Heterogeneity of Integrin αIIbβ3 Function in Pediatric Immune Thrombocytopenia Revealed by Continuous Flow Cytometry Analysis
Abstract
:1. Introduction
2. Results
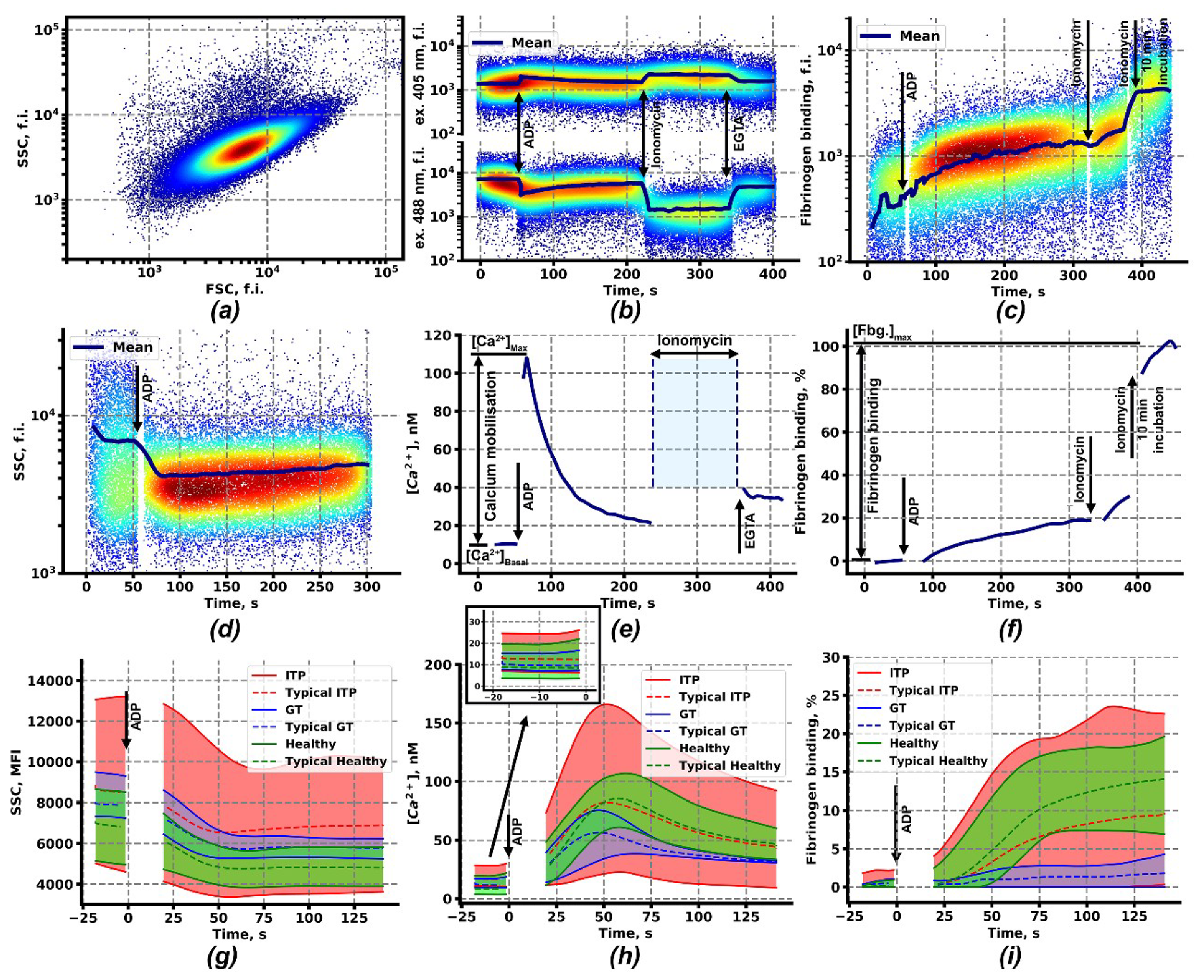
2.1. Calcium Signaling and Integrin Activation in Platelets of Immune Thrombocytopenia Patients
2.2. Clustering Analysis of Flow Cytometry Data Reveals Two Subgroups of Patients with ITP
2.3. Computational Models of Integrin Activation for ITP Subgroups
2.4. Ex Vivo Thrombus Growth and Leukocyte Activity for ITP Subgroups
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Materials
4.3. Continuous Flow Cytometry Assessment of the Platelet Intracellular Signaling and Functional Parameters
4.4. Ex vivo Thrombus Growth Analysis
4.5. Computational Modeling
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenylate Cyclase |
| CalDAGGEFI | Calcium Diacylglycerol Guanidine Exchange Factor I |
| CRP | Collagen-Related Peptide |
| DBCV | Density-Based Clustering Validation |
| FSC | Forward Scattering (Flow Cytometry Channel) |
| H-DBSCAN | Hierarchical Density-Based Spatial Clustering of Applications with Noise |
| IP3 | Inositol-1,4,5-Trisphosphate |
| ITP:HFB | High Fibrinogen Binding (ITP Population) |
| ITP:LFB | Low Fibrinogen Binding (ITP Population) |
| LRP | Leukocyte Rich Plasma |
| PAR | Protease-Activated Receptor |
| PI3K | Phosphoinositide-3-Kinase |
| PIP3 | Phosphoinositol-3,4,5-Trisphosphate |
| PKA | Protein Kinase A |
| PMN | Polymorphonuclear Neutrophils |
| Rap1GAP | Rap1 GTPase Activating Protein |
| RBC | Red Blood Cells |
| SSC | Side Scattering (Flow Cytometry Channel) |
| SSC-A | Area Signal of SSC |
| TRAP | Thrombin Receptor Activating Peptide |
| AC | Adenylate Cyclase |
| CalDAGGEFI | Calcium Diacylglycerol Guanidine Exchange Factor I |
| CRP | Collagen-Related Peptide |
| DBCV | Density-Based Clustering Validation |
| FSC | Forward Scattering (Flow Cytometry Channel) |
| H-DBSCAN | Hierarchical Density-Based Spatial Clustering of Applications with Noise |
References
- Cooper, N.; Bussel, J. The pathogenesis of immune thrombocytopaenic purpura. Br. J. Haematol. 2006, 133, 364–374. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report from an International Working Group; American Society of Hematology; American Society of Hematology: Colorado Springs, CO, USA, 2009; Volume 113. [Google Scholar]
- Panzer, S.; Rieger, M.; Vormittag, R.; Eichelberger, B.; Dunkler, D.; Pabinger, I. Platelet function to estimate the bleeding risk in autoimmune thrombocytopenia. Eur. J. Clin. Investig. 2007, 37, 814–819. [Google Scholar] [CrossRef]
- Cahill, M.R.; Macey, M.G.; Cavenagh, J.D.; Newland, A.C. Protein A immunoadsorption in chronic refractory ITP reverses increased platelet activation but fails to achieve sustained clinical benefit. Br. J. Haematol. 1998, 100, 358–364. [Google Scholar] [CrossRef]
