Microbiota and Malodor—Etiology and Management
Abstract
:1. Introduction
2. Origins of Body Odor Associated with Bacterial Metabolites
- Diet. Diet contains direct or indirect odorants (i.e., substrates for the production of odorants by bacteria).
- Composition and metabolic activity of bacteria in the gut, skin or mucosa. The production of specific odorant is often limited to specific genera or strains of bacteria.
- Gut function. Intestinal transit time and gut-blood barrier permeability affect penetration of bacterial metabolites and their precursors from the gut lumen to the bloodstream. Recently the concept of a leaky-gut has attracted a lot of attention, as several studies show that numerous diseases may affect intestinal barrier function and increase the penetration of bacterial metabolites to the circulation.
- Liver function. Liver metabolizes most of gut-derived bacterial metabolites, reducing the “odorant potential” of the metabolites (e.g., very odorant trimethylamine is transformed to almost neutral trimethylamine oxide; hydrogen sulfide is transformed to numerous sulfur compounds, etc.).
- Kidney function. Kidney excretion is crucial for the elimination of both bacterial metabolites and substrates for their production from blood.
3. Breath and Saliva
3.1. H2S–Smell of Rotten Eggs
3.2. Methanethiol (CH3SH/MT/MeSH) = Methyl Mercaptan–Putrid, Musty Smell
3.3. Other Volatile Sulfur Compounds (VSCs)–Sweet, Musty Smell of Cooked Onion
3.4. Trimethylamine–Fishy Smell
3.5. Indole and Skatole–Smell of Feces
3.6. Putrescine and Cadaverine–Smell of Rotten Meat or Fish
3.7. Acetone–Fruity Smell
3.8. Pyridine–Fishy, Sweaty Smell
3.9. Ammonia–Urine-Like Smell
4. Urine
4.1. H2S–Smell of Rotten Eggs
4.2. Methanethiol–Putrid, Musty Smell
4.3. Trimethylamine–Fishy Smell
4.4. Short Chain Fatty Acids–Cheesy Smell
4.5. Ammonia–Urine-Like Smell
4.6. Methionine and its Metabolites–Smell of Rancid Butter or Boiled Cabbage
4.7. Phenylacetate–Musty, Mousy Smell
4.8. Branched-Chain Amino Acids (Leucine, Isoleucine and Valine) and their Ketoacids–Smell of Caramelized Sugar or Maple Syrup
4.9. 3-Hydroxyisovaleric Acid–Smell of Male Cat Urine
4.10. Aldehydes (Acetaldehyde, Butylaldehyde, Isovaleraldehyde)–Urine-Like Smell
5. Sweat and Skin
5.1. (E)-3-Methyl-2-Hexenoic acid (E3M2H), (R)/(S)-3-Hydroxy-3-Methylhexanoic Acid ((R)/(S)-HMHA) and 3-Methyl-3-Sulfanylhexan-1-ol ((R)/(S)-MSH)–Rancid, Cheesy Smell of Sweat
5.2. Trimethylamine–Fishy Smell
5.3. Ammonia–Urine-Like Smell
5.4. Methionine and its Metabolites–Smell of Rancid Butter or Boiled Cabbage
5.5. 2-Nonenal–Greasy, Grassy Smell
6. Reproductive Fluids
7. Management
- Reduction of dietary substrates for odorant production.
- Frequent bowel movements to reduce passage time (to shorten time of gut bacterial metabolism and time of absorption of bacterial metabolites), treatment of constipation.
- Probiotic and prebiotic treatment (an attempt to modify gut bacterial composition).
- Increased water intake in order to increase excretion of metabolites with urine.
7.1. Hydrogen Sulfide
7.2. Methanethiol
7.3. Trimethylamine
7.4. Indole and Skatole
7.5. Putrescine and Cadaverine
7.6. Pyridine
7.7. Ammonia
7.8. (E)-3-Methyl-2-Hexenoic Acid (E3M2H), (R)/(S)-3-Hydroxy-3-Methylhexanoic Acid ((R)/(S)-HMHA) and 3-Methyl-3-Sulfanylhexan-1-ol ((R)/(S)-MSH)
8. Conclusions
9. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kursun, S.; Acar, B.; Atakan, C.; Oztas, B.; Paksoy, C.S. Relationship between genuine and pseudohalitosis and social anxiety disorder. J. Oral Rehabil. 2014, 41, 822–828. [Google Scholar] [CrossRef]
- McKeown, L. Social relations and breath odour. Int. J. Dent. Hyg. 2003, 1, 213–217. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Barbosa, M.D.; Westphal, M.A. Halitosis: A review of associated factors and therapeutic approach. Braz. Oral Res. 2008, 22 (Suppl. 1), 44–54. [Google Scholar] [CrossRef] [Green Version]
- Greenman, J.; Lenton, P.; Seemann, R.; Nachnani, S. Organoleptic assessment of halitosis for dental professionals--general recommendations. J. Breath Res. 2014, 8, 017102. [Google Scholar] [CrossRef]
- Rayman, S.; Almas, K. Halitosis among racially diverse populations: An update. Int. J. Dent. Hyg. 2008, 6, 2–7. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Extra-oral halitosis: An overview. J. Breath Res. 2010, 4, 017003. [Google Scholar] [CrossRef]
- Silva, M.F.; Leite, F.R.M.; Ferreira, L.B.; Pola, N.M.; Scannapieco, F.A.; Demarco, F.F.; Nascimento, G.G. Estimated prevalence of halitosis: A systematic review and meta-regression analysis. Clin. Oral Investig. 2018, 22, 47–55. [Google Scholar] [CrossRef]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Minh, T.o.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef] [Green Version]
- Gahl, W.A.; Bernardini, I.; Finkelstein, J.D.; Tangerman, A.; Martin, J.J.; Blom, H.J.; Mullen, K.D.; Mudd, S.H. Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J. Clin. Investig. 1988, 81, 390–397. [Google Scholar] [CrossRef]
