The Effect of Size, Maturation, Global Asphyxia, Cerebral Ischemia, and Therapeutic Hypothermia on the Pharmacokinetics of High-Dose Recombinant Erythropoietin in Fetal Sheep
Abstract
1. Introduction
2. Results
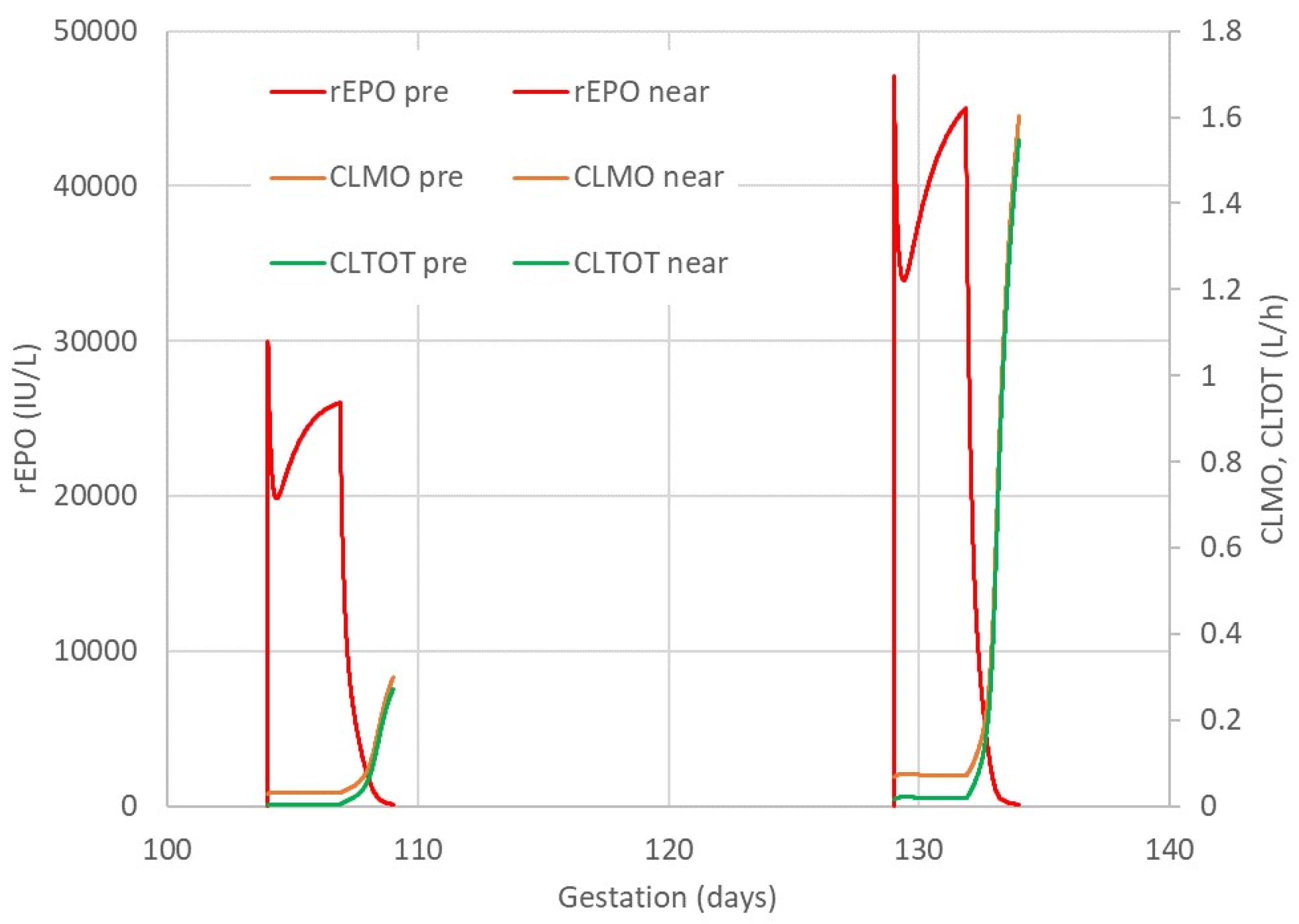
2.1. rEPO Plasma Concentration Profile
2.2. rEPO Bolus Treatment in Preterm Fetal Sheep
2.3. rEPO Pharmacokinetics
2.4. Fetal Hematological Variables
2.5. Post-Mortem Findings
3. Discussion
3.1. Body Size and rEPO Pharmacokinetics
3.2. Gestation Age and rEPO Pharmacokinetics
3.3. The Effect of Hypoxia-Ischemia on rEPO Pharmacokinetics
3.4. Therapeutic Hypothermia and rEPO Pharmacokinetics
3.5. rEPO Treatment-Associated Changes in rEPO Elimination
3.6. Higher rEPO Dose Required in Fetal Sheep Compared to Human Neonates
3.7. Conclusions
4. Methods
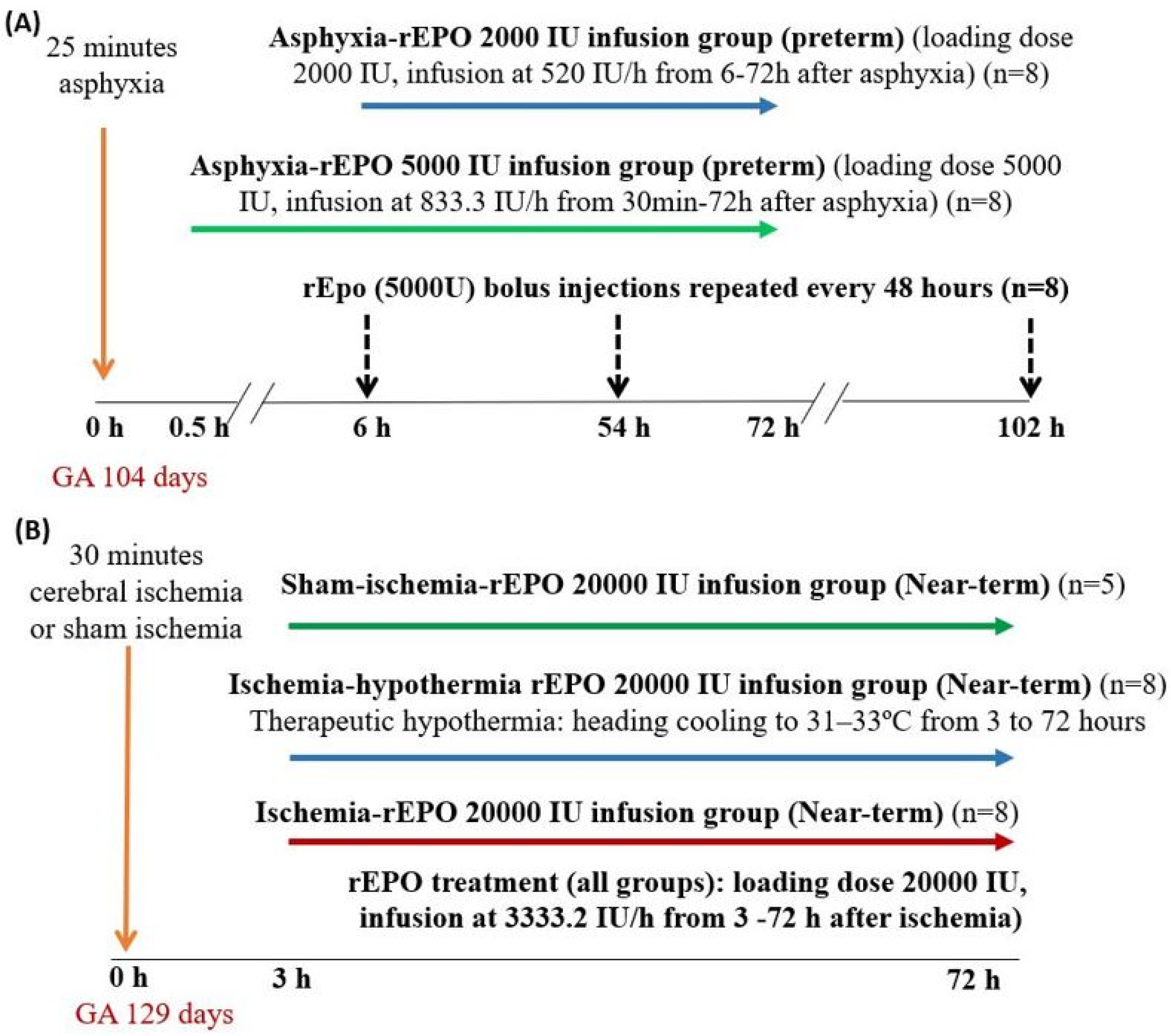
4.1. Studies in Preterm Fetal Sheep
4.2. Studies in Near-Term Fetal Sheep
4.3. Fetal Arterial Blood Sampling and rEPO Concentration Measurement
4.4. Pharmacokinetic Analysis
4.5. Histology
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohlsson, A.; Aher, S.M. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 2014, 4, CD004863. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Comstock, B.A.; Heagerty, P.J.; Mayock, D.E.; Goodman, A.M.; Hauge, S.; Gonzalez, F.; Wu, Y.W. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial—Background, Aims, and Study Protocol. Neonatology 2018, 113, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.; Hussain, A. Erythropoietin in perinatal hypoxic-ischemic encephalopathy: A systematic review and meta-analysis. J. Perinat. Med. 2019, 47, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Mathur, A.M.; Chang, T.; McKinstry, R.C.; Mulkey, S.B.; Mayock, D.E.; Van Meurs, K.P.; Rogers, E.E.; Gonzalez, F.F.; Comstock, B.A.; et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: A phase II trial. Pediatrics 2016, 137, e20160191. [Google Scholar] [CrossRef]
- Robinson, S.; Corbett, C.J.; Winer, J.L.; Chan, L.A.S.; Maxwell, J.R.; Anstine, C.V.; Yellowhair, T.R.; Andrews, N.A.; Yang, Y.; Sillerud, L.O.; et al. Neonatal erythropoietin mitigates impaired gait, social interaction and diffusion tensor imaging abnormalities in a rat model of prenatal brain injury. Exp. Neurol. 2018, 302, 1–13. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Corbett, C.J.; Firl, D.J.; Robinson, S. Postnatal Erythropoietin Mitigates Impaired Cerebral Cortical Development Following Subplate Loss from Prenatal Hypoxia-Ischemia. Cereb. Cortex 2015, 25, 2683–2695. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Fraser, M.; Yuill, C.A.; Bennet, L.; Gunn, A.J. Non-additive effects of adjunct erythropoietin therapy with therapeutic hypothermia after global cerebral ischaemia in near-term fetal sheep. J. Physiol. 2020, 598, 999–1015. [Google Scholar] [CrossRef]
