Leveraging Nuclear Receptors as Targets for Pathological Ocular Vascular Diseases
Abstract
:1. Introduction
2. Role of Nuclear Receptors in Angiogenesis
3. Nuclear Receptor Signaling in the Pathogenesis of Age-Related Macular Degeneration
3.1. Overview of Age-Related Macular Degeneration
3.2. Molecular Mechanisms and Etiology of AMD
3.3. Nuclear Receptors and AMD Pathobiology
3.3.1. Peroxisome Proliferator-Activated Receptors (PPARs)
3.3.2. Liver X Receptors (LXRs)
3.3.3. Estrogen Receptors (ERs)
3.3.4. Aryl Hydrocarbon Receptor (AhR)
3.3.5. Glucocorticoid Receptors (GR)
3.4. Case Studies and Clinical Trials Examining the Relationship between Nuclear Receptors and AMD
4. Nuclear Receptor Signaling in the Pathogenesis of Diabetic Retinopathy
4.1. Overview of Diabetic Retinopathy
4.2. Nuclear Receptors and Diabetic Retinopathy
4.2.1. Peroxisome Proliferator-Activated Receptors (PPARs)
4.2.2. Liver-X-Receptors (LXR)
4.2.3. Vitamin D Receptor
4.2.4. Retinoic Acid Receptor (RORs) and Rev-Erbs
4.2.5. Mineralocorticoid Receptors (MR) and Glucocorticoid Receptors (GR)
4.3. Human Studies Examining the Potential Role of Nuclear Receptors in Diabetic Retinopathy.
5. The Future of Nuclear Receptor Targeted Therapies for Ocular Neovascular Diseases
Author Contributions
Funding
Conflicts of Interest
References
- Potente, M.; Makinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel-Hett, S.; D’Amore, P.A. Signal transduction in vasculogenesis and developmental angiogenesis. Int. J. Dev. Biol. 2011, 55, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Hamik, A.; Wang, B.; Jain, M.K. Transcriptional regulators of angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1936–1947. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.S.; Lee, J.X.T.; Wahli, W.; Tan, N.S. Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment. Mol. Cancer 2019, 18, 51. [Google Scholar] [CrossRef]
- Sherman, M.H.; Downes, M.; Evans, R.M. Nuclear receptors as modulators of the tumor microenvironment. Cancer Prev. Res. (Phila.) 2012, 5, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.B.; Graham, J.D.; Clarke, C.L. Emerging functional roles of nuclear receptors in breast cancer. J. Mol. Endocrinol. 2017, 58, R169–R190. [Google Scholar] [CrossRef]
- Safe, S.; Jin, U.H.; Hedrick, E.; Reeder, A.; Lee, S.O. Minireview: Role of orphan nuclear receptors in cancer and potential as drug targets. Mol. Endocrinol. 2014, 28, 157–172. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heesterbeek, T.J.; Lores-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Gwairi, O.; Thach, L.; Zheng, W.; Osman, N.; Little, P.J. Cellular and Molecular Pathology of Age-Related Macular Degeneration: Potential Role for Proteoglycans. J. Ophthalmol. 2016, 2016, 2913612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Zimbron, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Canizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell. Longev. 2018, 2018, 8374647. [Google Scholar] [CrossRef]
- Malek, G.; Lad, E.M. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 2014, 71, 4617–4636. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. (Berl.) 2013, 91, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Campa, C.; Costagliola, C.; Incorvaia, C.; Sheridan, C.; Semeraro, F.; De Nadai, K.; Sebastiani, A.; Parmeggiani, F. Inflammatory mediators and angiogenic factors in choroidal neovascularization: Pathogenetic interactions and therapeutic implications. Mediat. Inflamm. 2010, 2010, 546826. [Google Scholar] [CrossRef] [Green Version]
- Holekamp, N.M. Review of neovascular age-related macular degeneration treatment options. Am. J. Manag. Care 2019, 25, S172–S181. [Google Scholar]
