Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis during In Vitro Maturation
Abstract
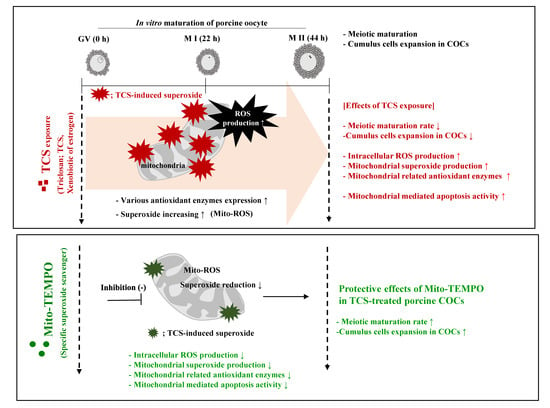
:1. Introduction
2. Results
2.1. Effects of TCS on Meiotic Maturation and Cumulus Expansion of Porcine Cocs
2.2. Effects of TCS on ROS Production and Antioxidant Enzyme Activity of Porcine Cocs
2.3. Effects of TCS on ROS-Derived Mitochondria Mediated Apoptosis in Porcine Cocs
2.4. Restoration of Impaired Meiotic Maturation and Cumulus Expansion in TCS-Exposed Porcine Cocs Using Mito-TEMPO, a Specific Superoxide Scavenger
2.5. TCS-Induced ROS and Superoxide Production in Porcine Cocs Was Reduced by the Protective Effect of Mito-TEMPO
2.6. Mito-TEMPO Inhibited the Mitochondrion-Mediated Apoptosis Induced by TCS in Porcine Cocs during IVM
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. IVM
4.3. Assessment of Cumulus Cell Expansion and Acetic-Orcein Staining
4.4. RNA Extraction and RT-PCR
4.5. DCF-DA and Mito-SOX Staining
4.6. Protein Extraction and Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| triclosan | (TCS) |
| in vitro maturation | (IVM) |
| reactive oxygen species | (ROS) |
| triphenylphosphonium chloride | (Mito-TEMPO) |
| cumulus oocyte complexes | (COCs) |
| dichlorofluorescein diacetate | (DCF-DA) |
| reverse-transcription polymerase chain reaction | (RT-PCR) |
| estrogen receptor | (ER) |
| Bisphenol A | (BPA) |
| 17β-estradiol | (E2) |
| germinal vesicle breakdown | (GVBD) |
| follicle-stimulating hormone | (FSH) |
| metaphase II | (M II) |
| hydrogen peroxide | (H2O2) |
| Tyrode’s lactate-N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid | (TL-HEPES) |
| North Carolina State University-23 | (NCSU-23) |
| pregnant mare’s serum gonadotropin | (PMSG) |
| human chorionic gonadotropin | (hCG) |
| dimethyl sulfoxide | (DMSO) |
| polyvinyl alcohol | (PVA) |
| phosphate-buffered saline | (PBS) |
| sodium dodecyl sulfate polyacrylamide gel electrophoresis | (SDS-PAGE) |
| enhanced chemiluminescence | (ECL) |
| one-way analysis of variance | (ANOVA) |
| standard deviation | (SD) |
| standard error of the mean | (SEM) |
References
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledon, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Bai, M.Z.; Huang, X.F.; Zhang, Y.; Liu, J.; Hu, M.H.; Zheng, W.Q.; Jin, F. Preimplantation Exposure to Bisphenol A and Triclosan May Lead to Implantation Failure in Humans. Biomed. Res. Int. 2015, 2015, 184845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.F.; Tian, Y. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environ. Pollut. 2015, 206, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.H.; Hsiao, C.D.; Kawakami, K.; Tse, W.K.F. Triclosan (TCS) exposure impairs lipid metabolism in zebrafish embryos. Aquat. Toxicol. 2016, 173, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- Wang, F.; Xu, R.; Zheng, F.; Liu, H. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp. Anim. 2018, 67, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Han, J.; Lee, M.C.; Seo, J.S.; Lee, J.S. Effects of triclosan (TCS) on fecundity, the antioxidant system, and oxidative stress-mediated gene expression in the copepod Tigriopus japonicus. Aquat. Toxicol. 2017, 189, 16–24. [Google Scholar] [CrossRef]