- Barić, I.; Staufner, C.; Augoustides-Savvopoulou, P.; Chien, Y.H.; Dobbelaere, D.; Grünert, S.C.; Opladen, T.; Petković Ramadža, D.; Rakić, B.; Wedell, A.; et al. Consensus recommendations for the diagnosis, treatment and follow-up of inherited methylation disorders. J. Inherit. Metab. Dis. 2017, 40, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Couce, M.L.; Bóveda, M.D.; García-Jimémez, C.; Balmaseda, E.; Vives, I.; Castiñeiras, D.E.; Fernández-Marmiesse, A.; Fraga, J.M.; Mudd, S.H.; Corrales, F.J. Clinical and metabolic findings in patients with methionine adenosyltransferase I/III deficiency detected by newborn screening. Mol Genet Metab 2013, 110, 218–221. [Google Scholar] [CrossRef]
- Katsinelos, P.; Tziomalos, K.; Chatzimavroudis, G.; Vasiliadis, T.; Katsinelos, T.; Pilpilidis, I.; Triantafillidis, I.; Paroutoglou, G.; Papaziogas, B. Eradication therapy in Helicobacter pylori-positive patients with halitosis: Long-term outcome. Med Princ Pract 2007, 16, 119–123. [Google Scholar] [CrossRef]
- Hoshi, K.; Yamano, Y.; Mitsunaga, A.; Shimizu, S.; Kagawa, J.; Ogiuchi, H. Gastrointestinal diseases and halitosis: Association of gastric Helicobacter pylori infection. Int. Dent. J. 2002, 52 (Suppl. 3), 207–211. [Google Scholar] [CrossRef]
- Longhini, A.B.; Ferguson, B.J. Clinical aspects of odontogenic maxillary sinusitis: A case series. Int. Forum Allergy Rhinol. 2011, 1, 409–415. [Google Scholar] [CrossRef]
- Magalhães, C.; Viana, M.; Alves, V.; Nakamura, R.; Duarte, D. Pediatric atrophic rhinosinusitis: What can we do? Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 763–765. [Google Scholar] [CrossRef]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef]
- Takeshita, T.; Suzuki, N.; Nakano, Y.; Yasui, M.; Yoneda, M.; Shimazaki, Y.; Hirofuji, T.; Yamashita, Y. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci. Rep. 2012, 2, 215. [Google Scholar] [CrossRef] [Green Version]
- Tangerman, A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3366–3377. [Google Scholar] [CrossRef]
- Tonzetich, J. Production and origin of oral malodor: A review of mechanisms and methods of analysis. J. Periodontol. 1977, 48, 13–20. [Google Scholar] [CrossRef]
- Solis, M.C.; Volpe, A.R. Determination of sulfur volatiles in putrefied saliva by a gas chromatography-microcoulometric titrating system. J. Periodontol. 1973, 44, 775–778. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Intra- and extra-oral halitosis: Finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J. Clin. Periodontol. 2007, 34, 748–755. [Google Scholar] [CrossRef]
- Nani, B.D.; Lima, P.O.; Marcondes, F.K.; Groppo, F.C.; Rolim, G.S.; Moraes, A.B.; Cogo-Müller, K.; Franz-Montan, M. Changes in salivary microbiota increase volatile sulfur compounds production in healthy male subjects with academic-related chronic stress. PLoS ONE 2017, 12, e0173686. [Google Scholar] [CrossRef]
- Gulsahi, A.; Evirgen, S.; Öztaş, B.; Genç, Y.; Çetinel, Y. Volatile sulphur compound levels and related factors in patients with chronic renal failure. J. Clin. Periodontol. 2014, 41, 814–819. [Google Scholar] [CrossRef]
- Yaegaki, K.; Sanada, K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J. Periodontal Res. 1992, 27, 233–238. [Google Scholar] [CrossRef]
- Awano, S.; Gohara, K.; Kurihara, E.; Ansai, T.; Takehara, T. The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis. Int. Dent. J. 2002, 52 (Suppl. 3), 212–216. [Google Scholar] [CrossRef]
- Codipilly, D.; Kleinberg, I. Generation of indole/skatole during malodor formation in the salivary sediment model system and initial examination of the oral bacteria involved. J. Breath Res. 2008, 2, 017017. [Google Scholar] [CrossRef]
- Codipilly, D.P.; Kaufman, H.W.; Kleinberg, I. Use of a novel group of oral malodor measurements to evaluate an anti-oral malodor mouthrinse (TriOralTM) in humans. J. Clin. Dent. 2004, 15, 98–104. [Google Scholar]
- Greenman, J.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S.; El-Maaytah, M. Study on the organoleptic intensity scale for measuring oral malodor. J. Dent. Res. 2004, 83, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Greenstein, R.B.; Goldberg, S.; Marku-Cohen, S.; Sterer, N.; Rosenberg, M. Reduction of oral malodor by oxidizing lozenges. J. Periodontol. 1997, 68, 1176–1181. [Google Scholar] [CrossRef]
- Goldberg, S.; Kozlovsky, A.; Gordon, D.; Gelernter, I.; Sintov, A.; Rosenberg, M. Cadaverine as a putative component of oral malodor. J. Dent. Res. 1994, 73, 1168–1172. [Google Scholar] [CrossRef]
- Kostelc, J.G.; Preti, G.; Zelson, P.R.; Stoller, N.H.; Tonzetich, J. Salivary volatiles as indicators of periodontitis. J. Periodontal. Res. 1980, 15, 185–192. [Google Scholar] [CrossRef]
- Anuradha, B.R.; Katta, S.; Kode, V.S.; Praveena, C.; Sathe, N.; Sandeep, N.; Penumarty, S. Oral and salivary changes in patients with chronic kidney disease: A clinical and biochemical study. J. Indian Soc. Periodonto.l 2015, 19, 297–301. [Google Scholar] [CrossRef]
- Kho, H.S.; Lee, S.W.; Chung, S.C.; Kim, Y.K. Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 316–319. [Google Scholar] [CrossRef]