- Khanna, S.; Komati, R.; Eichenbaum, D.A.; Hariprasad, I.; Ciulla, T.A.; Hariprasad, S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019, 4, e000398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovach, J.L.; Schwartz, S.G.; Flynn, H.W., Jr.; Scott, I.U. Anti-VEGF Treatment Strategies for Wet AMD. J. Ophthalmol. 2012, 2012, 786870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016, 10, 1857–1867. [Google Scholar] [PubMed] [Green Version]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group, S.-U.S. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Mones, J.M.; Tufail, A.; Jaffe, G.J. Anti-Vascular Endothelial Growth Factor Use and Atrophy in Neovascular Age-Related Macular Degeneration: Systematic Literature Review and Expert Opinion. Ophthalmology 2020, 127, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, M.A.; Kazmin, D.; Hu, P.; McDonnell, D.P.; Malek, G. Research resource: Nuclear receptor atlas of human retinal pigment epithelial cells: Potential relevance to age-related macular degeneration. Mol. Endocrinol. 2011, 25, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Malek, G. Rethinking Nuclear Receptors as Potential Therapeutic Targets for Retinal Diseases. J. Biomol. Screen 2016, 21, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Forrest, D.; Swaroop, A. Minireview: The role of nuclear receptors in photoreceptor differentiation and disease. Mol. Endocrinol. 2012, 26, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Zou, C.; Qin, B. The association between nuclear receptors and ocular diseases. Oncotarget 2017, 8, 27603–27615. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.S.A.; Wells, R.A. Cross-Talk between PPARs and the Partners of RXR: A Molecular Perspective. PPAR Res. 2009, 2009, 925309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Herzlich, A.A.; Ding, X.; Shen, D.; Ross, R.J.; Tuo, J.; Chan, C.C. Peroxisome Proliferator-Activated Receptor Expression in Murine Models and Humans with Age-related Macular Degeneration. Open Biol. J. 2009, 2, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Chong, E.W.; Kreis, A.J.; Wong, T.Y.; Simpson, J.A.; Guymer, R.H. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: A systematic review and meta-analysis. Arch. Ophthalmol. 2008, 126, 826–833. [Google Scholar] [CrossRef]
- Fu, Z.; Liegl, R.; Wang, Z.; Gong, Y.; Liu, C.H.; Sun, Y.; Cakir, B.; Burnim, S.B.; Meng, S.S.; Lofqvist, C.; et al. Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3862–3870. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Lofqvist, C.A.; Shao, Z.; Sun, Y.; Joyal, J.S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; SanGiovanni, J.P.; et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Shao, Z.; Fu, Z.; Edin, M.L.; Sun, Y.; Liegl, R.G.; Wang, Z.; Liu, C.H.; Burnim, S.B.; Meng, S.S.; et al. Fenofibrate Inhibits Cytochrome P450 Epoxygenase 2C Activity to Suppress Pathological Ocular Angiogenesis. EBioMedicine 2016, 13, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SanGiovanni, J.P.; Chen, J.; Sapieha, P.; Aderman, C.M.; Stahl, A.; Clemons, T.E.; Chew, E.Y.; Smith, L.E. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the omega-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS ONE 2013, 8, e53155. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Matlock, G.; Chen, Q.; Zhou, K.; Du, Y.; Wang, X.; Ma, J.X. Therapeutic Effects of PPARalpha Agonist on Ocular Neovascularization in Models Recapitulating Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5065–5075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, T.; He, S.; Hangai, M.; Ishibashi, T.; Xi, X.P.; Kim, S.; Hsueh, W.A.; Ryan, S.J.; Law, R.E.; Hinton, D.R. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2309–2317. [Google Scholar]
- Chang, J.Y.; Bora, P.S.; Bora, N.S. Prevention of Oxidative Stress-Induced Retinal Pigment Epithelial Cell Death by the PPARgamma Agonists, 15-Deoxy-Delta12,14-Prostaglandin J(2). PPAR Res. 2008, 2008, 720163. [Google Scholar] [CrossRef] [Green Version]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Fessler, M.B. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol. Ther. 2018, 181, 1–12. [Google Scholar] [CrossRef]
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.-E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell 2007, 25, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Ismail, E.N.; Yao, P.L.; Tayyari, F.; Radu, R.A.; Nusinowitz, S.; Boulton, M.E.; Apte, R.S.; Ruberti, J.W.; Handa, J.T.; et al. LXRs regulate features of age-related macular degeneration and may be a potential therapeutic target. JCI Insight 2020, 5, e131928. [Google Scholar] [CrossRef] [Green Version]