- Lin, D.; Xie, X.; Zhou, Q.; Liu, Y. Biochemical and genotoxic effect of triclosan on earthworms (Eisenia fetida) using contact and soil tests. Environ. Toxicol. 2012, 27, 385–392. [Google Scholar] [CrossRef]
- Teplova, V.V.; Belosludtsev, K.N.; Kruglov, A.G. Mechanism of triclosan toxicity: Mitochondrial dysfunction including complex II inhibition, superoxide release and uncoupling of oxidative phosphorylation. Toxicol. Lett. 2017, 275, 108–117. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Dumollard, R.; Carroll, J.; Duchen, M.R.; Campbell, K.; Swann, K. Mitochondrial function and redox state in mammalian embryos. Semin. Cell Dev. Biol. 2009, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Glasauer, A.; Hoover, P.; Yang, S.; Blatt, H.; Mullen, A.R.; Getsios, S.; Gottardi, C.J.; DeBerardinis, R.J.; Lavker, R.M.; et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 2013, 6, ra8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolstenholme, J.T.; Rissman, E.F.; Connelly, J.J. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011, 59, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Park, S.Y.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Choo, Y.K.; Koo, D.B. Melatonin Improves Oocyte Maturation and Mitochondrial Functions by Reducing Bisphenol A-Derived Superoxide in Porcine Oocytes In Vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Jones, D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998, 273, 11401–11404. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.G.; Park, H.J.; Kim, J.W.; Jung, J.M.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Kang, M.J.; Wee, G.; Yang, H.Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci Rep. 2018, 8, 10130. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Binelli, A. Environmental concentrations of 3,4-methylenedioxymethamphetamine (MDMA)-induced cellular stress and modulated antioxidant enzyme activity in the zebra mussel. Environ. Sci. Pollut. Res. Int. 2014, 21, 11099–11106. [Google Scholar] [CrossRef]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascon, A.J.; Ballesteros, E. Simultaneous determination of parabens, alkylphenols, phenylphenols, bisphenol A and triclosan in human urine, blood and breast milk by continuous solid-phase extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 119, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, X.; Chen, W.; Sun, Y.; Fan, C. Effects of triclosan on hormones and reproductive axis in female Yellow River carp (Cyprinus carpio): Potential mechanisms underlying estrogen effect. Toxicol. Appl. Pharmacol. 2017, 336, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Barbe, G.J.; Armstrong, D.T. Factors influencing resumption of meiotic maturation and cumulus expansion of porcine oocyte-cumulus cell complexes in vitro. Mol. Reprod. Dev. 1993, 36, 113–119. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, B.S.; Lee, S.R.; Yoon, S.B.; Huh, J.W.; Kim, S.U.; Kim, E.; Kim, S.H.; Choo, Y.K.; Koo, D.B.; et al. Supplementation with estradiol-17beta improves porcine oocyte maturation and subsequent embryo development. Fertil. Steril. 2011, 95, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar]
