Alteration of the Dopamine Receptors’ Expression in the Cerebellum of the Lysosomal Acid Phosphatase 2 Mutant (Naked–Ataxia (NAX)) Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Immunohistochemistry (IHC)
2.3. Primary Antibodies Used for IHC and WB Analysis
2.4. Protein Extraction and Western Blot Analysis
2.5. RNA Isolation and Analyses
2.6. Statistical Analysis
2.7. Imaging
3. Results
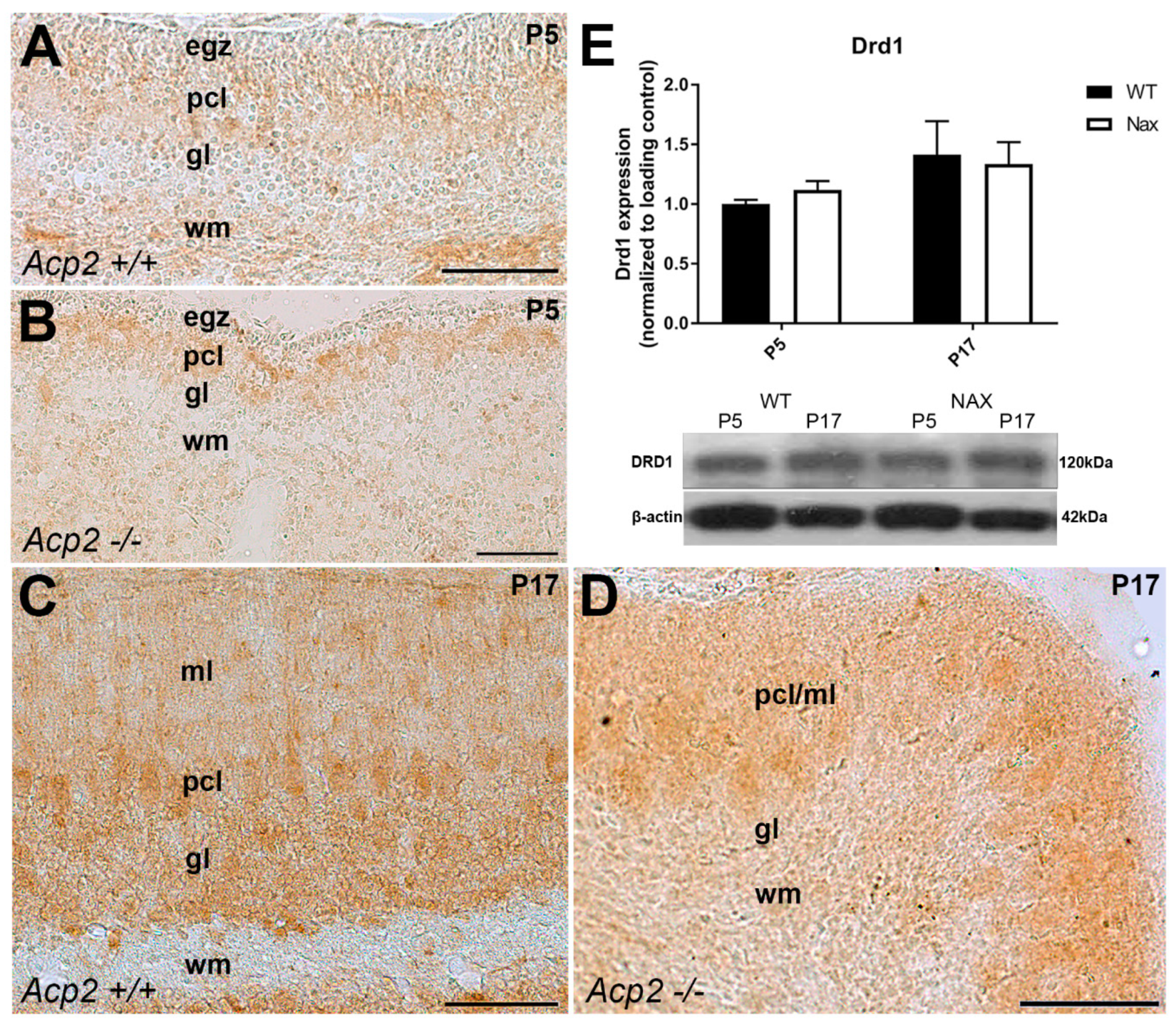
3.1. Dopamine Receptor D1 (DRD1) Expression in wt and nax Cerebella at P5 and P17
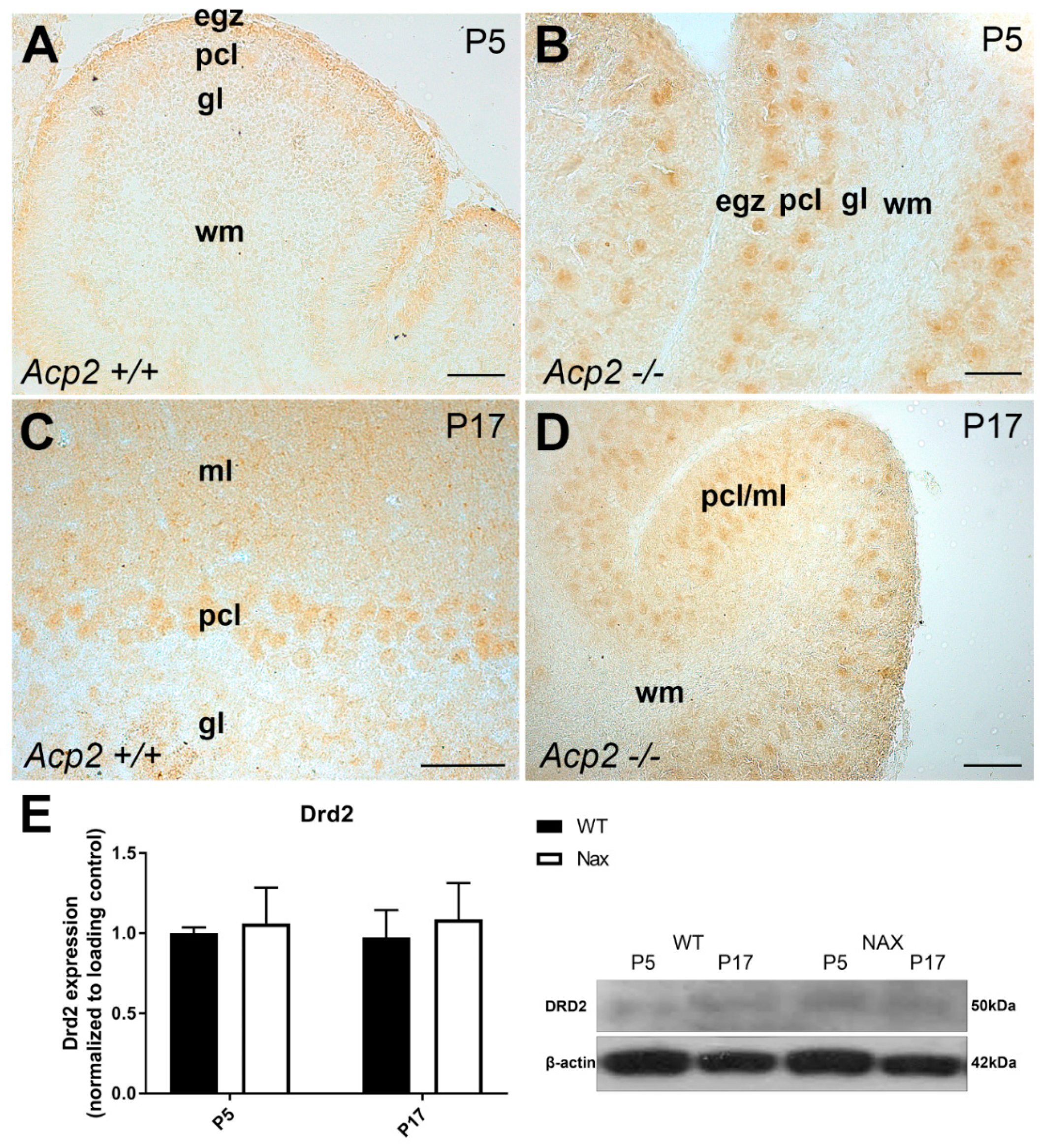
3.2. Dopamine Receptor D2 (DRD2) Expression in wt and nax Cerebella at P5 and P17
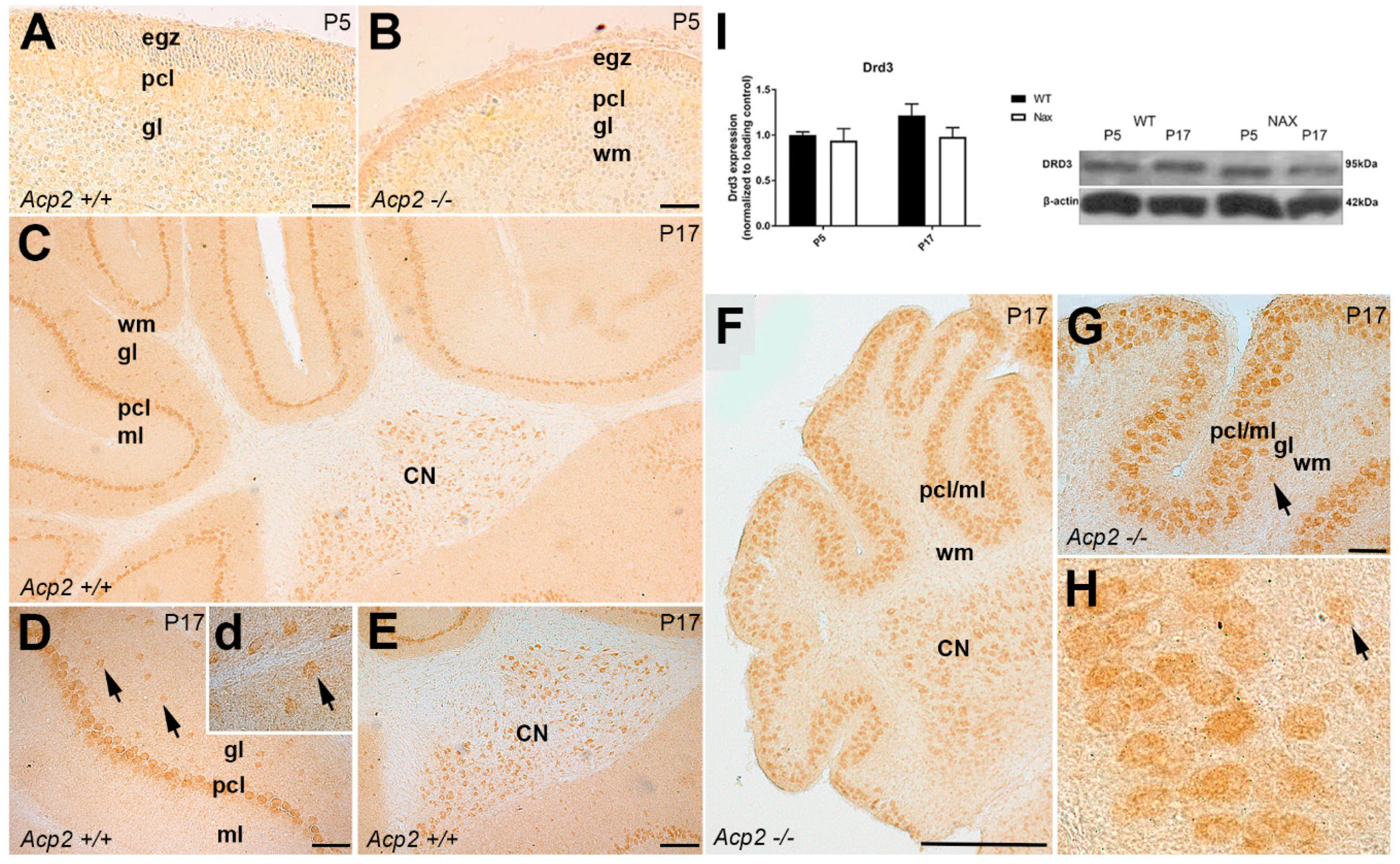
3.3. Dopamine Receptor D3 (DRD3) Expression in wt and nax Cerebella at P5 and P17
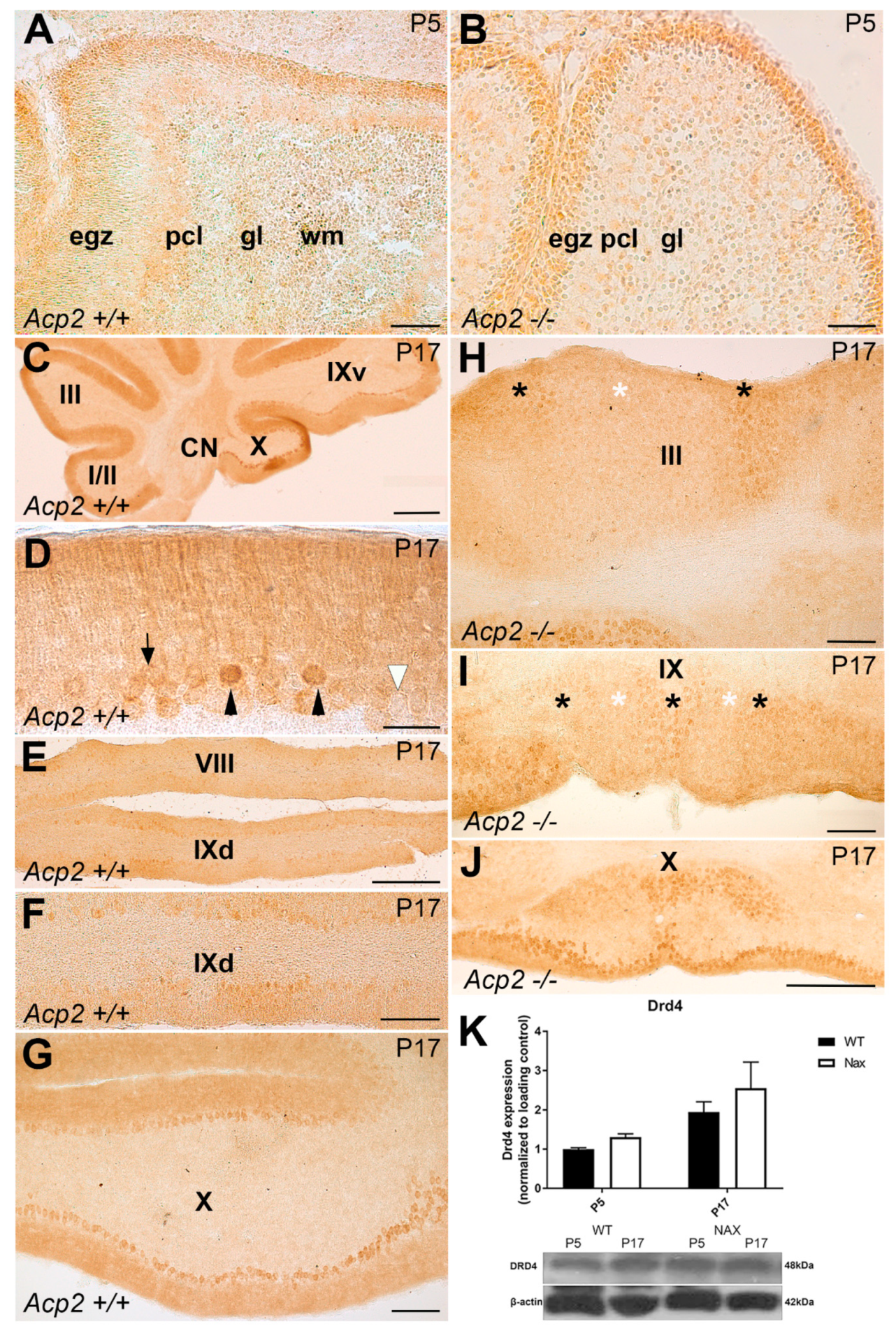
3.4. Dopamine Receptor D4 (DRD4) Expression in wt and nax Cerebella on P5 and P17
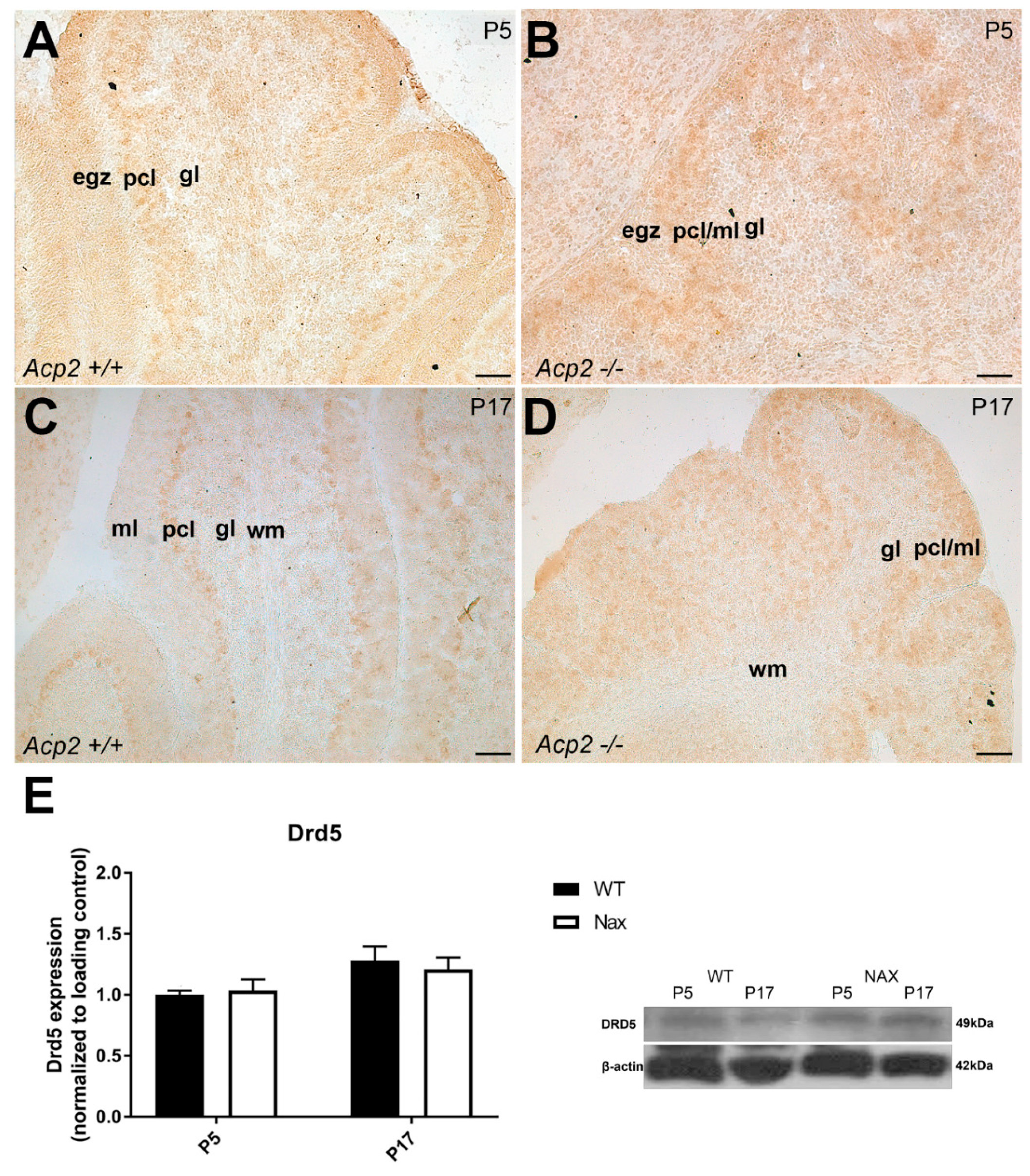
3.5. Dopamine receptor D5 (DRD5) expression in wt and nax cerebella on P5 and P17
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thach, W. On the mechanism of cerebellar contributions to cognition. Cerebellum 2007, 6, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Steinlin, M. Cerebellar disorders in childhood: Cognitive problems. Cerebellum 2008, 7, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain A J. Neurol. 2006, 129 Pt 2, 290–292. [Google Scholar] [CrossRef]
- Parisi, M.A. Editor Clinical and Molecular Features of Joubert Syndrome and related Disorders. In American Journal of Medical Genetics Part C: Seminars in Medical Genetics; Wiley Online Library: Toronto, ON, Canada, 2009. [Google Scholar]