- Song, X.Y.; Wu, W.F.; Gabbi, C.; Dai, Y.B.; So, M.; Chaurasiya, S.P.; Wang, L.; Warner, M.; Gustafsson, J.A. Retinal and optic nerve degeneration in liver X receptor beta knockout mice. Proc. Natl. Acad. Sci. USA 2019, 116, 16507–16512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Yang, H.; Chen, Z.; Zheng, C.; Lei, C.; Lei, B. Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Mukwaya, A.; Lennikov, A.; Xeroudaki, M.; Mirabelli, P.; Lachota, M.; Jensen, L.; Peebo, B.; Lagali, N. Time-dependent LXR/RXR pathway modulation characterizes capillary remodeling in inflammatory corneal neovascularization. Angiogenesis 2018, 21, 395–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sene, A.; Khan, A.A.; Cox, D.; Nakamura, R.E.; Santeford, A.; Kim, B.M.; Sidhu, R.; Onken, M.D.; Harbour, J.W.; Hagbi-Levi, S.; et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013, 17, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, C.V.; Walker, J.A.; Davidson, C. Oestrogen, ocular function and low-level vision: A review. J. Endocrinol. 2014, 223, R9–R18. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, M.; Miyamoto, N.; Mandai, M.; Kamizuru, H.; Ooto, S.; Yasukawa, T.; Takahashi, M.; Honda, Y. The role of estrogen and estrogen receptorbeta in choroidal neovascularization. Mol. Vis. 2004, 10, 923–932. [Google Scholar]
- Javitt, J.C.; Zhou, Z.; Maguire, M.G.; Fine, S.L.; Willke, R.J. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology 2003, 110, 1534–1539. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Owen, C.G. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology 2012, 119, 571–580. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Marin-Castano, M.E.; Pereira-Simon, S.; Hernandez, E.P.; Elliot, S.; Cousins, S.W. Gender and estrogen supplementation increases severity of experimental choroidal neovascularization. Exp. Eye Res. 2005, 80, 413–423. [Google Scholar] [CrossRef]
- Boekhoorn, S.S.; Vingerling, J.R.; Uitterlinden, A.G.; Van Meurs, J.B.; van Duijn, C.M.; Pols, H.A.; Hofman, A.; de Jong, P.T. Estrogen receptor alpha gene polymorphisms associated with incident aging macula disorder. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1012–1017. [Google Scholar] [CrossRef]
- Seitzman, R.L.; Mahajan, V.B.; Mangione, C.; Cauley, J.A.; Ensrud, K.E.; Stone, K.L.; Cummings, S.R.; Hochberg, M.C.; Hillier, T.A.; Sinsheimer, J.S.; et al. Study of Osteoporotic Fractures Research, G., Estrogen receptor alpha and matrix metalloproteinase 2 polymorphisms and age-related maculopathy in older women. Am. J. Epidemiol. 2008, 167, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Munoz, B.; Bressler, S.B.; West, S.K. Hormone replacement therapy, reproductive factors, and age-related macular degeneration: The Salisbury Eye Evaluation Project. Ophthalmic Epidemiol. 2005, 12, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.R.; Gallins, P.; Polk, M.; Ayala-Haedo, J.; Schwartz, S.G.; Kovach, J.L.; Spencer, K.; Wang, G.; Agarwal, A.; Postel, E.A.; et al. Inverse association of female hormone replacement therapy with age-related macular degeneration and interactions with ARMS2 polymorphisms. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1873–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snow, K.K.; Cote, J.; Yang, W.; Davis, N.J.; Seddon, J.M. Association between reproductive and hormonal factors and age-related maculopathy in postmenopausal women. Am. J. Ophthalmol. 2002, 134, 842–848. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Domenech, N.; Diago, T.; Montero, J.; Garcia-Canet, S.; Cervera-Taulet, E.; Gallego-Pinazo, R.; Arevalo, J.F. Hormonal Supplementation Triggering Choroidal Neovascularization in Healthy Young Females. Retin. Cases Br. Rep. 2019, 13, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cao, Z.; Wang, X. Role of aryl hydrocarbon receptor in cancer. Biochim. Biophys. Acta 2013, 1836, 197–210. [Google Scholar] [CrossRef]
- Roman, A.C.; Carvajal-Gonzalez, J.M.; Rico-Leo, E.M.; Fernandez-Salguero, P.M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem. 2009, 284, 25135–25148. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Kazmin, D.; Hu, P.; Thomas, R.S.; McDonnell, D.P.; Malek, G. Aryl hydrocarbon receptor knock-out exacerbates choroidal neovascularization via multiple pathogenic pathways. J. Pathol. 2015, 235, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Herrmann, R.; Bednar, A.; Saloupis, P.; Dwyer, M.A.; Yang, P.; Qi, X.; Thomas, R.S.; Jaffe, G.J.; Boulton, M.E.; et al. Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proc. Natl. Acad. Sci. USA 2013, 110, E4069–E4078. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Safe, S.; Malek, G. Suppression of aberrant choroidal neovascularization through activation of the aryl hydrocarbon receptor. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1583–1595. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in the eye. Steroids 2018, 133, 60–66. [Google Scholar] [CrossRef]