- Dalvit, G.C.; Cetica, P.D.; Pintos, L.N.; Beconi, M.T. Reactive oxygen species in bovine embryo in vitro production. Biocell 2005, 29, 209–212. [Google Scholar]
- Whitaker, B.D.; Knight, J.W. Mechanisms of oxidative stress in porcine oocytes and the role of anti-oxidants. Reprod. Fertil. Dev. 2008, 20, 694–702. [Google Scholar] [CrossRef]
- Oyawoye, O.; Abdel Gadir, A.; Garner, A.; Constantinovici, N.; Perrett, C.; Hardiman, P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum. Reprod. 2003, 18, 2270–2274. [Google Scholar] [CrossRef]
- Tamura, I.; Kanbara, Y.; Saito, M.; Horimoto, K.; Satoh, M.; Yamamoto, H.; Oyama, Y. Triclosan, an antibacterial agent, increases intracellular Zn(2+) concentration in rat thymocytes: Its relation to oxidative stress. Chemosphere 2012, 86, 70–75. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ajao, C.; Andersson, M.A.; Teplova, V.V.; Nagy, S.; Gahmberg, C.G.; Andersson, L.C.; Hautaniemi, M.; Kakasi, B.; Roivainen, M.; Salkinoja-Salonen, M. Mitochondrial toxicity of triclosan on mammalian cells. Toxicol. Rep. 2015, 2, 624–637. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. DPI induces mitochondrial superoxide-mediated apoptosis. Free Radic. Biol. Med. 2003, 34, 465–477. [Google Scholar] [CrossRef]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, B.; Han, X.; Mao, Z.; Chen, M.; Du, G.; Talbot, P.; Wang, X.; Xia, Y. The effects of triclosan on pluripotency factors and development of mouse embryonic stem cells and zebrafish. Arch. Toxicol. 2015, 89, 635–646. [Google Scholar] [CrossRef]
- Mlynarcikova, A.; Nagyova, E.; Fickova, M.; Scsukova, S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol. In Vitro 2009, 23, 371–377. [Google Scholar] [CrossRef]
- Salavati, M.; Ghafari, F.; Zhang, T.; Fouladi-Nashta, A.A. Effects of oxygen concentration on in vitro maturation of canine oocytes in a chemically defined serum-free medium. Reproduction 2012, 144, 547–556. [Google Scholar] [CrossRef] [Green Version]







| TCS (μM) | No. of COCs Examined | % of Cumulus Cells Expansion (n) | |||
|---|---|---|---|---|---|
| 3 step | 2 step | 1 step | 0 step | ||
| Non-treated | 130 | 72.1 ± 6.6 (93) a | 12.2 ± 4.1 (16) | 8.9 ± 4.1 (12) | 6.8 ± 1.4 (9) a |
| 1 | 111 | 69.1 ± 5.9 (75) a | 21.7 ± 10.7 (27) | 5.5 ± 5.3 (5) | 3.8 ± 5.0 (4) a |
| 10 | 134 | 35.9 ± 5.8 (49) c | 40.6 ± 11.1 (52) | 11.7 ± 5.5 (16) | 11.9 ± 5.1 (17) a |
| 100 | 146 | 11.5 ± 0.9 (17) d | 26.8 ± 6.4 (38) | 30.1 ± 2.8 (44) | 31.5 ± 5.3 (47) c |
| TCS (μM) | No. of Oocytes Examined | % of Oocytes (n) | |||
|---|---|---|---|---|---|
| GV | GVBD | M I | M II | ||
| Non-treated | 142 | 4.3 ± 3.3 (5) a | 4.0 ± 6.5 (4) a | 17.7 ± 4.6 (24) a | 77.5 ± 6.6 (109) a |
| 1 | 136 | 8.1 ± 0.8 (11) a | 4.6 ± 1.6 (6) a | 19.1 ± 5.3 (27) a | 66.1 ± 2.7 (92) a |
| 10 | 149 | 9.1 ± 5.0 (12) a | 11.8 ± 5.4 (16) a | 25.2 ± 10.7 (38) a | 55.1 ± 8.5 (83) d |
| 100 | 151 | 18.3 ± 5.3 (28) b | 17.2 ± 6.2 (25) b | 24.6 ± 5.6 (36) a | 39.8 ± 8.0 (62) d |
| TCS (100 μM) | Mito-TEMPO (0.1 μM) | No. of Oocytes Examined | % of Oocytes (n) | |||