- Endre, Z.H.; Pickering, J.W.; Storer, M.K.; Hu, W.P.; Moorhead, K.T.; Allardyce, R.; McGregor, D.O.; Scotter, J.M. Breath ammonia and trimethylamine allow real-time monitoring of haemodialysis efficacy. Physiol. Meas. 2011, 32, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Bevc, S.; Mohorko, E.; Kolar, M.; Brglez, P.; Holobar, A.; Kniepeiss, D.; Podbregar, M.; Piko, N.; Hojs, N.; Knehtl, M.; et al. Measurement of breath ammonia for detection of patients with chronic kidney disease. Clin. Nephrol. 2017, 88, 14–17. [Google Scholar] [CrossRef]
- Mitchell, S.; Ayesh, R.; Barrett, T.; Smith, R. Trimethylamine and foetor hepaticus. Scand. J. Gastroenterol. 1999, 34, 524–528. [Google Scholar]
- Mitchell, S.C. Trimethylaminuria (fish-odour syndrome) and oral malodour. Oral Dis. 2005, 11 (Suppl. 1), 10–13. [Google Scholar] [CrossRef]
- Preti, G.; Clark, L.; Cowart, B.J.; Feldman, R.S.; Lowry, L.D.; Weber, E.; Young, I.M. Non-oral etiologies of oral malodor and altered chemosensation. J. Periodontol. 1992, 63, 790–796. [Google Scholar] [CrossRef]
- Alkhouri, N.; Cikach, F.; Eng, K.; Moses, J.; Patel, N.; Yan, C.; Hanouneh, I.; Grove, D.; Lopez, R.; Dweik, R. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur. J. Gastroenterol. Hepatol. 2014, 26, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Polanowska, B.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Śliwka, I. The application of chromatographic breath analysis in the search of volatile biomarkers of chronic kidney disease and coexisting type 2 diabetes mellitus. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1060, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ruzsányi, V.; Péter Kalapos, M. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef]
- di Masi, A.; Ascenzi, P. H2S: A “double face” molecule in health and disease. Biofactors 2013, 39, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Washio, J.; Sato, T.; Koseki, T.; Takahashi, N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J. Med. Microbiol. 2005, 54, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awano, S.; Koshimune, S.; Kurihara, E.; Gohara, K.; Sakai, A.; Soh, I.; Hamasaki, T.; Ansai, T.; Takehara, T. The assessment of methyl mercaptan, an important clinical marker for the diagnosis of oral malodor. J. Dent. 2004, 32, 555–559. [Google Scholar] [CrossRef]
- Suarez, F.L.; Springfield, J.; Levitt, M.D. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut 1998, 43, 100–104. [Google Scholar] [CrossRef]
- Pelchat, M.L.; Bykowski, C.; Duke, F.F.; Reed, D.R. Excretion and perception of a characteristic odor in urine after asparagus ingestion: A psychophysical and genetic study. Chem Senses 2011, 36, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Mackay, R.J.; McEntyre, C.J.; Henderson, C.; Lever, M.; George, P.M. Trimethylaminuria: Causes and diagnosis of a socially distressing condition. Clin. Biochem. Rev. 2011, 32, 33–43. [Google Scholar]
- Ferreira, F.; Esteves, S.; Almeida, L.S.; Gaspar, A.; da Costa, C.D.; Janeiro, P.; Bandeira, A.; Martins, E.; Teles, E.L.; Garcia, P.; et al. Trimethylaminuria (fish odor syndrome): Genotype characterization among Portuguese patients. Gene 2013, 527, 366–370. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.B.; Beigelman, A.; Utterson, E.; Shinawi, M. Transient massive trimethylaminuria associated with food protein-induced enterocolitis syndrome. JIMD Rep 2014, 12, 11–15. [Google Scholar] [PubMed] [Green Version]
- Wise, P.M.; Eades, J.; Tjoa, S.; Fennessey, P.V.; Preti, G. Individuals reporting idiopathic malodor production: Demographics and incidence of trimethylaminuria. Am. J. Med. 2011, 124, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Tokatli, A.; Coşkun, T.; Ozalp, I. Isovaleric acidemia. Clinical presentation of 6 cases. Turk. J. Pediatr. 1998, 40, 111–119. [Google Scholar] [PubMed]
- Vatanavicharn, N.; Liammongkolkul, S.; Sakamoto, O.; Sathienkijkanchai, A.; Wasant, P. Phenotypic and mutation spectrums of Thai patients with isovaleric acidemia. Pediatr. Int. 2011, 53, 990–994. [Google Scholar] [CrossRef]
- Fleck, C.A. Fighting odor in wounds. Adv. Skin. Wound Care 2006, 19, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, A.; Kuboniwa, M.; Hashino, E.; Bamba, T.; Fukusaki, E.; Amano, A. Distinct signatures of dental plaque metabolic byproducts dictated by periodontal inflammatory status. Sci. Rep. 2017, 7, 42818. [Google Scholar] [CrossRef]
- Calenic, B.; Amann, A. Detection of volatile malodorous compounds in breath: Current analytical techniques and implications in human disease. Bioanalysis 2014, 6, 357–376. [Google Scholar] [CrossRef]
- Chen, K.C.; Forsyth, P.S.; Buchanan, T.M.; Holmes, K.K. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J. Clin. Investig. 1979, 63, 828–835. [Google Scholar] [CrossRef]
- Rooth, G.; Ostenson, S. Acetone in alveolar air, and the control of diabetes. Lancet 1966, 2, 1102–1105. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Umpierrez, G.E. Hyperosmolar hyperglycemic state: A historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014, 37, 3124–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath ethanol and acetone as indicators of serum glucose levels: An initial report. Diabetes Technol Ther 2005, 7, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y.; Endo, K.; Shimoda, M. Identification of distinctive volatile compounds in fish sauce. J. Agric. Food. Chem. 2002, 50, 5412–5416. [Google Scholar] [CrossRef] [PubMed]