- Oorschot, D.E.; Sizemore, R.J.; Amer, A.R. Treatment of Neonatal Hypoxic-Ischemic Encephalopathy with Erythropoietin Alone, and Erythropoietin Combined with Hypothermia: History, Current Status, and Future Research. Int. J. Mol. Sci. 2020, 21, 1487. [Google Scholar] [CrossRef]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.A.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef]
- Kellert, B.A.; McPherson, R.J.; Juul, S.E. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr. Res. 2007, 61, 451–455. [Google Scholar] [CrossRef]
- Statler, P.A.; McPherson, R.J.; Bauer, L.A.; Kellert, B.A.; Juul, S.E. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr. Res. 2007, 61, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; McPherson, R.J.; Bauer, L.A.; Ledbetter, K.J.; Gleason, C.A.; Mayock, D.E. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: Pharmacokinetics and safety. Pediatrics 2008, 122, 383–391. [Google Scholar] [CrossRef]
- Frymoyer, A.; Juul, S.E.; Massaro, A.N.; Bammler, T.K.; Wu, Y.W. High-dose erythropoietin population pharmacokinetics in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Pediatr. Res. 2017, 81, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Bauer, L.A.; Ballard, R.A.; Ferriero, D.M.; Glidden, D.V.; Mayock, D.E.; Chang, T.; Durand, D.J.; Song, D.; Bonifacio, S.L.; et al. Erythropoietin for neuroprotection in neonatal encephalopathy: Safety and pharmacokinetics. Pediatrics 2012, 130, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, H.; El-Mashad, A.R.; El-Bahrawy, H.; El-Gohary, T.; El-Barbary, A.; Aly, H. Human recombinant erythropoietin in asphyxia neonatorum: Pilot trial. Pediatrics 2010, 125, e1135–e1142. [Google Scholar] [CrossRef] [PubMed]
- El Shimi, M.S.; Awad, H.A.; Hassanein, S.M.; Gad, G.I.; Imam, S.S.; Shaaban, H.A.; El Maraghy, M.O. Single dose recombinant erythropoietin versus moderate hypothermia for neonatal hypoxic ischemic encephalopathy in low resource settings. J. Matern. Fetal Neonatal Med. 2014, 27, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Natalucci, G.; Latal, B.; Koller, B.; Ruegger, C.; Sick, B.; Held, L.; Bucher, H.U.; Fauchere, J.C.; Swiss, E.P.O. Neuroprotection