- Webb, S.J.; Sparks, B.F.; Friedman, S.D.; Shaw, D.W.; Giedd, J.; Dawson, G.; Dager, S.R. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. Neuroimaging 2009, 172, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Schmahmann, J.D. Consensus Paper. Cerebellar Reserve: From Cerebellar Physiology to Cerebellar Disorders. Cerebellum 2020, 19, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, N.; Jiao, X.; Rahimi-Balaei, M.; Amiri, S.; Mehr, S.E.; Yeganeh, B.; Marzban, H. Lysosomal acid phosphatase biosynthesis and dysfunction: A mini review focused on lysosomal enzyme dysfunction in brain. Curr. Mol. Med. 2016, 16, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; Hamilton, D.J.; Nixon, R.A. Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res. 1994, 640, 68–80. [Google Scholar] [CrossRef]
- Mannan, A.U.; Roussa, E.; Kraus, C.; Rickmann, M.; Maenner, J.; Nayernia, K.; Engel, W. Mutation in the gene encoding lysosomal acid phosphatase (Acp2) causes cerebellum and skin malformation in mouse. Neurogenetics 2004, 5, 229–238. [Google Scholar] [CrossRef]
- Makrypidi, G.; Damme, M.; Müller-Loennies, S.; Trusch, M.; Schmidt, B.; Schlüter, H.; Braulke, T. Mannose 6 dephosphorylation of lysosomal proteins mediated by acid phosphatases Acp2 and Acp5. Mol. Cell. Biol. 2012, 32, 774–782. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Prata, M.J.; Alves, S. Mannose–6–phosphate pathway: A review on its role in lysosomal function and dysfunction. Mol. Genet. Metab. 2012, 105, 542–550. [Google Scholar] [CrossRef]
- Ba, Q.; Raghavan, G.; Kiselyov, K.; Yang, G. Whole–Cell Scale Dynamic Organization of Lysosomes Revealed by Spatial Statistical Analysis. Cell Rep. 2018, 23, 3591–3606. [Google Scholar] [CrossRef] [PubMed]