- Milanowski, M.; Pomastowski, P.; Ligor, T.; Buszewski, B. Saliva - Volatile Biomarkers and Profiles. Crit. Rev. Anal. Chem. 2017, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Natsch, A.; Derrer, S.; Flachsmann, F.; Schmid, J. A broad diversity of volatile carboxylic acids, released by a bacterial aminoacylase from axilla secretions, as candidate molecules for the determination of human-body odor type. Chem. Biodivers. 2006, 3, 1–20. [Google Scholar] [CrossRef]
- Zeng, X.N.; Leyden, J.J.; Brand, J.G.; Spielman, A.I.; McGinley, K.J.; Preti, G. An investigation of human apocrine gland secretion for axillary odor precursors. J. Chem. Ecol. 1992, 18, 1039–1055. [Google Scholar] [CrossRef]
- Akutsu, T.; Sekiguchi, K.; Ohmori, T.; Sakurada, K. Individual comparisons of the levels of (E)-3-methyl-2-hexenoic acid, an axillary odor-related compound, in Japanese. Chem. Senses 2006, 31, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Begnaud, F.; Starkenmann, C.; Van de Waal, M.; Chaintreau, A. Chiral multidimensional gas chromatography (MDGC) and chiral GC-olfactometry with a double-cool-strand interface: Application to malodors. Chem. Biodivers. 2006, 3, 150–160. [Google Scholar] [CrossRef]
- Troccaz, M.; Starkenmann, C.; Niclass, Y.; van de Waal, M.; Clark, A.J. 3-Methyl-3-sulfanylhexan-1-ol as a major descriptor for the human axilla-sweat odour profile. Chem. Biodivers. 2004, 1, 1022–1035. [Google Scholar] [CrossRef]
- Troccaz, M.; Borchard, G.; Vuilleumier, C.; Raviot-Derrien, S.; Niclass, Y.; Beccucci, S.; Starkenmann, C. Gender-specific differences between the concentrations of nonvolatile (R)/(S)-3-methyl-3-sulfanylhexan-1-Ol and (R)/(S)-3-hydroxy-3-methyl-hexanoic acid odor precursors in axillary secretions. Chem. Senses 2009, 34, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Amano, A.; Yoshida, Y.; Oho, T.; Koga, T. Monitoring ammonia to assess halitosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 692–696. [Google Scholar] [CrossRef]
- Norberg, A.; Sandström, S.; Norberg, B.; Eriksson, S.; Sandman, P.O. The urine smell around patients with urinary incontinence. The production of ammonia in ordinary diapers and in diapers impregnated with copper acetate. Gerontology 1984, 30, 261–266. [Google Scholar] [CrossRef]
- Furukawa, S.; Sekine, Y.; Kimura, K.; Umezawa, K.; Asai, S.; Miyachi, H. Simultaneous and multi-point measurement of ammonia emanating from human skin surface for the estimation of whole body dermal emission rate. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1053, 60–64. [Google Scholar] [CrossRef]
- Turner, C.; Parekh, B.; Walton, C.; Spanel, P.; Smith, D.; Evans, M. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 526–532. [Google Scholar] [CrossRef]
- Brusilow, S.W.; Gordes, E.H. Ammonia secretion in sweat. Am. J. Physiol. 1968, 214, 513–517. [Google Scholar] [CrossRef]
- Mudd, S.H.; Levy, H.L.; Tangerman, A.; Boujet, C.; Buist, N.; Davidson-Mundt, A.; Hudgins, L.; Oyanagi, K.; Nagao, M.; Wilson, W.G. Isolated persistent hypermethioninemia. Am. J. Hum. Genet. 1995, 57, 882–892. [Google Scholar]
- Centerwall, S.A.; Centerwall, W.R. The discovery of phenylketonuria: The story of a young couple, two retarded children, and a scientist. Pediatrics 2000, 105, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Korein, J.; Sansaricq, C.; Kalmijn, M.; Honig, J.; Lange, B. Maple syrup urine disease: Clinical, EEG, and plasma amino acid correlations with a theoretical mechanism of acute neurotoxicity. Int. J. Neurosci. 1994, 79, 21–45. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Gass, J.M.; Vairo, F.P.E.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Grünert, S.C.; Stucki, M.; Morscher, R.J.; Suormala, T.; Bürer, C.; Burda, P.; Christensen, E.; Ficicioglu, C.; Herwig, J.; Kölker, S.; et al. 3-methylcrotonyl-CoA carboxylase deficiency: Clinical, biochemical, enzymatic and molecular studies in 88 individuals. Orphanet J. Rare Dis. 2012, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Pearson, M.A.; Aleck, K.A.; Heidenreich, R.A. Benign clinical presentation of 3-methylcrotonylglycinuria. J. Inherit. Metab. Dis. 1995, 18, 640–641. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.H.; Choi, S.O.; Sa, I.Y.; Oh, S.Y. Major odorants released as urinary volatiles by urinary incontinent patients. Sensors 2013, 13, 8523–8533. [Google Scholar] [CrossRef] [Green Version]
- Mitro, S.; Gordon, A.R.; Olsson, M.J.; Lundström, J.N. The smell of age: Perception and discrimination of body odors of different ages. PLoS ONE 2012, 7, e38110. [Google Scholar] [CrossRef] [Green Version]
- Haze, S.; Gozu, Y.; Nakamura, S.; Kohno, Y.; Sawano, K.; Ohta, H.; Yamazaki, K. 2-Nonenal newly found in human body odor tends to increase with aging. J. Investig. Dermatol. 2001, 116, 520–524. [Google Scholar] [CrossRef] [Green Version]
- van den Velde, S.; Quirynen, M.; van Hee, P.; van Steenberghe, D. Halitosis associated volatiles in breath of healthy subjects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 853, 54–61. [Google Scholar] [CrossRef]