Trial Group. Effect of Early Prophylactic High-Dose Recombinant Human Erythropoietin in Very Preterm Infants on Neurodevelopmental Outcome at 2 Years: A Randomized Clinical Trial. JAMA 2016, 315, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, R.; Widness, J.A.; Yan, X.; Schmidt, R.L.; Veng-Pedersen, P.; An, G. A mechanism-based population pharmacokinetics model of erythropoietin in premature infants and healthy adults following multiple intravenous doses. J. Clin. Pharmacol. 2019, 59, 835–846. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Fraser, M.; Galinsky, R.; Bennet, L.; Gunn, A.J. Partial white and grey matter protection with prolonged infusion of recombinant human erythropoietin after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 2017, 37, 1080–1094. [Google Scholar] [CrossRef]
- Davidson, J.O.; Wassink, G.; Draghi, V.; Dhillon, S.K.; Bennet, L.; Gunn, A.J. Limited benefit of slow rewarming after cerebral hypothermia for global cerebral ischemia in near-term fetal sheep. J. Cereb. Blood Flow Metab. 2019, 39, 2246–2257. [Google Scholar] [CrossRef]
- Bennet, L.; Galinsky, R.; Draghi, V.; Lear, C.A.; Davidson, J.O.; Unsworth, C.P.; Gunn, A.J. Time and sex dependent effects of magnesium sulphate on post-asphyxial seizures in preterm fetal sheep. J. Physiol. 2018, 596, 6079–6092. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Lear, C.A.; Beacom, M.J.; Ikeda, T.; Gunn, A.J.; Bennet, L. Evolving changes in fetal heart rate variability and brain injury after hypoxia-ischaemia in preterm fetal sheep. J. Physiol. 2018, 596, 6093–6104. [Google Scholar] [CrossRef] [PubMed]
- D’Cunha, R.; Schmidt, R.; Widness, J.A.; Mock, D.M.; Yan, X.; Cress, G.A.; Kuruvilla, D.; Veng-Pedersen, P.; An, G. Target-mediated disposition population pharmacokinetics model of erythropoietin in premature neonates following multiple intravenous and subcutaneous dosing regimens. Eur. J. Pharm. Sci. 2019, 138, 105013. [Google Scholar] [CrossRef]
- Anderson, B.J.; Allegaert, K.; Holford, N.H. Population clinical pharmacology of children: Modelling covariate effects. Eur. J. Pediatr. 2006, 165, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Allegaert, K.; Holford, N.H. Population clinical pharmacology of children: General principles. Eur. J. Pediatr. 2006, 165, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H.G.; Anderson, B.J. Allometric size: The scientific theory and extension to normal fat mass. Eur. J. Pharm. Sci. 2017, 109s, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.H. A size standard for pharmacokinetics. Clin. Pharmacokinet. 1996, 30, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Holford, N.; Heo, Y.A.; Anderson, B. A pharmacokinetic standard for babies and adults. J. Pharm. Sci. 2013, 102, 2941–2952. [Google Scholar] [CrossRef]