- Schröder, B.A.; Wrocklage, C.; Hasilik, A.; Saftig, P. The proteome of lysosomes. Proteomics 2010, 10, 4053–4076. [Google Scholar] [CrossRef] [PubMed]
- Lübke, T.; Lobel, P.; Sleat, D.E. Proteomics of the lysosome. Biochim. Et Biophys. Acta (Bba)–Mol. Cell Res. 2009, 1793, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.; Rahimi Balaei, M.; Mehdizadeh, M.; Marzban, H. Spatial and temporal expression of lysosomal acid phosphatase 2 (ACP2) reveals dynamic patterning of the mouse cerebellar cortex. Cerebellum 2013, 12, 870–881. [Google Scholar] [CrossRef]
- Bailey, K.; Balaei, M.R.; Mannan, A.; Del Bigio, M.R.; Marzban, H. Purkinje cell compartmentation in the cerebellum of the lysosomal Acid phosphatase 2 mutant mouse (nax–naked–ataxia mutant mouse). PLoS ONE 2014, 9, e94327. [Google Scholar] [CrossRef]
- Saftig, P.; Hartmann, D.; Lullmann–Rauch, R.; Wolff, J.; Evers, M.; Koster, A.; Peters, C. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J. Biol. Chem. 1997, 272, 18628–18635. [Google Scholar] [CrossRef]
- Rahimi-Balaei, M.; Jiao, X.; Ashtari, N.; Afsharinezhad, P.; Ghavami, S.; Marzban, H. Cerebellar expression of the neurotrophin receptor p75 in naked-ataxia mutant mouse. Int. J. Mol. Sci. 2016, 17, 115. [Google Scholar] [CrossRef]
- Matsui, H. Dopamine system, cerebellum, and nucleus ruber in fish and mammals. Dev. Growth Differ. 2017, 59, 219–227. [Google Scholar] [CrossRef]
- Locke, T.M.; Soden, M.E.; Miller, S.M.; Hunker, A.; Knakal, C.; Licholai, J.A.; Carlson, E.S. Dopamine D1 Receptor–Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biol. Psychiatry 2018, 84, 401–412. [Google Scholar] [CrossRef]