- Burke, D.G.; Halpern, B.; Malegan, D.; McCairns, E.; Danks, D.; Schlesinger, P.; Wilken, B. Profiles of urinary volatiles from metabolic disorders characterized by unusual odors. Clin. Chem. 1983, 29, 1834–1838. [Google Scholar] [CrossRef]
- Chalmers, R.A.; Bain, M.D.; Michelakakis, H.; Zschocke, J.; Iles, R.A. Diagnosis and management of trimethylaminuria (FMO3 deficiency) in children. J. Inherit. Metab. Dis. 2006, 29, 162–172. [Google Scholar] [CrossRef]
- Christodoulou, J. Trimethylaminuria: An under-recognised and socially debilitating metabolic disorder. J. Paediatr. Child Health 2012, 48, E153–E155. [Google Scholar] [CrossRef]
- Jousserand, G.; Antoine, J.C.; Camdessanché, J.P. Musty odour, mental retardation, and spastic paraplegia revealing phenylketonuria in adulthood. J. Neurol. 2010, 257, 302–304. [Google Scholar] [CrossRef]
- Strauss, K.A.; Wardley, B.; Robinson, D.; Hendrickson, C.; Rider, N.L.; Puffenberger, E.G.; Shellmer, D.; Shelmer, D.; Moser, A.B.; Morton, D.H. Classical maple syrup urine disease and brain development: Principles of management and formula design. Mol. Genet. Metab. 2010, 99, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Laiho, S.; Vaittinen, O.; Halonen, L.; Ortiz, F.; Forsblom, C.; Groop, P.H.; Lehto, M.; Metsälä, M. Biochemical pathways of breath ammonia (NH3) generation in patients with end-stage renal disease undergoing hemodialysis. J. Breath Res. 2016, 10, 036011. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.; Gungor, O.; Bozkurt, D.; Bozgul, S.; Dusunur, F.; Caliskan, H.; Berdeli, A.; Akcicek, F.; Basci, A.; Duman, S. Trimethylaminuria (fish malodour syndrome) in chronic renal failure. Hippokratia 2012, 16, 83–85. [Google Scholar] [PubMed]
- Shimamoto, C.; Hirata, I.; Katsu, K. Breath and blood ammonia in liver cirrhosis. Hepatogastroenterology 2000, 47, 443–445. [Google Scholar]
- Tangerman, A.; Meuwese-Arends, M.T.; Jansen, J.B. Cause and composition of foetor hepaticus. Lancet 1994, 343, 483. [Google Scholar] [CrossRef]
- Lukacz, E.S.; Santiago-Lastra, Y.; Albo, M.E.; Brubaker, L. Urinary Incontinence in Women: A Review. JAMA 2017, 318, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, M.E.; Ubagai, T.; Mudd, S.H.; Thomas, J.; Pao, V.Y.; Nguyen, T.K.; Levy, H.L.; Greene, C.; Freehauf, C.; Chou, J.Y. Methionine adenosyltransferase I/III deficiency: Novel mutations and clinical variations. Am J Hum Genet 2000, 66, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.M.; Lee, B.H.; Kim, G.H.; Kim, Y.M.; Choi, J.H.; Yoo, H.W. Chronic intermittent form of isovaleric aciduria in a 2-year-old boy. Korean J Pediatr 2013, 56, 351–354. [Google Scholar] [CrossRef]
- Mao, G.Y.; Yang, S.L.; Zheng, J.H. Etiology and management of axillary bromidrosis: A brief review. Int. J. Dermatol. 2008, 47, 1063–1068. [Google Scholar] [CrossRef]
- Kimura, K.; Sekine, Y.; Furukawa, S.; Takahashi, M.; Oikawa, D. Measurement of 2-nonenal and diacetyl emanating from human skin surface employing passive flux sampler-GCMS system. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1028, 181–185. [Google Scholar] [CrossRef]
- Marcin, U.; Mariusz, S. The Role of Brain Gaseous Transmitters in the Regulation of the Circulatory System. Curr. Pharm. Biotechnol. 2011, 12, 1322–1333. [Google Scholar]
- Kim, H.-Y.; Lee, Y.-J.; Hong, K.-H.; Kwon, Y.-K.; Ko, H.-S.; Lee, Y.-K.; Lee, C.-W. Studies on the Contents of Naturally Occurring of Sulfite in Foods. Korean J. Food Sci. Technol. 2000, 32, 544–549. [Google Scholar]
- Robbins, K.S.; Shah, R.; MacMahon, S.; de Jager, L.S. Development of a liquid chromatography-tandem mass spectrometry method for the determination of sulfite in food. J. Agric. Food Chem. 2015, 63, 5126–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.W.; Chan, B.T.; Chan, A.C. Determination of free and reversibly-bound sulfite in selected foods by high-performance liquid chromatography with fluorometric detection. J. AOAC Int. 2008, 91, 98–102. [Google Scholar] [PubMed]
- Tonzetich, J.; Johnson, P.W. Chemical analysis of thiol, disulphide and total sulphur content of human saliva. Arch. Oral Biol. 1977, 22, 125–131. [Google Scholar] [CrossRef]
- Torsten, M.; Gómez-Moreno, G.; Aguilar-Salvatierra, A. Drug-related oral malodour (halitosis): A literature review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4930–4934. [Google Scholar] [PubMed]
- Besouw, M.; Blom, H.; Tangerman, A.; de Graaf-Hess, A.; Levtchenko, E. The origin of halitosis in cystinotic patients due to cysteamine treatment. Mol. Genet. Metab. 2007, 91, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- Pospischil, E.; Johanson, G.; Nielsen, G.; Papameletiou, D.; Klein, C. SCOEL/REC/179 Trimethylamine Recommendation from the Scientific Committee on Occupational Exposure Limits; Publications Office of the European Union: Luxembourg, 2017.
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins (Basel) 2016, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015, 6, e02481. [Google Scholar] [CrossRef] [Green Version]