- Soo, J.Y.; Wiese, M.D.; Berry, M.J.; Morrison, J.L. Does poor fetal growth influence the extent of fetal exposure to maternal medications? Pharmacol. Res. 2018, 130, 74–84. [Google Scholar] [CrossRef]
- Alcorn, J.; McNamara, P.J. Ontogeny of hepatic and renal systemic clearance pathways in infants: Part I. Clin. Pharmacokinet. 2002, 41, 959–998. [Google Scholar] [CrossRef]
- Chen, N.; Aleksa, K.; Woodland, C.; Rieder, M.; Koren, G. Ontogeny of drug elimination by the human kidney. Pediatr. Nephrol. 2006, 21, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Widness, J.A.; Veng-Pedersen, P.; Schmidt, R.L.; Lowe, L.S.; Kisthard, J.A.; Peters, C. In vivo 125I-erythropoietin pharmacokinetics are unchanged after anesthesia, nephrectomy and hepatectomy in sheep. J. Pharmacol. Exp. Ther. 1996, 279, 1205–1210. [Google Scholar]
- Yoon, W.H.; Park, S.J.; Kim, I.C.; Lee, M.G. Pharmacokinetics of recombinant human erythropoietin in rabbits and 3/4 nephrectomized rats. Res. Commun. Mol. Pathol. Pharmacol. 1997, 96, 227–240. [Google Scholar] [PubMed]
- Jensen, J.D.; Jensen, L.W.; Madsen, J.K.; Poulsen, L. The metabolism of erythropoietin in liver cirrhosis patients compared with healthy volunteers. Eur. J. Haematol. 1995, 54, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kamiyama, H.; Okazaki, A.; Kumaki, K.; Kato, Y.; Sugiyama, Y. Mechanism for the nonlinear pharmacokinetics of erythropoietin in rats. J. Pharmacol. Exp. Ther. 1997, 283, 520–527. [Google Scholar]
- Walrafen, P.; Verdier, F.; Kadri, Z.; Chretien, S.; Lacombe, C.; Mayeux, P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood 2005, 105, 600–608. [Google Scholar] [CrossRef]
- Woo, S.; Krzyzanski, W.; Jusko, W.J. Target-mediated pharmacokinetic and pharmacodynamic model of recombinant human erythropoietin (rHuEPO). J. Pharmacokinet. Pharmacodyn. 2007, 34, 849–868. [Google Scholar] [CrossRef]
- Chapel, S.; Veng-Pedersen, P.; Hohl, R.J.; Schmidt, R.L.; McGuire, E.M.; Widness, J.A. Changes in erythropoietin pharmacokinetics following busulfan-induced bone marrow ablation in sheep: Evidence for bone marrow as a major erythropoietin elimination pathway. J. Pharmacol. Exp. Ther. 2001, 298, 820–824. [Google Scholar]
