG | Bacillus Calmette-Guérin |
| Ca2+ | Calcium |
| cAMP | Cyclic 3′,5′-AMP |
| CIA | Collagen-induced arthritis |
| DMARD | Disease-modifying antirheumaticdrug |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| ESR | Erythrocyte sedimentation rate |
| FLS | Fibroblast-like synoviocytes |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
| IL | Interleukin |
| Met-Enk | Met-enkephalin |
| MMP | Matrix metalloproteinase |
| MT1/2 | High-affinity G protein-coupled melatonin receptors |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| Nrf2 | Nuclear erythroid 2-related factor 2 |
| OA | Osteoarthritis |
| PI3K | Phosphatidylinositol 3-kinase |
| RA | Rheumatoid arthritis |
| ROS | Reactive oxygen species |
| TGF | Transforming growth factor |
| Th1 | Human T helper type 1 |
| TNF | Tumor necrosis factor |
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Mian, A.; Ibrahim, F.; Scott, D.L. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol. 2019, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. (Hoboken, N.J.) 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Lin, G.J.; Huang, S.H.; Chen, S.J.; Wang, C.H.; Chang, D.M.; Sytwu, H.K. Modulation by melatonin of the pathogenesis of inflammatory autoimmune diseases. Int. J. Mol. Sci. 2013, 14, 11742–11766. [Google Scholar] [CrossRef]
- Zhao, C.N.; Wang, P.; Mao, Y.M.; Dan, Y.L.; Wu, Q.; Li, X.M.; Wang, D.G.; Davis, C.; Hu, W.; Pan, H.F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Miller, S.C.; Pandi-Perumal, S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Kleszczynski, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Reiter, R.J.; Fischer, T.W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Slominski, A.T.; Semak, I.; Fischer, T.W.; Kim, T.K.; Kleszczynski, K.; Hardeland, R.; Reiter, R.J. Metabolism of melatonin in the skin: Why is it important? Exp. Dermatol. 2017, 26, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Mehrzadi, S.; Reiter, R.J.; Seidi, K.; Majidinia, M.; Baghi, H.B.; Khatami, N.; Yousefi, B.; Sadeghpour, A. Melatonin in regulation of inflammatory pathways in rheumatoid arthritis and osteoarthritis: Involvement of circadian clock genes. Br. J. Pharmacol. 2018, 175, 3230–3238. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Mehrzadi, S.; Reiter, R.J.; Khatami, N.; Yousefi, B. Melatonin: A pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal. Res. 2017, 63, e12416. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal. Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Sulli, A.; Pizzorni, C.; Villaggio, B.; Cutolo, M. Melatonin in rheumatoid arthritis: Synovial macrophages show melatonin receptors. Ann. N. Y. Acad. Sci. 2002, 966, 271–275. [Google Scholar] [CrossRef]
- Ha, E.; Choe, B.K.; Jung, K.H.; Yoon, S.H.; Park, H.J.; Park, H.K.; Yim, S.V.; Chung, J.H.; Bae, H.S.; Nam, M.; et al. Positive relationship between melatonin receptor type 1B polymorphism and rheumatoid factor in rheumatoid arthritis patients in the Korean population. J. Pineal. Res. 2005, 39, 201–205. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal. Res. 2019, 66, e12560. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Jetten, A.M. RORalpha is not a receptor for melatonin (response to DOI 10.1002/bies.201600018). Bioessays 2016, 38, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Gene regulation by melatonin. Ann. N. Y. Acad. Sci. 2000, 917, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Naji, L.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Guerrero, J.M.; Calvo, J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005, 39, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hashimoto, T.; Sakai, Y.; Hashiramoto, A. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 282495. [Google Scholar] [CrossRef]
- Berenbaum, F.; Meng, Q.J. The brain-joint axis in osteoarthritis: Nerves, circadian clocks and beyond. Nat. Rev. Rheumatol. 2016, 12, 508–516. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Kamrava, S.K.; Joghataei, M.T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R.J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal. Res. 2016, 61, 411–425. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, W. Effects and mechanisms of melatonin on inflammatory and immune responses of adjuvant arthritis rat. Int. Immunopharmacol. 2002, 2, 1443–1449. [Google Scholar] [CrossRef]