- Martens, B.; Drebert, Z. Glucocorticoid-mediated effects on angiogenesis in solid tumors. J. Steroid Biochem. Mol. Biol. 2019, 188, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Cutolo, M. Glucocorticoids and chronic inflammation. Rheumatology (Oxford) 2016, 55, ii6–ii14. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.L.; Choi, Y.; Jeong, K.W. Crosstalk between Aryl Hydrocarbon Receptor and Glucocorticoid Receptor in Human Retinal Pigment Epithelial Cells. Int. J. Endocrinol. 2017, 2017, 5679517. [Google Scholar] [CrossRef] [PubMed]
- Takata, S.; Masuda, T.; Nakamura, S.; Kuchimaru, T.; Tsuruma, K.; Shimazawa, M.; Nagasawa, H.; Kizaka-Kondoh, S.; Hara, H. The effect of triamcinolone acetonide on laser-induced choroidal neovascularization in mice using a hypoxia visualization bio-imaging probe. Sci. Rep. 2015, 5, 9898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciulla, T.A.; Criswell, M.H.; Danis, R.P.; Hill, T.E. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch. Ophthalmol. 2001, 119, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Capuano, V.; Serra, R.; Oubraham, H.; Zambrowski, O.; Amana, D.; Zerbib, J.; Souied, E.H.; Querques, G. Dexamethasone Intravitreal Implant for Choroidal Neovascularization during Pregnancy. Retin. Cases Br. Rep. 2019, 13, 300–307. [Google Scholar] [CrossRef]
- Bakri, S.J.; Ekdawi, N.S. Intravitreal triamcinolone and bevacizumab combination therapy for refractory choroidal neovascularization with retinal angiomatous proliferation. Eye (Lond.) 2008, 22, 978–980. [Google Scholar] [CrossRef] [Green Version]
- Augustin, A.J.; Puls, S.; Offermann, I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: Verteporfin PDT, bevacizumab, and dexamethasone. Retina 2007, 27, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehmann, D.; Garcia, R. Triple therapy for neovascular age-related macular degeneration (verteporfin photodynamic therapy, intravitreal dexamethasone, and intravitreal bevacizumab). Can. J. Ophthalmol. 2010, 45, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Augustin, A. Anecortave acetate in the treatment of age-related macular degeneration. Clin. Interv. Aging 2006, 1, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.R.; Hudson, H.L.; Jerdan, J.A.; Anecortave Acetate Clinical Study Group. Anecortave acetate for the treatment of exudative age-related macular degeneration—A review of clinical outcomes. Surv. Ophthalmol. 2007, 52, S79–S90. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.G.; Adamis, A.P. Ocular Angiogenesis: Vascular Endothelial Growth Factor and Other Factors. Dev. Ophthalmol. 2016, 55, 28–37. [Google Scholar]
- Clark, A.F. Mechanism of action of the angiostatic cortisene anecortave acetate. Surv. Ophthalmol. 2007, 52, S26–S34. [Google Scholar] [CrossRef]
- Hong, F.; Xu, P.; Zhai, Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar]
- Tomany, S.C.; Wang, J.J.; Van Leeuwen, R.; Klein, R.; Mitchell, P.; Vingerling, J.R.; Klein, B.E.; Smith, W.; De Jong, P.T. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology 2004, 111, 1280–1287. [Google Scholar] [CrossRef]
- Spaide, R.F.; Sorenson, J.; Maranan, L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2005, 112, 301–304. [Google Scholar] [CrossRef]