- Frelinger, A.L.; Grace, R.F.; Gerrits, A.J.; Berny-Lang, M.A.; Brown, T.; Carmichael, S.L.; Neufeld, E.J.; Michelson, A.D. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood 2015, 126, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Frelinger, A.L.; Grace, R.F.; Gerrits, A.J.; Carmichael, S.L.; Forde, E.E.; Michelson, A.D. Platelet Function in ITP, Independent of Platelet Count, Is Consistent Over Time and Is Associated with Both Current and Subsequent Bleeding Severity. Thromb. Haemost. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Suntsova, E.V.; Demina, I.M.; Ignatova, A.A.; Ershov, N.M.; Trubina, N.M.; Dobrynina, J.; Serkova, I.V.; Supik, Z.S.; Orekhova, E.V.; Hachatryan, L.A.; et al. Bleeding tendency and platelet function during treatment with romiplostim in children with severe immune thrombocytopenic purpura. Int. J. Hematol. 2017, 105, 841–848. [Google Scholar] [CrossRef]
- Ignatova, A.A.; Ponomarenko, E.A.; Polokhov, D.M.; Suntsova, E.V.; Zharkov, P.A.; Fedorova, D.V.; Balashova, E.N.; Rudneva, A.E.; Ptushkin, V.V.; Nikitin, E.A.; et al. Flow cytometry for pediatric platelets. Platelets 2019, 30, 428–437. [Google Scholar] [CrossRef]
- Ignatova, A.A.; Demina, I.A.; Ptushkin, V.V.; Khaspekova, S.G.; Shustova, O.N.; Pankrashkina, M.M.; Ryabykh, A.A.; Obydennyi, S.I.; Strelkova, O.S.; Polokhov, D.M.; et al. Evolution of platelet function in adult patients with chronic immune thrombocytopenia on romiplostim treatment. Br. J. Haematol. 2019, 187, e38–e42. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-J.; Bai, J.; Guo, Q.-L.; Huang, Z.; Yang, H.; Bai, Y.-Q. Role of platelet function and platelet membrane glycoproteins in children with primary immune thrombocytopenia. Mol. Med. Rep. 2016, 14, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Nishiura, N.; Kashiwagi, H.; Akuta, K.; Hayashi, S.; Kato, H.; Kanakura, Y.; Tomiyama, Y. Reevaluation of platelet function in chronic immune thrombocytopenia: Impacts of platelet size, platelet-associated anti-αIIbβ3 antibodies and thrombopoietin receptor agonists. Br. J. Haematol. (Pre-print) 2020. [Google Scholar] [CrossRef]
- van Bladel, E.R.; Laarhoven, A.G.; van der Heijden, L.B.; Heitink-Pollé, K.M.; Porcelijn, L.; van der Schoot, C.E.; de Haas, M.; Roest, M.; Vidarsson, G.; de Groot, P.G.; et al. Functional platelet defects in children with severe chronic ITP as tested with 2 novel assays applicable for low platelet counts. Blood 2014, 123, 1556–1563. [Google Scholar] [CrossRef] [Green Version]
- Panzer, S.; Höcker, L.; Rieger, M.; Vormittag, R.; Koren, D.; Dunkler, D.; Pabinger, I. Agonist-inducible platelet activation in chronic idiopathic autoimmune thrombocytopenia. Eur. J. Haematol. 2007, 79, 198–204. [Google Scholar] [CrossRef]