- Bang, J.; Chang, H.W.; Jung, H.R.; Cho, C.H.; Hur, J.A.; Lee, S.I.; Choi, T.H.; Kim, S.H.; Ha, E. Melatonin attenuates clock gene cryptochrome1, which may aggravate mouse anti-type II collagen antibody-induced arthritis. Rheumatol. Int. 2012, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Caliani, A.J.; Jimenez-Jorge, S.; Molinero, P.; Guerrero, J.M.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Osuna, C. Dual effect of melatonin as proinflammatory and antioxidant in collagen-induced arthritis in rats. J. Pineal. Res. 2005, 38, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Villaggio, B.; Candido, F.; Valenti, S.; Giusti, M.; Felli, L.; Sulli, A.; Accardo, S. Melatonin influences interleukin-12 and nitric oxide production by primary cultures of rheumatoid synovial macrophages and THP-1 cells. Ann. N.Y. Acad. Sci. 1999, 876, 246–254. [Google Scholar] [CrossRef] [PubMed]
- El-Awady, H.M.; El-Wakkad, A.S.; Saleh, M.T.; Muhammad, S.I.; Ghaniema, E.M. Serum melatonin in juvenile rheumatoid arthritis: Correlation with disease activity. Pak. J. Biol. Sci. 2007, 10, 1471–1476. [Google Scholar] [CrossRef]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Stone, T.W.; Darlington, L.G. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br. J. Clin. Pharmacol. 2007, 64, 517–526. [Google Scholar] [CrossRef]
- Afkhamizadeh, M.; Sahebari, M.; Seyyed-Hoseini, S.R. Morning melatonin serum values do not correlate with disease activity in rheumatoid arthritis: A cross-sectional study. Rheumatol. Int. 2014, 34, 1145–1151. [Google Scholar] [CrossRef]
- Hand, L.E.; Dickson, S.H.; Freemont, A.J.; Ray, D.W.; Gibbs, J.E. The circadian regulator Bmal1 in joint mesenchymal cells regulates both joint development and inflammatory arthritis. Arthritis. Res. Ther. 2019, 21, 5. [Google Scholar] [CrossRef]
- Kouri, V.P.; Olkkonen, J.; Kaivosoja, E.; Ainola, M.; Juhila, J.; Hovatta, I.; Konttinen, Y.T.; Mandelin, J. Circadian timekeeping is disturbed in rheumatoid arthritis at molecular level. PLoS ONE 2013, 8, e54049. [Google Scholar] [CrossRef]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Constant darkness enhances autoimmunity to type II collagen and exaggerates development of collagen-induced arthritis in DBA/1 mice. J. Neuroimmunol. 1990, 27, 79–84. [Google Scholar] [CrossRef]
- Hansson, I.; Holmdahl, R.; Mattsson, R. The pineal hormone melatonin exaggerates development of collagen-induced arthritis in mice. J. Neuroimmunol. 1992, 39, 23–30. [Google Scholar] [CrossRef]
- Hansson, I.; Holmdahl, R.; Mattsson, R. Pinealectomy ameliorates collagen II-induced arthritis in mice. Clin Exp. Immunol. 1993, 92, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; deLyra, J.L.; Markus, R.P.; Mariano, M. Circadian rhythm in experimental granulomatous inflammation is modulated by melatonin. J. Pineal. Res. 1997, 23, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Maestroni, G.J.; Villaggio, B.; Hertens, E.; Craviotto, C.; Pizzorni, C.; Briata, M.; Seriolo, B.; Cutolo, M. Melatonin serum levels in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2002, 966, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Maestroni, G.J. The melatonin-cytokine connection in rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Secchi, M.E.; Soldano, S.; Seriolo, B.; Straub, R.H.; Otsa, K.; Maestroni, G.J. Circadian rhythms: Glucocorticoids and arthritis. Ann. N. Y. Acad. Sci. 2006, 1069, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rudra, D.S.; Pal, U.; Maiti, N.C.; Reiter, R.J.; Swarnakar, S. Melatonin inhibits matrix metalloproteinase-9 activity by binding to its active site. J. Pineal. Res. 2013, 54, 398–405. [Google Scholar] [CrossRef]
- Zhou, M.; Qin, S.; Chu, Y.; Wang, F.; Chen, L.; Lu, Y. Immunolocalization of MMP-2 and MMP-9 in human rheumatoid synovium. Int. J. Clin. Exp. Pathol. 2014, 7, 3048–3056. [Google Scholar]
- Nah, S.S.; Won, H.J.; Park, H.J.; Ha, E.; Chung, J.H.; Cho, H.Y.; Baik, H.H. Melatonin inhibits human fibroblast-like synoviocyte proliferation via extracellular signal-regulated protein kinase/P21(CIP1)/P27(KIP1) pathways. J. Pineal. Res. 2009, 47, 70–74. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Otsa, K.; Cutolo, M. Melatonin treatment does not improve rheumatoid arthritis. Br. J. Clin. Pharmacol. 2008, 65, 797–798. [Google Scholar] [CrossRef]
- Korkmaz, A. Melatonin as an adjuvant therapy in patients with rheumatoid arthritis. Br. J. Clin. Pharmacol. 2008, 66, 316–317. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Caputi, A.P. Protective effect of melatonin on zymosan-induced cellular damage. Biol. Signals Recept. 1999, 8, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabo, C. Protective effect of melatonin in carrageenan-induced models of local inflammation: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal. Res. 1997, 23, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhu, Y.; Tao, L.; Zhao, S.; Qiu, S. miR-590-3p mediates melatonin-induced cell apoptosis by targeting septin 7 in the human osteoblast cell line hFOB 1.19. Mol. Med. Rep. 2018, 17, 7202–7208. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).