- Gallemore, R.P.; Wallsh, J.; Hudson, H.L.; Ho, A.C.; Chace, R.; Pearlman, J. Combination verteporfin photodynamic therapy ranibizumab-dexamethasone in choroidal neovascularization due to age-related macular degeneration: Results of a phase II randomized trial. Clin. Ophthalmol. 2017, 11, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Crawford, T.N.; Alfaro, D.V.; Kerrison, J.B.; Jablon, E.P. Diabetic retinopathy and angiogenesis. Curr. Diabetes Rev. 2009, 5, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Ballard, D.J.; Melton, L.J.; Dwyer, M.S.; Trautmann, J.C.; Chu, C.P.; O’Fallon, W.M.; Palumbo, P.J. Risk factors for diabetic retinopathy: A population-based study in Rochester, Minnesota. Diabetes Care 1986, 9, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Atchison, E.; Barkmeier, A. The Role of Systemic Risk Factors in Diabetic Retinopathy. Curr. Ophthalmol. Rep. 2016, 4, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S.B. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Bio. Med. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2017, 2017, 9702820. [Google Scholar] [CrossRef]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the cardiovascular system. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X.; et al. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.-C.; Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staels, B.; Koenig, W.; Habib, A.; Merval, R.; Lebret, M.; Torra, I.P.; Delerive, P.; Fadel, A.; Chinetti, G.; Fruchart, J.C.; et al. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 1998, 393, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; De Bosscher, K.; Besnard, S.; Vanden Berghe, W.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.C.; Tedgui, A.; Haegeman, G.; Staels, B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J. Biol. Chem. 1999, 274, 32048–32054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadian, S.; Mahadevan, N.; Balakumar, P. Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy. Eur. J. Pharmacol. 2013, 698, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Group, A.S.; Group, A.E.S.; Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar]
- Noonan, J.E.; Jenkins, A.J.; Ma, J.-X.; Keech, A.C.; Wang, J.J.; Lamoureux, E.L. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013, 62, 3968–3975. [Google Scholar] [CrossRef] [Green Version]
- Bishop-Bailey, D.; Swales, K.E. The Role of PPARs in the Endothelium: Implications for Cancer Therapy. PPAR Res. 2008, 2008, 904251. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, M.E.; McCollum, G.W.; Savage, S.R.; Penn, J.S. Peroxisome proliferator-activated receptor-β/δ regulates angiogenic cell behaviors and oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4197–4207. [Google Scholar] [CrossRef] [Green Version]
- Spiegelman, B.M. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes 1998, 47, 507–514. [Google Scholar] [CrossRef]
- Costa, V.; Ciccodicola, A. Is PPARG the key gene in diabetic retinopathy? Br. J. Pharmacol. 2012, 165, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Casamassimi, A.; Esposito, K.; Villani, A.; Capone, M.; Iannella, R.; Schisano, B.; Ciotola, M.; Di Palo, C.; Corrado, F.C.; et al. Characterization of a novel polymorphism in PPARG regulatory region associated with type 2 diabetes and diabetic retinopathy in Italy. J. Biomed. Biotechnol. 2009, 2009, 126917. [Google Scholar] [CrossRef] [PubMed]
- Malecki, M.T.; Cyganek, K.; Mirkiewicz-Sieradzka, B.; Wolkow, P.P.; Wanic, K.; Skupien, J.; Solnica, B.; Sieradzki, J. Alanine variant of the Pro12Ala polymorphism of the PPARgamma gene might be associated with decreased risk of diabetic retinopathy in type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 80, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Katome, T.; Namekata, K.; Mitamura, Y.; Semba, K.; Egawa, M.; Naito, T.; Harada, C.; Harada, T. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J. Diabetes Its Complicat. 2015, 29, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Sanders, T.; Kahook, K.; Akeel, S.; Elmarakby, A.; Al-Shabrawey, M. Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: Role of NADPH oxidase. Investig. Ophthalmol. Vis. Sci. 2009, 50, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Hammer, S.S.; Beli, E.; Kady, N.; Wang, Q.; Wood, K.; Lydic, T.A.; Malek, G.; Saban, D.R.; Wang, X.X.; Hazra, S.; et al. The Mechanism of Diabetic Retinopathy Pathogenesis Unifying Key Lipid Regulators, Sirtuin 1 and Liver X Receptor. EBioMedicine 2017, 22, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Malek, G.; Busik, J.; Grant, M.B.; Choudhary, M. Models of retinal diseases and their applicability in drug discovery. Expert Opin. Drug Discov. 2018, 13, 359–377. [Google Scholar] [CrossRef]