- Melquist, S.; Craig, D.W.; Huentelman, M.J.; Crook, R.; Pearson, J.V.; Baker, M.; Corneveaux, J. Identification of a novel risk locus for progressive supranuclear palsy by a pooled genomewide scan of 500,288 single–nucleotide polymorphisms. Am. J. Hum. Genet. 2007, 80, 769–778. [Google Scholar] [CrossRef]
- Stamelou, M.; Hoglinger, G. A Review of Treatment Options for Progressive Supranuclear Palsy. Cns Drugs 2016, 30, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Graybiel, A.M.; Duyckaerts, C.; Javoy–Agid, F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA 1987, 84, 5976–5980. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, S.; Oda, Y. Cholinergic neuronal loss in the basal forebrain and mesopontine tegmentum of progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2003, 105, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.; Matusch, A.; Elmenhorst, D.; Hurlemann, R.; Eggert, K.M.; Zilles, K.; Bauer, A. Nigrostriatal upregulation of 5–HT2A receptors correlates with motor dysfunction in progressive supranuclear palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Rahimi–Balaei, M.; Afsharinezhad, P.; Bailey, K.; Buchok, M.; Yeganeh, B.; Marzban, H. Embryonic stages in cerebellar afferent development. Cerebellum Ataxias 2015, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Ikai, Y.; Takada, M.; Shinonaga, Y.; Mizuno, N. Dopaminergic and non–dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience 1992, 51, 719–728. [Google Scholar] [CrossRef]
- Ikai, Y.; Takada, M.; Mizuno, N. Single neurons in the ventral tegmental area that project to both the cerebral and cerebellar cortical areas by way of axon collaterals. Neuroscience 1994, 61, 925–934. [Google Scholar] [CrossRef]