- Nalbant, D.; Saleh, M.; Goldman, F.D.; Widness, J.A.; Veng-Pedersen, P. Evidence of receptor-mediated elimination of erythropoietin by analysis of erythropoietin receptor mRNA expression in bone marrow and erythropoietin clearance during anemia. J. Pharmacol. Exp. Ther. 2010, 333, 528–532. [Google Scholar] [CrossRef]
- Juul, S.E.; Yachnis, A.T.; Christensen, R.D. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum. Dev. 1998, 52, 235–249. [Google Scholar] [CrossRef]
- David, R.B.; Lim, G.B.; Moritz, K.M.; Koukoulas, I.; Wintour, E.M. Quantitation of the mRNA levels of Epo and EpoR in various tissues in the ovine fetus. Mol. Cell. Endocrinol. 2002, 188, 207–218. [Google Scholar] [CrossRef]
- Gal, P.; Boer, H.R.; Toback, J.; Wells, T.J.; Erkan, N.V. Effect of asphyxia on theophylline clearance in newborns. South. Med. J. 1982, 75, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Gal, P.; Toback, J.; Erkan, N.V.; Boer, H.R. The influence of asphyxia on phenobarbital dosing requirements in neonates. Dev. Pharmacol. Ther. 1984, 7, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Spandou, E.; Papoutsopoulou, S.; Soubasi, V.; Karkavelas, G.; Simeonidou, C.; Kremenopoulos, G.; Guiba-Tziampiri, O. Hypoxia-ischemia affects erythropoietin and erythropoietin receptor expression pattern in the neonatal rat brain. Brain Res. 2004, 1021, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Miller, R.H.; Robinson, S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic-ischemic injury. J. Neurosurg. Pediatr. 2010, 6, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Melendez, M.; Yan, E.; Walker, D.W. Expression of erythropoietin and its receptor in the brain of late-gestation fetal sheep, and responses to asphyxia caused by umbilical cord occlusion. Dev. Neurosci. 2005, 27, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Poloyac, S.M. The effect of therapeutic hypothermia on drug metabolism and response: Cellular mechanisms to organ function. Expert Opin. Drug Metab. Toxicol. 2011, 7, 803–816. [Google Scholar] [CrossRef]
- Sarkar, S.; Donn, S.M.; Bapuraj, J.R.; Bhagat, I.; Barks, J.D. Distribution and severity of hypoxic-ischaemic lesions on brain MRI following therapeutic cooling: Selective head versus whole body cooling. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F335–F339. [Google Scholar] [CrossRef]