- Lyu, M.E.; Li, Y.; Lyu, C.C.; Liu, W.J.; Guan, Y.; Wang, S.X.; Yang, R.C. [Relative analysis of platelet activation with bleeding risk in patients with primary immune thrombocytopenia]. Zhonghua Xue Ye Xue Za Zhi 2017, 38, 33–38. [Google Scholar]
- Connor, D.E.; Ma, D.D.F.; Joseph, J.E. Flow cytometry demonstrates differences in platelet reactivity and microparticle formation in subjects with thrombocytopenia or thrombocytosis due to primary haematological disorders. Thromb. Res. 2013, 132, 572–577. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [Green Version]
- Durrant, T.N.; van den Bosch, M.T.; Hers, I. Integrin α(IIb)β(3) outside-in signaling. Blood 2017, 130, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.P.; Herbert, J.M.J.; Pollitt, A.Y. GPVI and CLEC-2 in hemostasis and vascular integrity. J. Thromb. Haemost. JTH 2010, 8, 1456–1467. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Chauhan, A.K.; Yang, J.J.; De Meyer, S.F.; Kollnberger, M.; Wakefield, T.W.; Lammle, B.; Massberg, S.; Wagner, D.D. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011, 117, 1400–1407. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, Y.; Asazuma, N.; Suzuki-Inoue, K.; Berndt, M.C. Platelet GPIb-IX-V-dependent signaling. J. Thromb. Haemost. JTH 2005, 3, 1745–1751. [Google Scholar] [CrossRef]
- Senis, Y.A.; Mazharian, A.; Mori, J. Src family kinases: At the forefront of platelet activation. Blood 2014, 124, 2013–2024. [Google Scholar] [CrossRef] [Green Version]
- Lian, L.; Wang, Y.; Draznin, J.; Eslin, D.; Bennett, J.S.; Poncz, M.; Wu, D.; Abrams, C.S. The relative role of PLC and PI3K in platelet activation. Blood 2005, 106, 110–117. [Google Scholar] [CrossRef]
- Cunningham, M.; McIntosh, K.; Bushell, T.; Sloan, G.; Plevin, R. Proteinase-activated receptors (PARs) as targets for antiplatelet therapy. Biochem. Soc. Trans. 2016, 44, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arter. Thromb.Vasc.Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Canault, M.; Ghalloussi, D.; Grosdidier, C.; Guinier, M.; Perret, C.; Chelghoum, N.; Germain, M.; Raslova, H.; Peiretti, F.; Morange, P.E.; et al. Human CalDAG-GEFI gene (RASGRP2) mutation affects platelet function and causes severe bleeding. J. Exp. Med. 2014, 211, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Cook, A.A.; Deng, W.; Ren, J.; Li, R.; Sondek, J.; Bergmeier, W. Calcium-induced structural rearrangements release autoinhibition in the Rap-GEF, CalDAG-GEFI. J. Biol. Chem. 2018, 293, 8521–8529. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, M. Inherited Disorders of Platelet Function. In Platelets; Elsevier: Amsterdam, Netherlands, 2019; pp. 877–904. [Google Scholar]