- Bielinska, K.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Huc, T.; Jaworska, K.; Motooka, D.; Nakamura, S.; Ufnal, M. High salt intake increases plasma trimethylamine N-oxide (TMAO) concentration and produces gut dysbiosis in rats. Nutrition 2018, 54, 33–39. [Google Scholar] [CrossRef]
- Rath, S.; Heidrich, B.; Pieper, D.H.; Vital, M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart. J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Addou, S.; Lee, D.; Orengo, C.; Shephard, E.A.; Phillips, I.R. Trimethylaminuria and a human FMO3 mutation database. Hum. Mutat. 2003, 22, 209–213. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Smith, R.L. Trimethylaminuria: The fish malodor syndrome. Drug Metab. Dispo.s 2001, 29, 517–521. [Google Scholar]
- Yamazaki, H.; Fujieda, M.; Cashman, J.R.; Kamataki, T. Mild trimethylaminuria observed in a Japanese cohort with liver damage. Am. J. Med. 2005, 118, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Cashman, J.R.; Yamazaki, H. Transient trimethylaminuria related to menstruation. BMC Med. Genet. 2007, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Mayatepek, E.; Kohlmüller, D. Transient trimethylaminuria in childhood. Acta Paediatr. 1998, 87, 1205–1207. [Google Scholar] [CrossRef]
- Arseculeratne, G.; Wong, A.K.; Goudie, D.R.; Ferguson, J. Trimethylaminuria (fish-odor syndrome): A case report. Arch. Dermatol. 2007, 143, 81–84. [Google Scholar] [CrossRef]
- Piotr, K.; Marcin, U. Indoles - Gut Bacteria Metabolites of Tryptophan with Pharmacotherapeutic Potential. Curr. Drug Metab. 2018, 19, 883–890. [Google Scholar]
- Cooke, M.; Leeves, N.; White, C. Time profile of putrescine, cadaverine, indole and skatole in human saliva. Arch. Oral Biol. 2003, 48, 323–327. [Google Scholar] [CrossRef]
- Hayes, M.L.; Hyatt, A.T. The decarboxylation of amino acids by bacteria derived from human dental plaque. Arch. Oral Biol. 1974, 19, 361–369. [Google Scholar] [CrossRef]
- Spaněl, P.; Dryahina, K.; Rejšková, A.; Chippendale, T.W.; Smith, D. Breath acetone concentration; biological variability and the influence of diet. Physiol. Meas. 2011, 32, N23–N31. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Vaittinen, O.; Metsälä, M.; Lehto, M.; Forsblom, C.; Groop, P.H.; Halonen, L. Ammonia in breath and emitted from skin. J. Breath Res. 2013, 7, 017109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrover, R.; Cocozzella, D.; Ridruejo, E.; García, A.; Rome, J.; Podestá, J.J. Breath-ammonia testing of healthy subjects and patients with cirrhosis. Dig. Dis. Sci. 2012, 57, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Spacek, L.A.; Mudalel, M.; Tittel, F.; Risby, T.H.; Solga, S.F. Clinical utility of breath ammonia for evaluation of ammonia physiology in healthy and cirrhotic adults. J. Breath Res. 2015, 9, 047109. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.C.; Miekisch, W. Exhaled volatile substances mirror clinical conditions in pediatric chronic kidney disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef]
- Chen, W.; Metsälä, M.; Vaittinen, O.; Halonen, L. The origin of mouth-exhaled ammonia. J. Breath Res. 2014, 8, 036003. [Google Scholar] [CrossRef]
- Liu, J.; Lkhagva, E.; Chung, H.J.; Kim, H.J.; Hong, S.T. The Pharmabiotic Approach to Treat Hyperammonemia. Nutrients 2018, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Awano, N.; Wada, M.; Mori, H.; Nakamori, S.; Takagi, H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005, 71, 4149–4152. [Google Scholar] [CrossRef] [Green Version]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar]
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut Bacteria and Hydrogen Sulfide: The New Old Players in Circulatory System Homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef] [PubMed]
- Španěl, P.; Smith, D. What is the real utility of breath ammonia concentration measurements in medicine and physiology? J. Breath Res. 2018, 12, 027102. [Google Scholar] [CrossRef] [PubMed]
- Nouvenne, A.; Ticinesi, A.; Morelli, I.; Guida, L.; Borghi, L.; Meschi, T. Fad diets and their effect on urinary stone formation. Transl. Androl. Urol. 2014, 3, 303–312. [Google Scholar] [PubMed]
- Wyka, J.; Malczyk, E.; Misiarz, M.; Zołoteńka-Synowiec, M.; Całyniuk, B.; Baczyńska, S. Assessment of food intakes for women adopting the high protein Dukan diet. Rocz Panstw Zakl Hig. 2015, 66, 137–142. [Google Scholar] [PubMed]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- He, X.; Slupsky, C.M. Metabolic Fingerprint of Dimethyl Sulfone (DMSO2) in Microbial–Mammalian Co-metabolism. J. Proteome Res. 2014, 13, 5281–5292. [Google Scholar] [CrossRef] [Green Version]
- Forsgren-Brusk, U.; Yhlen, B.; Blomqvist, M.; Larsson, P. Method for Bacterial Growth and Ammonia Production and Effect of Inhibitory Substances in Disposable Absorbent Hygiene Products. J Wound Ostomy Continence Nurs. 2017, 44, 78–83. [Google Scholar] [CrossRef]
- Semkova, K.; Gergovska, M.; Kazandjieva, J.; Tsankov, N. Hyperhidrosis, bromhidrosis, and chromhidrosis: Fold (intertriginous) dermatoses. Clin. Dermatol. 2015, 33, 483–491. [Google Scholar] [CrossRef]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef]