- Mittleman, G.; Goldowitz, D.; Heck, D.H.; Blaha, C.D. Cerebellar modulation of frontal cortex dopamine efflux in mice: Relevance to autism and schizophrenia. Synapse 2008, 62, 544–550. [Google Scholar] [CrossRef]
- Giompres, P.; Delis, F. Dopamine transporters in the cerebellum of mutant mice. Cerebellum 2005, 4, 105–111. [Google Scholar] [CrossRef]
- Panagopoulos, N.T.; Papadopoulos, G.C.; Matsokis, N.A. Dopaminergic innervation and binding in the rat cerebellum. Neurosci. Lett. 1991, 130, 208–212. [Google Scholar] [CrossRef]
- Sibley, D.R.; Monsma Jr, F.J.; McVittie, L.D.; Gerfen, C.R.; Burch, R.M.; Mahan, L.C. Molecular neurobiology of dopamine receptor subtypes. Neurochem. Int. 1992, 20, 17–22. [Google Scholar] [CrossRef]
- Gingrich, J.A.; Caron, M.G. Recent advances in the molecular biology of dopamine receptors. Annu. Rev. Neurosci. 1993, 16, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Marzban, H.; Rahimi–Balaei, M.; Hawkes, R. Early trigeminal ganglion afferents enter the cerebellum before the Purkinje cells are born and target the nuclear transitory zone. Brain Struct. Funct. 2019, 224, 2421–2436. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra–fast all–in–one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed–and–vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Rahimi–Balaei, M.; Buchok, M.; Vihko, P.; Parkinson, F.E.; Marzban, H. Loss of prostatic acid phosphatase and alpha–synuclein cause motor circuit degeneration without altering cerebellar patterning. PLoS ONE 2019, 14, e0222234. [Google Scholar] [CrossRef]
- Afshar, P.; Ashtari, N.; Jiao, X.; Rahimi–Balaei, M.; Zhang, X.; Yaganeh, B.; Marzban, H. Overexpression of Human SOD1 Leads to Discrete Defects in the Cerebellar Architecture in the Mouse. Front. Neuroanat. 2017, 11, 22. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef]