- Kannan, M.; Ahmad, F.; Yadav, B.K.; Kumar, R.; Choudhry, V.P.; Saxena, R. Molecular defects in ITGA2B and ITGB3 genes in patients with Glanzmann thrombasthenia. J. Thromb. Haemost. 2009, 7, 1878–1885. [Google Scholar] [CrossRef]
- Nurden, A.T.; Pillois, X.; Wilcox, D.A. Glanzmann thrombasthenia: State of the art and future directions. Semin. Thromb. Hemost. 2013, 39, 642–655. [Google Scholar]
- Nurden, A.T. Acquired Glanzmann thrombasthenia: From antibodies to anti-platelet drugs. Blood Rev. 2019, 36, 10–22. [Google Scholar] [CrossRef]
- Niessner, H.; Clemetson, K.J.; Panzer, S.; Mueller-Eckhardt, C.; Santoso, S.; Bettelheim, P. Acquired thrombasthenia due to GPIIb/IIIa-specific platelet autoantibodies. Blood 1986, 68, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Spangenberg, P.; Kirchmaier, C.M.; Schirmer, A.; Meyer, M.; Breddin, H.K. Functional studies on platelets of a patient with an acquired disorder of platelet function associated with autoantibodies against membrane glycoprotein IIb/IIIa complex. Thromb. Res. 1993, 69, 435–442. [Google Scholar] [CrossRef]
- Akuta, K.; Kashiwagi, H.; Yujiri, T.; Nishiura, N.; Morikawa, Y.; Kato, H.; Honda, S.; Kanakura, Y.; Tomiyama, Y. A unique phenotype of acquired Glanzmann thrombasthenia due to non-function-blocking anti-αIIbβ3 autoantibodies. J. Thromb. Haemost. 2019, 17, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillan, R.; Lopez-Dee, J.; Bowditch, R. Clonal restriction of platelet-associated anti-GPIIb/IIIa autoantibodies in patients with chronic ITP. Thromb. Haemost. 2001, 85, 821–823. [Google Scholar]
- Li, J.; Van Der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sullivan, J.A.; Ni, H. Pathophysiology of immune thrombocytopenia. Curr. Opin. Hematol. 2018, 25, 373–381. [Google Scholar] [CrossRef]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachs, U.J. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP): Complement activation in ITP. Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef]
- Tomita, E.; Akatsuka, J.I.; Kokubun, Y. Differential diagnosis of various thrombocytopenias in childhood by analysis of platelet volume. Pediatr. Res. 1980, 14, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Sveshnikova, A.N.; Balatskiy, A.V.; Demianova, A.S.; Shepelyuk, T.O.; Shakhidzhanov, S.S.; Balatskaya, M.N.; Pichugin, A.V.; Ataullakhanov, F.I.; Panteleev, M.A. Systems biology insights into the meaning of the platelet’s dual-receptor thrombin signaling. J. Thromb. Haemost. 2016, 14, 2045–2057. [Google Scholar] [CrossRef]
- Sveshnikova, A.N.; Ataullakhanov, F.I.; Panteleev, M.A. Compartmentalized calcium signaling triggers subpopulation formation upon platelet activation through PAR1. Mol. Biosyst. 2015, 11, 1052–1060. [Google Scholar] [CrossRef]