- Iwai, M.; Stetler, R.A.; Xing, J.; Hu, X.; Gao, Y.; Zhang, W.; Chen, J.; Cao, G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010, 41, 1032–1037. [Google Scholar] [CrossRef]
- Tankiewicz-Kwedlo, A.; Hermanowicz, J.; Surazynski, A.; Rozkiewicz, D.; Pryczynicz, A.; Domaniewski, T.; Pawlak, K.; Kemona, A.; Pawlak, D. Erythropoietin accelerates tumor growth through increase of erythropoietin receptor (EpoR) as well as by the stimulation of angiogenesis in DLD-1 and Ht-29 xenografts. Mol. Cell. Biochem. 2016, 421, 1–18. [Google Scholar] [CrossRef]
- El-Komy, M.H.; Schmidt, R.L.; Widness, J.A.; Veng-Pedersen, P. Differential pharmacokinetic analysis of in vivo erythropoietin receptor interaction with erythropoietin and continuous erythropoietin receptor activator in sheep. Biopharm. Drug Dispos. 2011, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Moritz, K.M.; Lim, G.B.; Wintour, E.M. Developmental regulation of erythropoietin and erythropoiesis. Am. J. Physiol. 1997, 273, R1829–R1844. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.M.; Varner, M.W. Principles of Pharmacokinetics in the Pregnant Woman and Fetus. Clin. Perinatol. 2019, 46, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Widness, J.A.; Malone, T.A.; Mufson, R.A. Impermeability of the ovine placenta to 35S-recombinant erythropoietin. Pediatr. Res. 1989, 25, 649–651. [Google Scholar] [CrossRef]
- Widness, J.A.; Sawyer, S.T.; Schmidt, R.L.; Chestnut, D.H. Lack of maternal to fetal transfer of 125I-labelled erythropoietin in sheep. J. Dev. Physiol. 1991, 15, 139–143. [Google Scholar]
- Saito-Benz, M.; Flanagan, P.; Berry, M.J. Management of anaemia in pre-term infants. Br. J. Haematol. 2020, 188, 354–366. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Juul, S.E.; McPherson, R.J.; Farrell, F.X.; Jolliffe, L.; Ness, D.J.; Gleason, C.A. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol. Neonate 2004, 85, 138–144. [Google Scholar] [CrossRef]
- Karlsson, M.O.; Jonsson, E.N.; Wiltse, C.G.; Wade, J.R. Assumption testing in population pharmacokinetic models: Illustrated with an analysis of moxonidine data from congestive heart failure patients. J. Pharmacokinet. Biopharm. 1998, 26, 207–246. [Google Scholar] [CrossRef]
- Bauer, R.J.; Boeckmann, A.; Beal, S.; Sheiner, L.B. (Eds.) NONMEM User’s Guides. (1989–2019); Icon Development Solutions: Ellicott City, MD, USA, 2019. [Google Scholar]
- Holford, N.H.G. Wings for NONMEM Version 742 for NONMEM 7.4, 7.3 and 7.2. 2017. Available online: http://wfn.sourceforge.net (accessed on 13 February 2019).