- Leyden, J.J.; McGinley, K.J.; Hölzle, E.; Labows, J.N.; Kligman, A.M. The microbiology of the human axilla and its relationship to axillary odor. J. Investig. Dermatol. 1981, 77, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Honig, P.J.; Frieden, I.J.; Kim, H.J.; Yan, A.C. Streptococcal intertrigo: An underrecognized condition in children. Pediatrics 2003, 112, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Al-Sarraf, A.; Sinclair, G.; Frohlich, J. Fish odour syndrome. CMAJ 2011, 183, 929–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallegange, R.C.; Verhulst, N.O.; Takken, W. Sweaty skin: An invitation to bite? Trends Parasitol 2011, 27, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Czarnowski, D.; Górski, J.; Jóźwiuk, J.; Boroń-Kaczmarska, A. Plasma ammonia is the principal source of ammonia in sweat. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 135–137. [Google Scholar] [CrossRef]
- Subramanian, C.; Nyirjesy, P.; Sobel, J.D. Genital malodor in women: a modern reappraisal. J. Low Genit. Tract. Dis. 2012, 16, 49–55. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakano, Y.; Watanabe, T.; Yoneda, M.; Hirofuji, T.; Hanioka, T. Two mechanisms of oral malodor inhibition by zinc ions. J. Appl. Oral Sci. 2018, 26, e20170161. [Google Scholar] [CrossRef] [Green Version]
- Shinada, K.; Ueno, M.; Konishi, C.; Takehara, S.; Yokoyama, S.; Zaitsu, T.; Ohnuki, M.; Wright, F.A.; Kawaguchi, Y. Effects of a mouthwash with chlorine dioxide on oral malodor and salivary bacteria: A randomized placebo-controlled 7-day trial. Trials 2010, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.M.; Wahlqvist, M.L.; Chang, H.Y.; Yeh, N.H.; Lee, M.S. Choline and betaine food sources and intakes in Taiwanese. Asia Pac. J. Clin. Nutr. 2012, 21, 547–557. [Google Scholar]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messenger, J.; Clark, S.; Massick, S.; Bechtel, M. A review of trimethylaminuria: (fish odor syndrome). J. Clin. Aesthet. Dermatol. 2013, 6, 45–48. [Google Scholar] [PubMed]
- Strasser, B.; Gostner, J.M.; Fuchs, D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Greenman, J.; El-Maaytah, M.; Duffield, J.; Spencer, P.; Rosenberg, M.; Corry, D.; Saad, S.; Lenton, P.; Majerus, G.; Nachnani, S. Assessing the relationship between concentrations of malodor compounds and odor scores from judges. J. Am. Dent. Assoc. 2005, 136, 749–757. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Kircheis, G.; Hilger, N.; McPhail, M.J.W. Efficacy of l-Ornithine l-Aspartate for the Treatment of Hepatic Encephalopathy and Hyperammonemia in Cirrhosis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Exp. Hepatol. 2018, 8, 301–313. [Google Scholar] [CrossRef]
- Guillet, G.; Zampetti, A.; Aballain-Colloc, M.L. Correlation between bacterial population and axillary and plantar bromidrosis: Study of 30 patients. Eur. J. Dermatol. 2000, 10, 41–42. [Google Scholar]
- Kim, I.H.; Seo, S.L.; Oh, C.H. Minimally invasive surgery for axillary osmidrosis: Combined operation with CO2 laser and subcutaneous tissue remover. Dermatol. Surg. 1999, 25, 875–879. [Google Scholar] [CrossRef]
- Fan, Y.M.; Wu, Z.H.; Li, S.F.; Chen, Q.X. Axillary osmidrosis treated by partial removal of the skin and subcutaneous tissue en bloc and apocrine gland subcision. Int. J. Dermatol. 2001, 40, 714–716. [Google Scholar] [CrossRef]
- Rezende, R.M.; Luz, F.B. Surgical treatment of axillary hyperhidrosis by suction-curettage of sweat glands. An. Bras. Dermatol 2014, 89, 940–954. [Google Scholar] [CrossRef] [Green Version]
- Park, D.H.; Kim, T.M.; Han, D.G.; Ahn, K.Y. A comparative study of the surgical treatment of axillary osmidrosis by instrument, manual, and combined subcutaneous shaving procedures. Ann. Plast. Surg. 1998, 41, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, M.; Teichmann, B.; Pause, B.M.; Plewig, G. Amelioration of body odor after intracutaneous axillary injection of botulinum toxin A. Arch. Dermatol. 2003, 139, 57–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
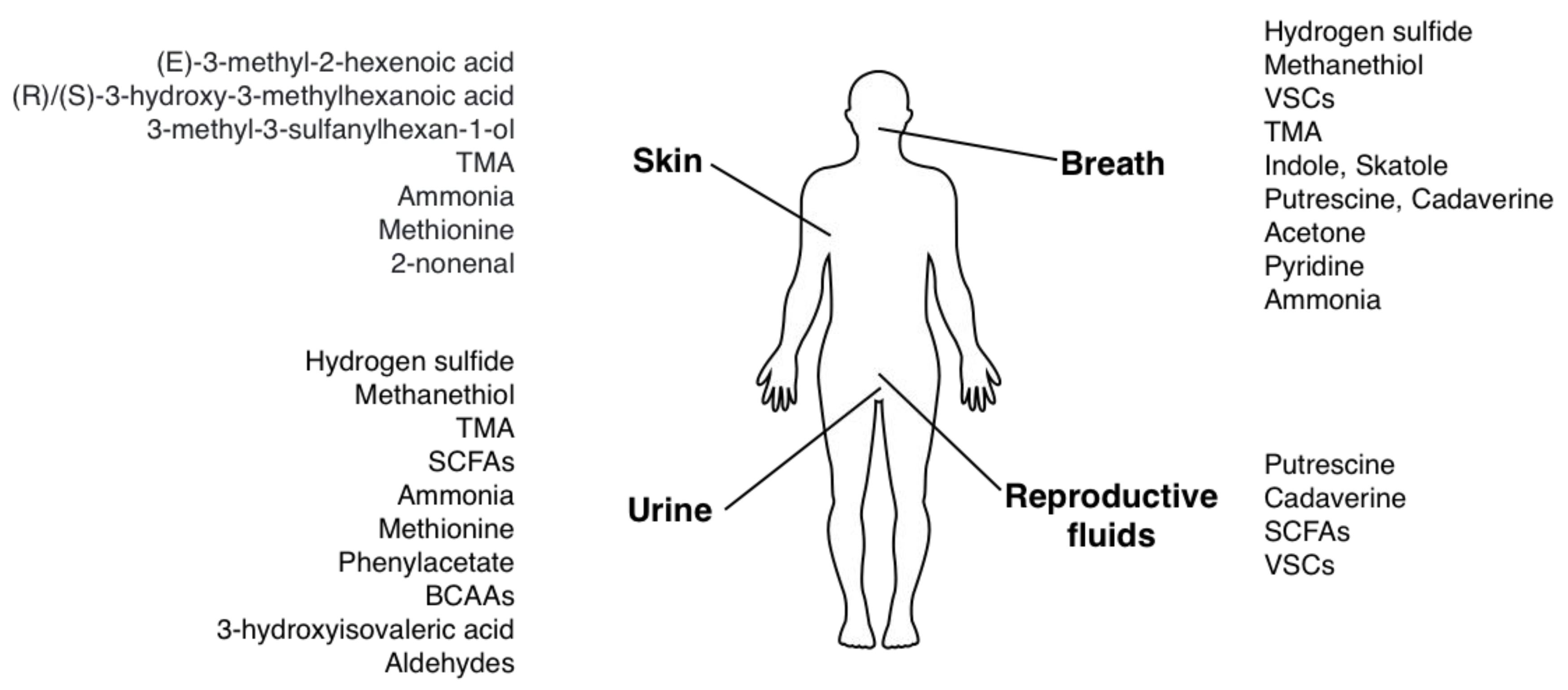
| Substance | Smell | Body Site | References |
|---|---|---|---|
| Hydrogen sulfide | Rotten eggs | Breath, Saliva, Flatus, Urine | [18,19,44,45] |
| Other volatile sulfur compounds (VSCs): ethanethiol, S-ethyl thioacetate, diethyl disulfide, dimethylsufide | Cooked onion or vegetables, ocean; musty, unpleasantly sweet smell | Breath, Saliva | [20,23,26] |
| Methanethiol | Putrid, barnyard; musty smell | Breath, Saliva, Flatus, Urine | [20,26,46,47,48] |
| Trimethylamine | Rotten fish | Breath, Urine, Sweat | [38,49,50,51,52,53] |
| Indole, Skatole | Fecal matter | Breath, Saliva | [28,30] |
| Short chain fatty acids (butyric, propionic, acetic, isovaleric, isocaproic acid) | Human vomit, sweat, goat-like, sweaty feet odor; cheesy smell | Sweat, Urine, Vaginal discharge | [54,55,56] |
| Putrescine, Cadaverine | Rotten meat, spoiled fish | Breath, Saliva, Vaginal fluid (in bacterial vaginosis) | [32,57,58,59] |
| Acetone | Acetone, fruity smell of rotten apple | Breath | [60,61,62] |
| Pyridine | Fishy odor | Breath, Saliva | [63,64] |
| (E)-3-methyl-2-hexenoic acid (E3M2H) | Peculiar pungent odor | Sweat | [65,66,67] |