- Shakhidzhanov, S.S.; Shaturny, V.I.; Panteleev, M.A.; Sveshnikova, A.N. Modulation and pre-amplification of PAR1signaling by ADP acting via the P2Y12receptor during platelet subpopulation formation. Biochim. Et Biophys. Acta Gen. Subj. 2015, 1850, 2518–2529. [Google Scholar] [CrossRef]
- Martyanov, A.; Balabin, F.A.; Dunster, J.L.; Panteleev, M.A.; Gibbins, J.; Sveshnikova, A.N. Diffusional and Chemical Control in the Tyrosine Kinase Network of Platelet CLEC-2 Signalling. Biorxiv 2019, 529859. [Google Scholar]
- Jackson, S.P.; Nesbitt, W.S.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. JTH 2009, 7 (Suppl. 1), 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Woollard, K.J.; Thomas, S.; Oxley, D.; Jackson, S.P. Conversion of platelets from a proaggregatory to a proinflammatory adhesive phenotype: Role of PAF in spatially regulating neutrophil adhesion and spreading. Blood 2007, 110, 1879–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbousset, R.; Mezouar, S.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Involvement of neutrophils in thrombus formation in living mice. Pathol. Biol. 2014, 62, 1–9. [Google Scholar] [CrossRef]
- Morozova, D.S.; Martyanov, A.A.; Korobkina, J.-J.D.; Obydennyy, S.I.; Sveshnikova, A.N. Towards a quantitative ex vivo assessment of the innate immune system participation in thrombus formation. In Proceedings of the ECTH 2019 Abstract Book, Glasgow, UK, 2–4 October 2019; Volume 1, p. 41. [Google Scholar]
- Alberio, L.; Ravanat, C.; Hechler, B.; Mangin, P.H.; Lanza, F.; Gachet, C. Delayed-onset of procoagulant signalling revealed by kinetic analysis of COAT platelet formation. Thromb. Haemost. 2017, 117, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Assinger, A.; Volf, I.; Schmid, D. A novel, rapid method to quantify intraplatelet calcium dynamics by ratiometric flow cytometry. PLoS ONE 2015, 10, e0122527. [Google Scholar] [CrossRef] [Green Version]
- Abbasian, N.; Millington-Burgess, S.L.; Chabra, S.; Malcor, J.-D.; Harper, M.T. Supramaximal calcium signaling triggers procoagulant platelet formation. Blood Adv. 2020, 4, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Shattil, S.J.; Hoxie, J.A.; Cunningham, M.; Brass, L.F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J. Biol. Chem. 1985, 260, 11107–11114. [Google Scholar]
- Filkova, A.A.; Martyanov, A.A.; Garzon Dasgupta, A.K.; Panteleev, M.A.; Sveshnikova, A.N. Quantitative dynamics of reversible platelet aggregation: Mathematical modelling and experiments. Sci. Rep. 2019, 9, 6217. [Google Scholar] [CrossRef]
- Chen, Y.; Ju, L.A.; Zhou, F.; Liao, J.; Xue, L.; Su, Q.P.; Jin, D.; Yuan, Y.; Lu, H.; Jackson, S.P.; et al. An integrin αIIbβ3 intermediate affinity state mediates biomechanical platelet aggregation. Nat. Mater. 2019, 18, 760–769. [Google Scholar] [CrossRef]