- Holford, N.H.G. The Visual Predictive Check—Superiority to Standard Diagnostic (Rorschach) Plots; PAGE: Pamplona, Spain, 2005; Volume 14. Available online: www.page-meeting.org/?abstract=738 (accessed on 13 February 2019).
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Parke, J.; Holford, N.H.; Charles, B.G. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 1999, 59, 19–29. [Google Scholar] [CrossRef]
- Thai, H.T.; Mentre, F.; Holford, N.H.; Veyrat-Follet, C.; Comets, E. Evaluation of bootstrap methods for estimating uncertainty of parameters in nonlinear mixed-effects models: A simulation study in population pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2014, 41, 15–33. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Description | Units | Original | Bootstrap Average | 2.5% ile | 97.5% ile | RSE |
|---|---|---|---|---|---|---|---|
| CL | rEPO first-order clearance | L/h/70 kg | 0.471 | 0.506 | 0.399 | 0.663 | 15.6% |
| Vmax | rEPO elimination capacity | IU/h/70kg | 4830 | 5342 | 2501 | 11,130 | 49.6% |
| Km | rEPO Km | IU/L | 441 | 560 | 157 | 1394 | 103.5% |
| V1 | rEPO central volume | L/70 kg | 7.67 | 7.98 | 6.49 | 9.98 | 11.3% |
| Q | rEPO distribution clearance | L/h/70 kg | 0.379 | 0.383 | 0.229 | 0.547 | 24.7% |
| V2 | rEPO peripheral volume | L/70 kg | 13.4 | 13.2 | 8.1 | 17.7 | 18.8% |
| SLOPE FO | Slope of maturation for first-order elimination | 1/day | 0.0194 | 0.0156 | −0.0111 | 0.0363 | 81.9% |
| SLOPE MO | Slope of maturation for mixed-order elimination | 1/day | 0.0396 | 0.0371 | 0.0127 | 0.0530 | 34.9% |
| WT0 preterm | Initial weight in preterm | kg | 1.50 | 1.49 | 1.25 | 1.73 | 8.0% |
| WT0 near-term | Initial weight near-term | kg | 3.82 | 3.81 | 3.37 | 4.24 | 5.9% |
| WT slope | Weight slope | kg/d | 0.072 | 0.075 | 0.021 | 0.132 | 40.0% |
| PPV CL | PPV Clearance | . | 0.023 | 0.028 | 0.000 | 0.139 | 134.7% |
| PPV VMAX | PPV Vmax | . | 0.520 | 0.489 | 0.288 | 0.683 | 22.8% |
| PPV km | PPV km | . | 1.08 | 1.04 | 0.583 | 1.46 | 20.5% |
| PPV V1 | PPV V1 | . | 0.211 | 0.151 | 0.00200 | 0.300 | 61.2% |
| PPV WT0 | PPV initial weight | . | 0.046 | 0.081 | 0.000 | 0.191 | 80.5% |
| PPV WTslope | PPV slope of weight | . | 0.727 | 0.597 | 0.007 | 1.263 | 75.3% |
| R12 | Correlation CL with Vmax | . | 0.804 | 0.496 | −0.976 | 1.00 | 127.6% |
| R13 | Correlation CL with km | . | 0.992 | 0.693 | −0.760 | 1.00 | 68.4% |
| R23 | Correlation Vmax with km | . | 0.872 | 0.834 | 0.572 | 0.986 | 28.3% |
| RUV PROP rEPO | Proportional residual error for rEPO | . | 0.211 | 0.203 | 0.160 | 0.244 | 11.0% |
| RUV ADD rEPO | Additive residual error for rEPO | IU/L | 5.08 | 4.92 | 2.56 | 7.96 | 28.0% |
| RUV ADD WT | Additive residual error for post-mortem weight | 0.367 | 0.368 | 0.217 | 0.489 | 19.6% |
| Parameter | Description | Original | Bootstrap Average | 2.5% ile | 97.5% ile | RSE |