- Hazra, S.; Rasheed, A.; Bhatwadekar, A.; Wang, X.; Shaw, L.C.; Patel, M.; Caballero, S.; Magomedova, L.; Solis, N.; Yan, Y.; et al. Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes 2012, 61, 3270–3279. [Google Scholar] [CrossRef] [Green Version]
- Von Essen, M.R.; Geisler, C. VDR, the Vitamin D Receptor. In Encyclopedia of Signaling Molecules; Springer International Publishing: Seoul, Korea, 2018. [Google Scholar]
- Merrigan, S.L.; Kennedy, B.N. Vitamin D receptor agonists regulate ocular developmental angiogenesis and modulate expression of dre-miR-21 and VEGF. Br. J. Pharmacol. 2017, 174, 2636–2651. [Google Scholar] [CrossRef] [Green Version]
- Duez, H.; Staels, B. Rev-erb-alpha: An integrator of circadian rhythms and metabolism. J. Appl. Physiol. (Bethesda Md. 1985) 2009, 107, 1972–1980. [Google Scholar] [CrossRef] [Green Version]
- Solt, L.A.; Kojetin, D.J.; Burris, T.P. The REV-ERBs and RORs: Molecular links between circadian rhythms and lipid homeostasis. Future Med. Chem. 2011, 3, 623–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Wu, N.; Lazar, M.A. Nuclear receptor Rev-erbalpha: A heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal. 2010, 8, e001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wärnmark, A.; Treuter, E.; Wright, A.P.H.; Gustafsson, J.-A. Activation functions 1 and 2 of nuclear receptors: Molecular strategies for transcriptional activation. Mol. Endocrinol. (Baltim. Md.) 2003, 17, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Burris, T.P. Action of RORs and their ligands in (patho) physiology. Trends Endocrinol. Metab. 2012, 23, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santori, F.R. Nuclear hormone receptors put immunity on sterols. Eur. J. Immunol. 2015, 45, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Besnard, S.; Silvestre, J.S.; Duriez, M.; Bakouche, J.; Lemaigre-Dubreuil, Y.; Mariani, J.; Levy, B.I.; Tedgui, A. Increased ischemia-induced angiogenesis in the staggerer mouse, a mutant of the nuclear receptor Roralpha. Circ. Res. 2001, 89, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, C.-H.; SanGiovanni, J.P.; Evans, L.P.; Tian, K.T.; Zhang, B.; Stahl, A.; Pu, W.T.; Kamenecka, T.M.; Solt, L.A.; et al. Nuclear receptor RORα regulates pathologic retinal angiogenesis by modulating SOCS3-dependent inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, 10401–10406. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, C.-H.; Wang, Z.; Meng, S.S.; Burnim, S.B.; SanGiovanni, J.P.; Kamenecka, T.M.; Solt, L.A.; Chen, J. RORα modulates semaphorin 3E transcription and neurovascular interaction in pathological retinal angiogenesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 4492–4502. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A.; Joyal, J.-S.; Chen, J.; Sapieha, P.; Juan, A.M.; Hatton, C.J.; Pei, D.T.; Hurst, C.G.; Seaward, M.R.; Krah, N.M.; et al. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood 2012, 120, 2925–2929. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Okada, M.; Kataoka, H.; Hirashima, M.; Yoshida, Y.; Mann, F.; Gomi, F.; Nishida, K.; Nishikawa, S.-I.; Uemura, A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Investig. 2011, 121, 1974–1985. [Google Scholar] [CrossRef]
- Kim, J.; Oh, W.-J.; Gaiano, N.; Yoshida, Y.; Gu, C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes. Dev. 2011, 25, 1399–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriya, J.; Minamino, T.; Tateno, K.; Okada, S.; Uemura, A.; Shimizu, I.; Yokoyama, M.; Nojima, A.; Okada, M.; Koga, H.; et al. Inhibition of semaphorin as a novel strategy for therapeutic angiogenesis. Circu. Res. 2010, 106, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Boo, K.; Yu, Y.S.; Oh, S.K.; Kim, H.; Jeon, Y.; Bhin, J.; Hwang, D.; Kim, K.I.; Lee, J.-S.; et al. RORα controls hepatic lipid homeostasis via negative regulation of PPARγ transcriptional network. Nature Commun. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnard, S.; Heymes, C.; Merval, R.; Rodriguez, M.; Galizzi, J.-P.; Boutin, J.A.; Mariani, J.; Tedgui, A. Expression and regulation of the nuclear receptor RORalpha in human vascular cells. FEBS Lett. 2002, 511, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar]