|---|---|---|---|---|---|---|
| GV | GVBD | M I | M II | |||
| - | - | 110 | 7.8 ± 3.2 (8) a | 5.0 ± 2.5 (5) a | 16.0 ± 5.2 (18) a | 74.5 ± 3.0 (79) a |
| + | - | 139 | 6.4 ± 1.8 (9) a | 25.6 ± 11.6 (36) b | 29.3 ± 11.8 (40) c | 38.8 ± 5.2 (54) d |
| - | + | 106 | 6.8 ± 2.8 (8) a | 7.9 ± 7.0 (10) a | 9.0 ± 4.8 (10) a | 76.2 ± 9.7 (78) a |
| + | + | 134 | 5.2 ± 0.4 (7) a | 7.8 ± 2.8 (11) b | 23.1 ± 1.5 (31) b | 63.8 ± 1.3 (85) a |
| TCS (100 μM) | Mito-TEMPO (0.1 μM) | No. of COCs Examined | % of Cumulus Cells Expansion (n) | |||
|---|---|---|---|---|---|---|
| 3 step | 2 step | 1 step | 0 step | |||
| - | - | 145 | 71.8 ± 8.2 (104) a | 13.7 ± 5.0 (25) a | 9.7 ± 6.1 (18) a | 4.8 ± 2.4 (11) a |
| + | - | 163 | 14.5 ± 8.6 (24) d | 27.1 ± 4.7 (54) b | 24.6 ± 4.9 (49) b | 33.8 ± 5.7 (69) d |
| - | + | 154 | 74.7 ± 9.1 (115) a | 8.9 ± 2.4 (17) a | 10.1 ± 7.5 (18) a | 5.7 ± 4.8 (11) a |
| + | + | 154 | 55.7 ± 5.3 (86) a | 15.6 ± 3.8 (30) a | 15.0 ± 4.8 (27) a | 13.6 ± 2.9 (29) a |
| Genes | Primer Sequences | Tm °C | Gene Bank Accession No. | Base Pairs |
|---|---|---|---|---|
| Has2 | F(5′–3′): TGGCTGTACAATGCGATGTG R(5′–3′): TGGGTGGTGTGATTTTCACC | 55 | (NM_214053.1) | 402 |
| Tnfaip6 | F(5′–3′): TCTTCCTGTGGGAAGAGGCT R(5′–3′): GTCCGTCTGAACAGAAGCGA | 55 | (NM_001159607.1) | 337 |
| Ptx3 | F(5′–3′): TCAGTGCCTGCATTTGGGTC R(5′–3′): TTCTGAACAAGGGCATGTAG | 58 | (NM_001244783.1) | 225 |
| Sod2 | F(5′–3′): GCAGCTCGAGCAGGAATCTGG R(5′–3′): ACGCGGCCTACGTGAACAA | 59.7 | (NM_214127.2) | 163 |
| Prdx3 | F(5′–3′): AGTGGATTCCCACTTCAGCC R(5′–3′): AACCCATGGAGAAGTCTGCC | 55.1 | (NM_001244531.1) | 290 |
| Prdx5 | F(5′–3′): ACCTTCCAGGGTTTGTGGAG R(5′–3′): CCTGAATGTGGAGCCAGATG | 55 | (NM_214144.1) | 285 |
| Bax | F(5′–3′): AAGCGCATTGGAGATGAACT R(5′–3′): CTGGACTTCCTTCGAGATCG | 50 | (XM_003127290.4) | 251 |
| Bcl-xl | F(5′–3′): AGGGCATTCAGTGACCTGAC R(5′–3′): CACCTAGAGCCTTGGATCCA | 55 | (NM_214285.1) | 242 |
| Caspase3 | F(5′–3′): GAGGCAGACTTCTTGTATGC R(5′–3′): TTCCATGTATTGTGTCCATGC | 50 | (NM_214131.1) | 238 |
| Gapdh | F(5′–3′): TCGGAGTGAACGGATTTC R(5′–3′): CCTGGAAGATGGTGATGG | 53.7 | (NM_001206359.1) | 230 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Song, B.-S.; Kim, J.-W.; Yang, S.-G.; Kim, S.-U.; Koo, D.-B. Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis during In Vitro Maturation. Int. J. Mol. Sci. 2020, 21, 3050. https://doi.org/10.3390/ijms21093050
Park H-J, Song B-S, Kim J-W, Yang S-G, Kim S-U, Koo D-B. Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis during In Vitro Maturation. International Journal of Molecular Sciences. 2020; 21(9):3050. https://doi.org/10.3390/ijms21093050
Chicago/Turabian StylePark, Hyo-Jin, Bong-Seok Song, Jin-Woo Kim, Seul-Gi Yang, Sun-Uk Kim, and Deog-Bon Koo. 2020. "Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis during In Vitro Maturation" International Journal of Molecular Sciences 21, no. 9: 3050. https://doi.org/10.3390/ijms21093050
APA StylePark, H.-J., Song, B.-S., Kim, J.-W., Yang, S.-G., Kim, S.-U., & Koo, D.-B. (2020). Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis during In Vitro Maturation. International Journal of Molecular Sciences, 21(9), 3050. https://doi.org/10.3390/ijms21093050