| 3-methyl-3-sulfanylhexan-1-ol ((R)/(S)-MSH) | Tropical fruit or grapefruit (enantiomer R), onion, clary sage, chicken-sulfury (enantiomer S) | Sweat | [65] |
| (R)/(S)-3-hydroxy-3-methylhexanoic acid ((R)/(S)-HMHA | Cheesy, rancid odor | Sweat | [68,69,70] |
| Ammonia | Urine-like, ammoniacal, fetid | Breath, Saliva, Urine, Stool, Sweat | [34,71,72,73,74,75] |
| Methionine (transformed into dimethylsulfide) | Boiled cabbage, rancid butter, oast house, rotten mushrooms | Breath, Sweat, Urine | [11,12,76] |
| Phenylacetate | Musty, mousy, sweaty | Urine, Infant skin | [77] |
| Branched-chain amino acids (leucine, isoleucine and valine) | Maple syrup, caramelized/burnt sugar, fenugreek, curry | Urine, Ear wax | [78,79] |
| 3-hydroxyisovaleric acid | Male cat urine | Urine | [80,81] |
| Aldehydes (2-nonenal) | Foul, urine-like | Urine, Skin | [82,83,84] |
| Condition | Smell | Body Site | References |
|---|---|---|---|
| Intra-oral halitosis | Rotten eggs, cooked onion or vegetables, putrid, musty, urine-like smell, fecal matter, rotten meat or fish | Breath, Saliva | [8,21,27,32,85] |
| Trimethylaminuria | Rotten fish | Breath, Urine, Sweat | [39,49,86,87,88] |
| Phenylketonuria | Musty, mousy, sweaty smell | Urine, Infant skin | [77,86,89] |
| Maple syrup urine disease | Maple syrup, caramelized/burnt sugar, fenugreek, curry | Urine, Ear wax | [78,79,86,90] |
| Diabetic ketoacidosis | Acetone, fruity smell of rotten apple | Breath | [9,10,62] |
| End-stage renal disease | Urine-like smell, ammoniacal, rotten fish | Breath | [35,91,92] |
| Liver failure | Urine-like smell, ammoniacal, rotten fish | Breath | [38,93,94] |
| Urinary incontinence | Rotten eggs, putrid, musty, urine-like smell | Urine | [72,82,95] |
| Methionine adenosyltransferase (MAT) I/III deficiency | Putrid, musty, smell of rancid butter or boiled cabbage | Breath, Urine, Sweat, Skin | [11,12,13,96] |
| Isovaleric acidemia | Human vomit, sweat, goat-like, sweaty feet odor; cheesy smell | Sweat, Urine | [54,55,86,97] |
| 3-methylcrotonyl-CoA carboxylase (3-MCC) deficiency | Smell of male cat urine | Urine | [80,81] |
| Bromhidrosis | Rancid, cheesy smell of sweat | Sweat, Skin | [65,66,67,68,69,70,98] |
| Aging | Greasy, grassy smell | Sweat, Skin | [84,99] |
| Bacterial vaginosis | Fishy smell | Vaginal discharge | [59] |
| Gynecological lesions | Rotting smell | Vaginal discharge | [56] |
| Bacterial Odorant | Conditions | Management | References |
|---|---|---|---|
| Hydrogen sulfide | Intra-oral halitosis (periodontic disease, excessive tongue coating), UI and UTIs | Basic dental hygiene, treatment of periodontal disease, mouthwashes with chlorine dioxide or Zn2+ ions; avoidance of sulfites and sulfides in diet | [5,101,102,103,147,148] |
| Methanethiol | Intra-oral halitosis (periodontic disease, excessive tongue coating), treatment with cysteamine, hepatic methionine adenosyltransferase deficiency, UI | Basic dental hygiene, treatment of periodontal disease, avoidance of asparagus in diet | [19,23,48] |
| Trimethylamine | Trimethylaminuria, bacterial vaginosis, liver failure, ESRD | Avoidance of products containing choline, betaine, l-carnitine or TMAO in diet; administration of probiotics and activated charcoal; use of low pH soaps and deodorants | [88,149,150,151,152,153] |
| Indole, Skatole | Intra-oral halitosis | Decreasing tryptophan intake to allowed minimum; treatment of periodontal disease | [28,154,155] |
| Putrescine, Cadaverine | Intra-oral halitosis, bacterial vaginosis, gynecological lesions | Basic dental hygiene, antibiotics for bacterial vaginosis, treatment of underlying gynecological problems | [121] |
| Pyridine | Intra-oral halitosis (periodontic disease) | Basic dental hygiene, treatment of periodontal disease | [33] |
| Ammonia | Intra-oral halitosis (periodontic disease, excessive tongue coating), extra-oral halitosis, liver failure, ESRD, UI, bromhidrosis | Avoidance of high-protein diets; l-Ornithine l-Aspartate, rifaximin, lactulose; basic dental hygiene, treatment of periodontal disease | [71,129,133,157] |
| E3M2H, (R)/(S)-HMHA, R)/(S)-MSH | Bromhidrosis | Personal hygiene with the use of antiperspirants and topical antibacterial agents; avoidance of garlic, onion, alcohol and curry in diet; subdermal coagulation, surgical methods, BTX-A | [139,158,159,160,161,162,163] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogilnicka, I.; Bogucki, P.; Ufnal, M. Microbiota and Malodor—Etiology and Management. Int. J. Mol. Sci. 2020, 21, 2886. https://doi.org/10.3390/ijms21082886
Mogilnicka I, Bogucki P, Ufnal M. Microbiota and Malodor—Etiology and Management. International Journal of Molecular Sciences. 2020; 21(8):2886. https://doi.org/10.3390/ijms21082886
Chicago/Turabian StyleMogilnicka, Izabella, Pawel Bogucki, and Marcin Ufnal. 2020. "Microbiota and Malodor—Etiology and Management" International Journal of Molecular Sciences 21, no. 8: 2886. https://doi.org/10.3390/ijms21082886
APA StyleMogilnicka, I., Bogucki, P., & Ufnal, M. (2020). Microbiota and Malodor—Etiology and Management. International Journal of Molecular Sciences, 21(8), 2886. https://doi.org/10.3390/ijms21082886