- Jensen, L.D.; Cao, Y. Clock controls angiogenesis. Cell Cycle (Georget. Tex.) 2013, 12, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshi, T.; Toukairin, Y.; Arai, T.; Nogami, M. Circadian rhythms of angiogenic factors in skin and wound tissue in per2-mutant mice. Biomed. Res. Clin. Pract. 2017, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.D.; Gyllenhaal, C.; Block, K. Circadian angiogenesis. Biomol. Concepts 2014, 5, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Busik, J.V.; Tikhonenko, M.; Bhatwadekar, A.; Opreanu, M.; Yakubova, N.; Caballero, S.; Player, D.; Nakagawa, T.; Afzal, A.; Kielczewski, J.; et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J. Exp. Med. 2009, 206, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2014, 4, 965–994. [Google Scholar] [PubMed] [Green Version]
- Richardson, R.V.; Batchen, E.J.; Denvir, M.A.; Gray, G.A.; Chapman, K.E. Cardiac GR and MR: From Development to Pathology. Trends Endocrinol. Metab. 2016, 27, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, R.; Rao, A.D.; Baimas-George, M.; Hurwitz, S.; Foster, C.; Shah, R.V.; Jerosch-Herold, M.; Kwong, R.Y.; Di Carli, M.F.; Adler, G.K. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015, 64, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson-Berka, J.L.; Tan, G.; Jaworski, K.; Harbig, J.; Miller, A.G. Identification of a retinal aldosterone system and the protective effects of mineralocorticoid receptor antagonism on retinal vascular pathology. Circ. Res. 2009, 104, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Felinski, E.A.; Antonetti, D.A. Glucocorticoid regulation of endothelial cell tight junction gene expression: Novel treatments for diabetic retinopathy. Curr. Eye Res. 2005, 30, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.P.; Ashton, P.; Kompella, U.B. Fluocinolone Inhibits VEGF Expression via Glucocorticoid Receptor in Human Retinal Pigment Epithelial (ARPE-19) Cells and TNF-α–Induced Angiogenesis in Chick Chorioallantoic Membrane (CAM). J. Ocul. Pharmacol. Ther. 2009, 25, 97–104. [Google Scholar] [CrossRef]
- Taverna, M.J.; Sola, A.; Guyot-Argenton, C.; Pacher, N.; Bruzzo, F.; Slama, G.; Reach, G.; Selam, J.-L. Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia 2002, 45, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Taverna, M.J.; Selam, J.-L.; Slama, G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 4803–4808. [Google Scholar] [CrossRef] [Green Version]
- Cyganek, K.; Mirkiewicz-Sieradzka, B.; Malecki, M.T.; Wolkow, P.; Skupien, J.; Bobrek, J.; Czogala, M.; Klupa, T.; Sieradzki, J. Clinical risk factors and the role of VDR gene polymorphisms in diabetic retinopathy in Polish type 2 diabetes patients. Acta Diabetol. 2006, 43, 114–119. [Google Scholar] [CrossRef]
- Bućan, K.; Ivanisević, M.; Zemunik, T.; Boraska, V.; Skrabić, V.; Vatavuk, Z.; Galetović, D.; Znaor, L. Retinopathy and nephropathy in type 1 diabetic patients—Association with polymorphysms of vitamin D-receptor, TNF, Neuro-D and IL-1 receptor 1 genes. Coll. Antropol. 2009, 33, 99–105. [Google Scholar]
- Jia, J.; Ding, H.; Yang, K.; Mao, L.; Zhao, H.; Zhan, Y.; Shen, C. Vitamin D Receptor Genetic Polymorphism Is Significantly Associated with Risk of Type 2 Diabetes Mellitus in Chinese Han Population. Arch. Med. Res. 2015, 46, 572–579. [Google Scholar] [CrossRef]
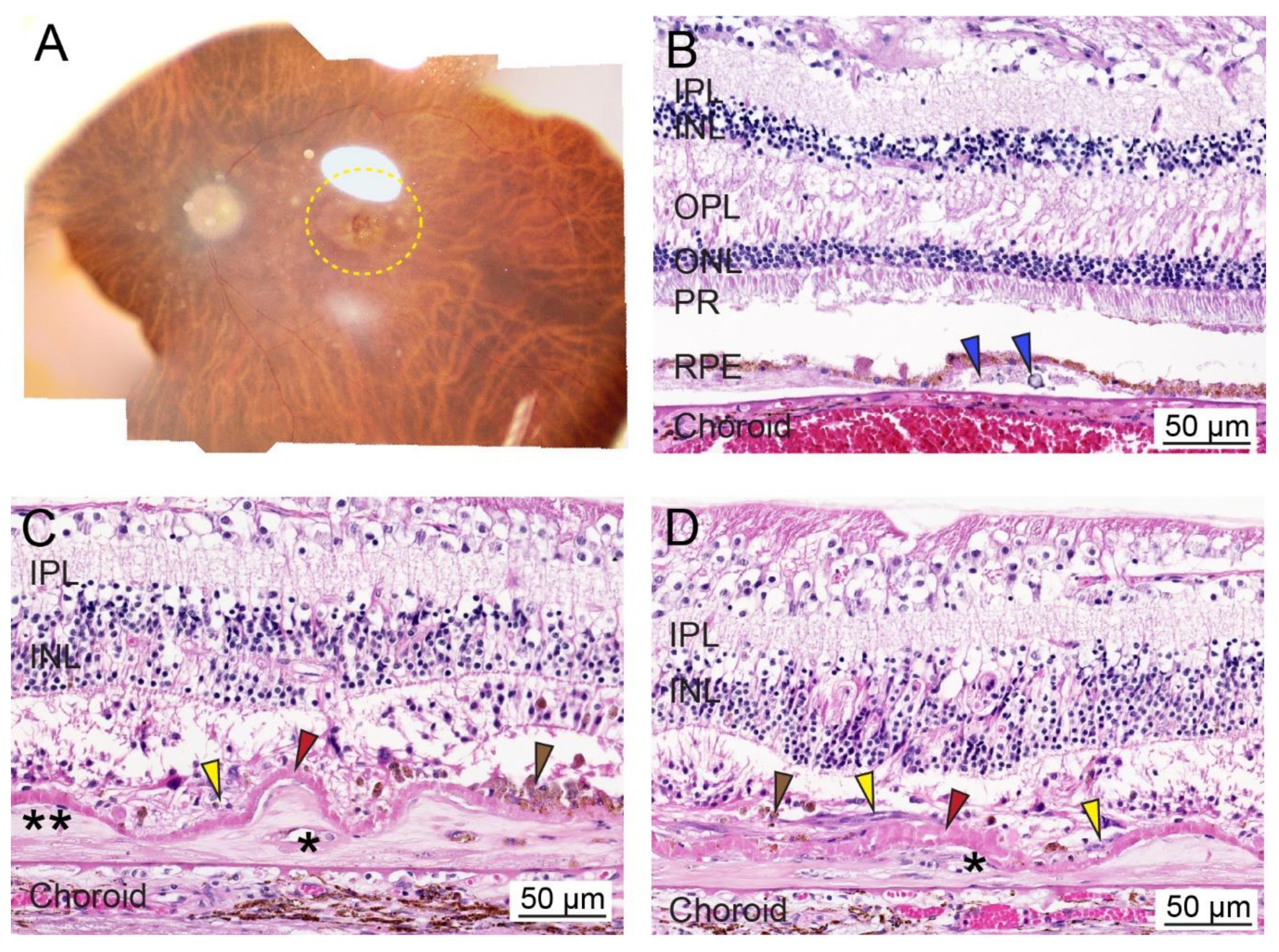

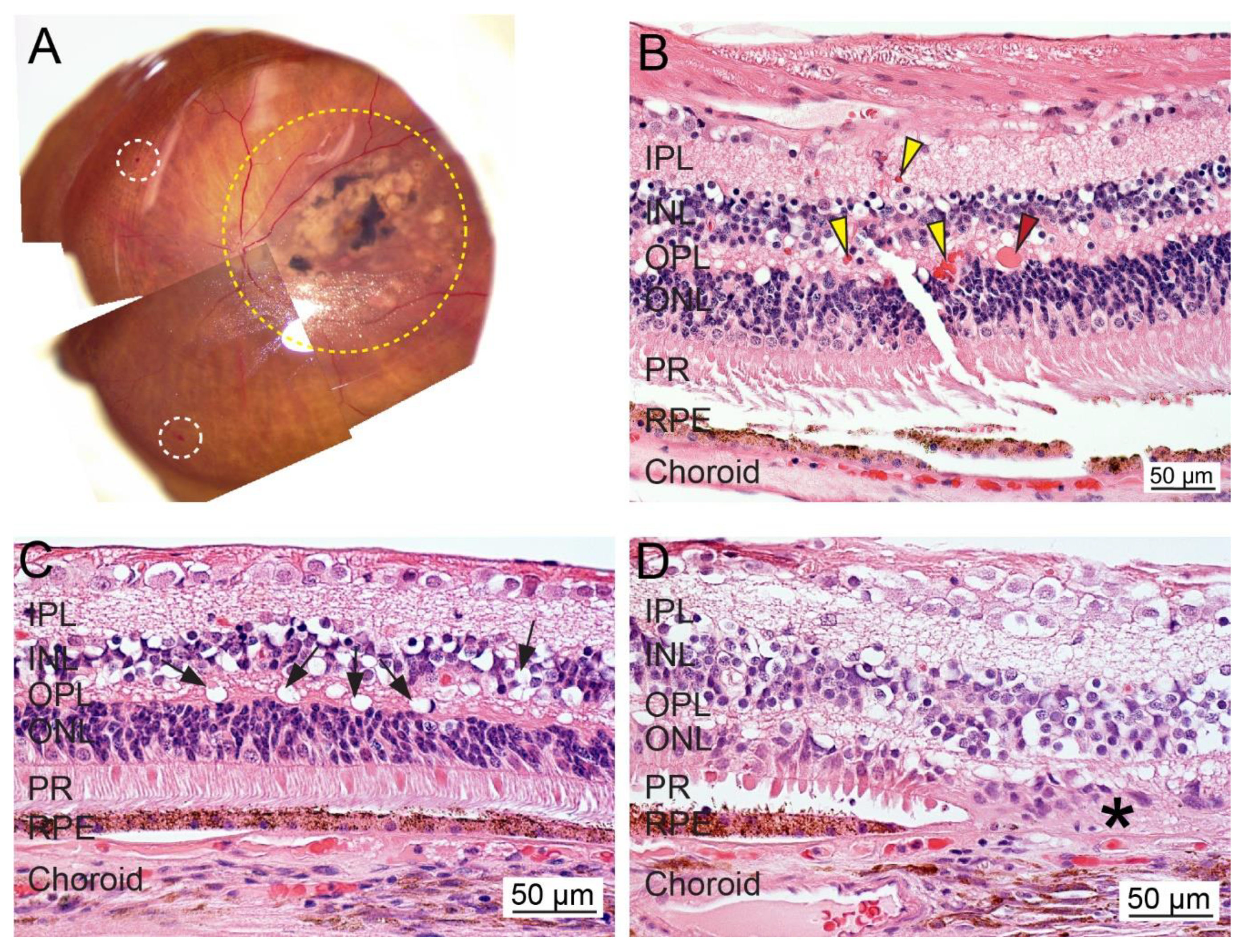

| Reference | Target | Type of Study | Cohort Size | Results and Interpretation |
|---|---|---|---|---|
| Hong et al., 2018. Review [87] | PPARs | unknown | unknown | PPARα agonist macuneos (Biophytis) is under clinical trial phase I for treating AMD. |
| The Eye Disease Case–control Study Group. 1992 [88] | ERs | Case-control study | n = 1036 | Women in the U.S. exposed to exogenous estrogen exhibited lower risk of neovascular AMD. |
| Snow et al., 2002 [64] | ERs | Cross-sectional study on postmenopausal women with AMD | n = 394 | Women under postmenopausal estrogen therapy experienced lower grade of AMD. |
| Tomany et al., 2004 [89] | ERs | Population-based cohort study (meta-analysis) | n = 9523 | No significant associations between the use of hormone therapy and the incidence of late AMD was reported. |