- Jones, C.I.; Garner, S.F.; Angenent, W.; Bernard, A.; Berzuini, C.; Burns, P.; Farndale, R.W.; Hogwood, J.; Rankin, A.; Stephens, J.C.; et al. Mapping the platelet profile for functional genomic studies and demonstration of the effect size of the GP6 locus. J. Thromb. Haemost. 2007, 5, 1756–1765. [Google Scholar] [CrossRef] [Green Version]
- Furihata, K.; Clemetson, K.J.; Deguchi, H.; Kunicki, T.J.; Drummond, G.R.; Cai, H.; Davis, M.E.; Harrison, D.G. Variation in Human Platelet Glycoprotein VI Content Modulates Glycoprotein VI–Specific Prothrombinase Activity. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Takagi, S.; Iwai, N.; Baba, S.; Mannami, T.; Ono, K.; Tanaka, C.; Miyata, T.; Miyazaki, S.; Nonogi, H.; Goto, Y. A GPVI polymorphism is a risk factor for myocardial infarction in Japanese. Atherosclerosis 2002, 165, 397–398. [Google Scholar] [CrossRef]
- Trifiro, E.; Williams, S.A.; Cheli, Y.; Furihata, K.; Pulcinelli, F.M.; Nugent, D.J.; Kunicki, T.J. The low-frequency isoform of platelet glycoprotein VIb attenuates ligand-mediated signal transduction but not receptor expression or ligand binding. Blood 2009, 114, 1893–1899. [Google Scholar] [CrossRef] [Green Version]
- Massberg, S.; Konrad, I.; Bültmann, A.; Schulz, C.; Münch, G.; Peluso, M.; Lorenz, M.; Schneider, S.; Besta, F.; Müller, I.; et al. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 2004, 18, 397–399. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Karunakaran, D.; Shen, Y.; Arthur, J.F.; Andrews, R.K.; Berndt, M.C. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J. Thromb. Haemost. 2007, 5, 1530–1537. [Google Scholar] [CrossRef]
- Södergren, A.L.; Ramström, S. Platelet subpopulations remain despite strong dual agonist stimulation and can be characterised using a novel six-colour flow cytometry protocol. Sci. Rep. 2018, 8, 1441. [Google Scholar] [CrossRef] [Green Version]
- Porcelijn, L.; Huiskes, E.; Oldert, G.; Schipperus, M.; Zwaginga, J.J.; de Haas, M. Detection of platelet autoantibodies to identify immune thrombocytopenia: State of the art. Br. J. Haematol. 2018, 182, 423–426. [Google Scholar] [CrossRef]
- Hamidpour, M.; Khalili, G.; Tajic, N.; Shamsian, B.B.S.; Hamidpour, R. Comparative of three methods (ELIZA, MAIPA and flow cytometry) to determine anti-platelet antibody in children with ITP. Am. J. Blood Res. 2014, 4, 86–92. [Google Scholar]
- Goerge, T.; Ho-Tin-Noe, B.; Carbo, C.; Benarafa, C.; Remold-O’Donnell, E.; Zhao, B.-Q.; Cifuni, S.M.; Wagner, D.D. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008, 111, 4958–4964. [Google Scholar] [CrossRef] [Green Version]
- Ho-Tin-Noé, B.; Boulaftali, Y.; Camerer, E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018, 131, 277–288. [Google Scholar] [CrossRef]