- Delis, F.; Mitsacos, A.; Giompres, P. Dopamine receptor and transporter levels are altered in the brain of Purkinje Cell Degeneration mutant mice. Neuroscience 2004, 125, 255–268. [Google Scholar] [CrossRef]
- Mysliveček, J.; Cendelín, J.; Korelusová, I.; Kunová, M.; Markvartová, V.; Vožeh, F. Changes of dopamine receptors in mice with olivocerebellar degeneration. Prague Med. Rep. 2007, 108, 57–66. [Google Scholar]
- Camps, M.; Kelly, P.; Palacios, J. Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J. Neural Transm. Gen. Sect. JNT 1990, 80, 105–127. [Google Scholar] [CrossRef]
- El–Ghundi, M.; O’Dowd, B.F.; George, S.R. Insights into the role of dopamine receptor systems in learning and memory. Rev. Neurosci. 2007, 18, 37–66. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yamada, M.; Suhara, T. Functional significance of central D1 receptors in cognition: Beyond working memory. J. Cereb. Blood Flow Metab. 2012, 32, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Castner, S. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience 2006, 139, 263–276. [Google Scholar] [CrossRef]
- Rani, M.; Kanungo, M. Expression of D2 dopamine receptor in the mouse brain. Biochem. Biophys. Res. Commun. 2006, 344, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Pilon, C.; Le Foll, B.; Gros, C.; Triller, A.; Schwartz, J.-C.; Sokoloff, P. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J. Neurosci. 2000, 20, 8677–8684. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Levesque, D.; Lammers, C.; Griffon, N.; Martres, M.-P.; Schwartz, J.-C.; Sokoloff, P. Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 1995, 65, 731–745. [Google Scholar] [CrossRef]