|---|---|---|---|---|---|---|
| FV1 ASP | Asphyxia on V1 | 1.030 | 0.982 | 0.773 | 1.210 | 12.0% |
| FV2 ASP | Asphyxia on V2 | 0.419 | 0.480 | 0.174 | 0.886 | 36.6% |
| FQ ASP | Asphyxia on Q | 0.570 | 0.646 | 0.157 | 1.242 | 41.7% |
| FCL ASP | Asphyxia on CL | 1.390 | 1.266 | 0.385 | 2.169 | 35.4% |
| FVM ASP | Asphyxia on Vmax | 2.040 | 2.146 | 0.723 | 4.270 | 45.4% |
| FKM ASP | Asphyxia on km | 2.890 | 3.774 | 0.295 | 19.800 | 110.3% |
| FV1 ISC | Ischemia on V1 | 0.872 | 0.862 | 0.648 | 1.080 | 13.5% |
| FV2 ISC | Ischemia on V2 | 1.200 | 1.174 | 0.889 | 1.685 | 17.9% |
| FQ ISC | Ischemia on Q | 1.790 | 1.749 * | 1.107 | 2.651 | 23.8% |
| FCL ISC | Ischemia on CL | 1.510 | 1.463 * | 1.110 | 1.942 | 14.7% |
| FVM ISC | Ischemia on Vmax | 0.505 | 0.543 | 0.171 | 0.984 | 43.9% |
| FKM ISC | Ischemia on km | 0.850 | 0.967 | 0.224 | 2.888 | 68.8% |
| FV1 COOL | Hypothermia on V1 | 1.320 | 1.265 | 0.886 | 2.083 | 25.3% |
| FV2 COOL | Hypothermia on V2 | 1.170 | 1.169 | 0.731 | 1.820 | 20.5% |
| FQ COOL | Hypothermia on Q | 1.330 | 1.337 | 0.769 | 2.132 | 25.6% |
| FCL COOL | Hypothermia on CL | 1.200 | 1.181 | 0.888 | 1.502 | 15.8% |
| FVM COOL | Hypothermia on Vmax | 0.775 | 0.831 | 0.330 | 1.495 | 38.6% |
| FKM COOL | Hypothermia on km | 0.884 | 1.026 | 0.233 | 2.875 | 64.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, S.K.; Wassink, G.; Lear, C.A.; Davidson, J.O.; Holford, N.H.G.; Gunn, A.J.; Bennet, L. The Effect of Size, Maturation, Global Asphyxia, Cerebral Ischemia, and Therapeutic Hypothermia on the Pharmacokinetics of High-Dose Recombinant Erythropoietin in Fetal Sheep. Int. J. Mol. Sci. 2020, 21, 3042. https://doi.org/10.3390/ijms21093042
Dhillon SK, Wassink G, Lear CA, Davidson JO, Holford NHG, Gunn AJ, Bennet L. The Effect of Size, Maturation, Global Asphyxia, Cerebral Ischemia, and Therapeutic Hypothermia on the Pharmacokinetics of High-Dose Recombinant Erythropoietin in Fetal Sheep. International Journal of Molecular Sciences. 2020; 21(9):3042. https://doi.org/10.3390/ijms21093042
Chicago/Turabian StyleDhillon, Simerdeep K., Guido Wassink, Christopher A. Lear, Joanne O. Davidson, Nicholas H.G. Holford, Alistair J. Gunn, and Laura Bennet. 2020. "The Effect of Size, Maturation, Global Asphyxia, Cerebral Ischemia, and Therapeutic Hypothermia on the Pharmacokinetics of High-Dose Recombinant Erythropoietin in Fetal Sheep" International Journal of Molecular Sciences 21, no. 9: 3042. https://doi.org/10.3390/ijms21093042
APA StyleDhillon, S. K., Wassink, G., Lear, C. A., Davidson, J. O., Holford, N. H. G., Gunn, A. J., & Bennet, L. (2020). The Effect of Size, Maturation, Global Asphyxia, Cerebral Ischemia, and Therapeutic Hypothermia on the Pharmacokinetics of High-Dose Recombinant Erythropoietin in Fetal Sheep. International Journal of Molecular Sciences, 21(9), 3042. https://doi.org/10.3390/ijms21093042