- Gros, A.; Syvannarath, V.; Lamrani, L.; Ollivier, V.; Loyau, S.; Goerge, T.; Nieswandt, B.; Jandrot-Perrus, M.; Ho-Tin-Noé, B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood 2015, 126, 1017–1026. [Google Scholar] [CrossRef]
- Deppermann, C.; Kraft, P.; Volz, J.; Schuhmann, M.K.; Beck, S.; Wolf, K.; Stegner, D.; Stoll, G.; Nieswandt, B. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood 2017, 129, 1702–1706. [Google Scholar] [CrossRef]
- Nechipurenko, D.Y.; Receveur, N.; Yakimenko, A.O.; Shepelyuk, T.O.; Yakusheva, A.A.; Kerimov, R.R.; Obydennyy, S.I.; Eckly, A.; Léon, C.; Gachet, C.; et al. Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 37–47. [Google Scholar] [CrossRef]
- Kuharsky, A.L.; Fogelson, A.L. Surface-mediated control of blood coagulation: The role of binding site densities and platelet deposition. Biophys. J. 2001, 80, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Flamm, M.H.; Colace, T.V.; Chatterjee, M.S.; Jing, H.; Zhou, S.; Jaeger, D.; Brass, L.F.; Sinno, T.; Diamond, S.L. Multiscale prediction of patient-specific platelet function under flow. Blood 2012, 120, 190–198. [Google Scholar] [CrossRef]
- Stalker, T.J.; Traxler, E.A.; Wu, J.; Wannemacher, K.M.; Cermignano, S.L.; Voronov, R.; Diamond, S.L.; Brass, L.F. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 2013, 121, 1875–1885. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, M.; Panteleev, M.; Sveshnikova, A.; Ignatova, A.; Martyanov, A.; Zharkov, P.; Fedorova, D. Novel continuous flow cytometry approach to ITP diagnosis is capable of identifying subtypes of the disease and moving forward its understanding. In Proceedings of the ECTH 2019 Abstract Book, Glasgow, UK, 2–4 October 2019; Volume 1, p. 255. [Google Scholar]
- Morozova, D.; Demianova, A.; Canault, M.; Panteleev, M.; Alessie, M.-C.; Sveshnikova, A. Identification of Platelet Intracellular Signaling Pathways Controlling the Reversible and Irreversible Platelet Aggregation. In Proceedings of the Res Pract Thromb Haemost, Dublin, Ireland, 18–21 July 2018; Volume 2, p. 46. [Google Scholar]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar]
- Schoenmakers, T.J.; Visser, G.J.; Flik, G.; Theuvenet, A.P. CHELATOR: An improved method for computing metal ion concentrations in physiological solutions. BioTechniques 1992, 12, 870–874. [Google Scholar]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Stefanini, L.; Paul, D.S.; Robledo, R.F.; Chan, E.R.; Getz, T.M.; Campbell, R.A.; Kechele, D.O.; Casari, C.; Piatt, R.; Caron, K.M.; et al. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J. Clin. Investig. 2015, 125, 1419–1432. [Google Scholar] [CrossRef] [Green Version]