- Bouthenet, M.-L.; Souil, E.; Martres, M.-P.; Sokoloff, P.; Giros, B.; Schwartz, J.-C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res. 1991, 564, 203–219. [Google Scholar] [CrossRef]
- Ariano, M.A.; Wang, J.; Noblett, K.L.; Larson, E.R.; Sibley, D.R. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti–receptor antisera. Brain Res. 1997, 752, 26–34. [Google Scholar] [CrossRef]
- Defagot, M.C.; Malchiodi, E.L.; Villar, M.J.; Antonelli, M.C. Distribution of D4 dopamine receptor in rat brain with sequence–specific antibodies. Mol. Brain Res. 1997, 45, 1–12. [Google Scholar] [CrossRef]
- Barili, P.; Bronzetti, E.; Ricci, A.; Zaccheo, D.; Amenta, F. Microanatomical localization of dopamine receptor protein immunoreactivity in the rat cerebellar cortex. Brain Res. 2000, 854, 130–138. [Google Scholar] [CrossRef]
- Pohl, S.; Mitchison, H.M.; Kohlschutter, A.; Van Diggelen, O.; Braulke, T.; Storch, S. Increased expression of lysosomal acid phosphatase in CLN3–defective cells and mouse brain tissue. J. Neurochem. 2007, 103, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Yeganeh, B.; Zeki, A.A.; Shojaei, S.; Kenyon, N.J.; Ott, S.; Dixon, I.M. Autophagy and the unfolded protein response promote profibrotic effects of TGF–beta1 in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L493–L504. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Sharma, P.; Yeganeh, B.; Ojo, O.O.; Jha, A.; Mutawe, M.M.; Halayko, A.J. Airway mesenchymal cell death by mevalonate cascade inhibition: Integration of autophagy, unfolded protein response and apoptosis focusing on Bcl2 family proteins. Biochim Biophys Acta. 2014, 1843, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Yeganeh, B.; Stelmack, G.L.; Kashani, H.H.; Sharma, P.; Cunnington, R.; Freed, D.H. Apoptosis, autophagy and ER stress in mevalonate cascade inhibition–induced cell death of human atrial fibroblasts. Cell Death Dis. 2012, 3, e330. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdizadeh, M.; Ashtari, N.; Jiao, X.; Rahimi Balaei, M.; Marzban, A.; Qiyami-Hour, F.; Kong, J.; Ghavami, S.; Marzban, H. Alteration of the Dopamine Receptors’ Expression in the Cerebellum of the Lysosomal Acid Phosphatase 2 Mutant (Naked–Ataxia (NAX)) Mouse. Int. J. Mol. Sci. 2020, 21, 2914. https://doi.org/10.3390/ijms21082914
Mehdizadeh M, Ashtari N, Jiao X, Rahimi Balaei M, Marzban A, Qiyami-Hour F, Kong J, Ghavami S, Marzban H. Alteration of the Dopamine Receptors’ Expression in the Cerebellum of the Lysosomal Acid Phosphatase 2 Mutant (Naked–Ataxia (NAX)) Mouse. International Journal of Molecular Sciences. 2020; 21(8):2914. https://doi.org/10.3390/ijms21082914
Chicago/Turabian StyleMehdizadeh, Mehdi, Niloufar Ashtari, Xiaodan Jiao, Maryam Rahimi Balaei, Asghar Marzban, Farshid Qiyami-Hour, Jiming Kong, Saeid Ghavami, and Hassan Marzban. 2020. "Alteration of the Dopamine Receptors’ Expression in the Cerebellum of the Lysosomal Acid Phosphatase 2 Mutant (Naked–Ataxia (NAX)) Mouse" International Journal of Molecular Sciences 21, no. 8: 2914. https://doi.org/10.3390/ijms21082914
APA StyleMehdizadeh, M., Ashtari, N., Jiao, X., Rahimi Balaei, M., Marzban, A., Qiyami-Hour, F., Kong, J., Ghavami, S., & Marzban, H. (2020). Alteration of the Dopamine Receptors’ Expression in the Cerebellum of the Lysosomal Acid Phosphatase 2 Mutant (Naked–Ataxia (NAX)) Mouse. International Journal of Molecular Sciences, 21(8), 2914. https://doi.org/10.3390/ijms21082914