| Boekhoorn et al., 2007. The Rotterdam Study [60] | ERs | Population-based cohort study | n = 4571 | ERα polymorphisms (ESR1 PvuII-XbaI) are associated with an increased incidence of wet AMD. |
| Edwards et al., 2010 [63] | ERs | Case-control study | n = 799 | Hormone replacement therapy or oral contraceptives have a protective role in women with neovascular AMD. |
| Spaide et al., 2005 [90] | GRs | Small cohort study | n = 26 | CNV patients treated with combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide (GR agonist) exhibited improved vision and reduced treatment frequency. |
| Augustin et al., 2007 [81] | GRs | Small cohort study | n = 104 | One cycle of triple therapy with verteporfin (photodynamic therapy), dexamethasone (GR agonist), and bevacizumab (anti-VEGF) improved the visual acuity of CNV patients. |
| Ehmann et al., 2010 [82] | GRs | Small cohort study | n = 30 | One cycle of triple therapy with verteporfin (photodynamic therapy), dexamethasone (GR agonist), and bevacizumab (anti-VEGF) improved visual acuity of CNV patients. |
| Gallemore et al., 2017. The RADICAL Study [91] | GRs | Randomized control study | n = 162 | Combined therapy with verteporfin (photodynamic therapy), ranibizumab (anti-VEGF) and dexamethasone (GR agonist) significantly reduced retreatment visits than ranibizumab treatment alone in CNV patients. |
| Capuano et al., 2019 [79] | GRs | Small cohort study | n = 3 | Intravitreal implants of dexamethasone (GR agonist) improved the vision of pregnant CNV patients. |
| Reference | Target | Type of Study | Study size | Results and Interpretation |
|---|---|---|---|---|
| ACCORD Study Group et al., 2014 [106] | PPARα | Randomized, controlled clinical trial | n= 1593 type II diabetes mellitus patients (806 fenofibrate treatment; 787 placebo) | Patients treated with fenofibrate, a potent PPARα agonist, were less likely to develop diabetic retinopathy (adjusted OR = 0.60; 95% CI 0.42–0.87; p = 0.006). |
| Costa V et al., 2009 [112] | PPARγ | Case control | n = 670 (211 type II diabetes; 205 obese; 254 control individuals) | Pro12Ala polymorphism of the PPARγ gene may be associated with decreased risk of DR. |
| Malecki MT et al., 2008 [113] | PPARγ | Case control | n = 159 (38 type II diabetes without DR; 121 with DR) | Polymorphism A-2819 in the PPARγ gene is associated with DR. |
| Taverna et al., 2002 [147] | VDR | Cross-sectional | n = 200 c-peptide negative type I diabetics | Homozygous wild-type (TT) individuals had lower odds of “severe” diabetic retinopathy (OR = 0.50; 95% CI, 0.26–0.94; p = 0.02). |
| Taverna et al., 2005 [148] | VDR | Cross-sectional | n = 254 c-peptide negative type I diabetics | Those with severe DR were less likely to have the FF genotype than those individuals with none or mild DR (OR = 0.54; 95% CI, 0.32–0.90). |
| Cyganek et al., 2006 [149] | VDR | Cross-sectional | n = 267 type II diabetics | FOKI, TAQI, BSMI, and APA1 polymorphisms of VDR were not associated with DR. |
| Bućan et al., 2009 [150] | VDR | Cross-sectional | n = 120 type I diabetics | FOKI, TAQI, and TRU91 polymorphisms were not significantly associated with DR. BSMI was weakly associated with DR (n = 7, p < 0.05). |
| Jia et al., 2015 [151] | VDR | Case control | Cases = 81 Controls = 113 | TAQI T allele (OR = 2.78; 95% CI, 1.15–6.72) and BSMI b allele (OR = 3.20; 95% CI, 1.19–8.60) in VDR gene are associated with diabetic retinopathy. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, P.-L.; Peavey, J.; Malek, G. Leveraging Nuclear Receptors as Targets for Pathological Ocular Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 2889. https://doi.org/10.3390/ijms21082889
Yao P-L, Peavey J, Malek G. Leveraging Nuclear Receptors as Targets for Pathological Ocular Vascular Diseases. International Journal of Molecular Sciences. 2020; 21(8):2889. https://doi.org/10.3390/ijms21082889
Chicago/Turabian StyleYao, Pei-Li, Jeremy Peavey, and Goldis Malek. 2020. "Leveraging Nuclear Receptors as Targets for Pathological Ocular Vascular Diseases" International Journal of Molecular Sciences 21, no. 8: 2889. https://doi.org/10.3390/ijms21082889
APA StyleYao, P.-L., Peavey, J., & Malek, G. (2020). Leveraging Nuclear Receptors as Targets for Pathological Ocular Vascular Diseases. International Journal of Molecular Sciences, 21(8), 2889. https://doi.org/10.3390/ijms21082889