- Stefanini, L.; Bergmeier, W. RAP1-GTPase signaling and platelet function. J. Mol. Med. 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Siljander, P.R.-M.; Munnix, I.C.A.; Smethurst, P.A.; Deckmyn, H.; Lindhout, T.; Ouwehand, W.H.; Farndale, R.W.; Heemskerk, J.W.M. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood 2004, 103, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- McInnes, L.; Healy, J.; Astels, S. hdbscan: Hierarchical density based clustering. J. Open Source Softw. 2017, 2, 205. [Google Scholar] [CrossRef]
- Moulavi, D.; A Jaskowiak, P.; Campello, R.; Zimek, A.; Sander, J. Density-Based Clustering Validation. In Proceedings of the 14th SIAM Interantional conference on Data Mining (SDM), Philadelphia, PA, USA, 22 January 2014; Volume 1, pp. 839–847. [Google Scholar]


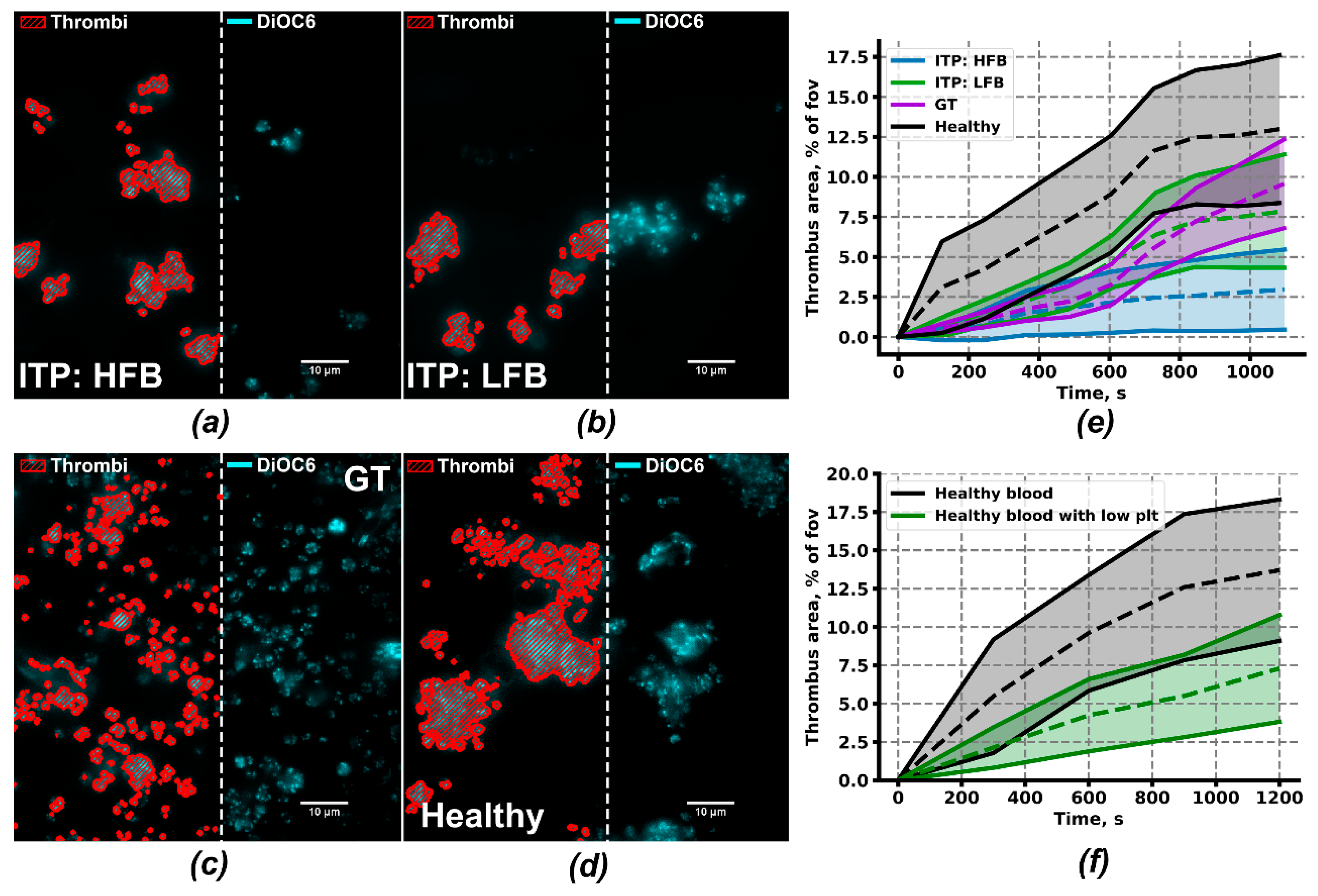
| Parameter | ITP:HFB | ITP:LFB | GT | Low Plt1 | Healthy |
|---|---|---|---|---|---|
| Fraction of thrombus area, % | 3 ± 2***2,3 | 10 ± 4* | 10 ± 3* | 7 ± 3** | 15 ± 4 |
| Fraction of continuously growing individual thrombi | 72 ± 26ns | 44 ± 15 ns | 59 ± 27 ns | 60 ± 14 ns | 74 ± 36 |
| Maximal thrombus growth, fold change | 3.5 ± 0.9 ns | 1.5 ± 0.5** | 1.5 ± 0.4** | 1.47 ± 0.29* | 2.7 ± 0.3 |
| Growth velocity, %/min | 25 ± 12 ns | 9 ± 5 ns | 7 ± 5 ns | 7 ± 4* | 17 ± 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martyanov, A.A.; Morozova, D.S.; Sorokina, M.A.; Filkova, A.A.; Fedorova, D.V.; Uzueva, S.S.; Suntsova, E.V.; Novichkova, G.A.; Zharkov, P.A.; Panteleev, M.A.; et al. Heterogeneity of Integrin αIIbβ3 Function in Pediatric Immune Thrombocytopenia Revealed by Continuous Flow Cytometry Analysis. Int. J. Mol. Sci. 2020, 21, 3035. https://doi.org/10.3390/ijms21093035
Martyanov AA, Morozova DS, Sorokina MA, Filkova AA, Fedorova DV, Uzueva SS, Suntsova EV, Novichkova GA, Zharkov PA, Panteleev MA, et al. Heterogeneity of Integrin αIIbβ3 Function in Pediatric Immune Thrombocytopenia Revealed by Continuous Flow Cytometry Analysis. International Journal of Molecular Sciences. 2020; 21(9):3035. https://doi.org/10.3390/ijms21093035
Chicago/Turabian StyleMartyanov, Alexey A., Daria S. Morozova, Maria A. Sorokina, Aleksandra A. Filkova, Daria V. Fedorova, Selima S. Uzueva, Elena V. Suntsova, Galina A. Novichkova, Pavel A. Zharkov, Mikhail A. Panteleev, and et al. 2020. "Heterogeneity of Integrin αIIbβ3 Function in Pediatric Immune Thrombocytopenia Revealed by Continuous Flow Cytometry Analysis" International Journal of Molecular Sciences 21, no. 9: 3035. https://doi.org/10.3390/ijms21093035
APA StyleMartyanov, A. A., Morozova, D. S., Sorokina, M. A., Filkova, A. A., Fedorova, D. V., Uzueva, S. S., Suntsova, E. V., Novichkova, G. A., Zharkov, P. A., Panteleev, M. A., & Sveshnikova, A. N. (2020). Heterogeneity of Integrin αIIbβ3 Function in Pediatric Immune Thrombocytopenia Revealed by Continuous Flow Cytometry Analysis. International Journal of Molecular Sciences, 21(9), 3035. https://doi.org/10.3390/ijms21093035






