Role of the Furosemide Stress Test in Renal Injury Prognosis
Abstract
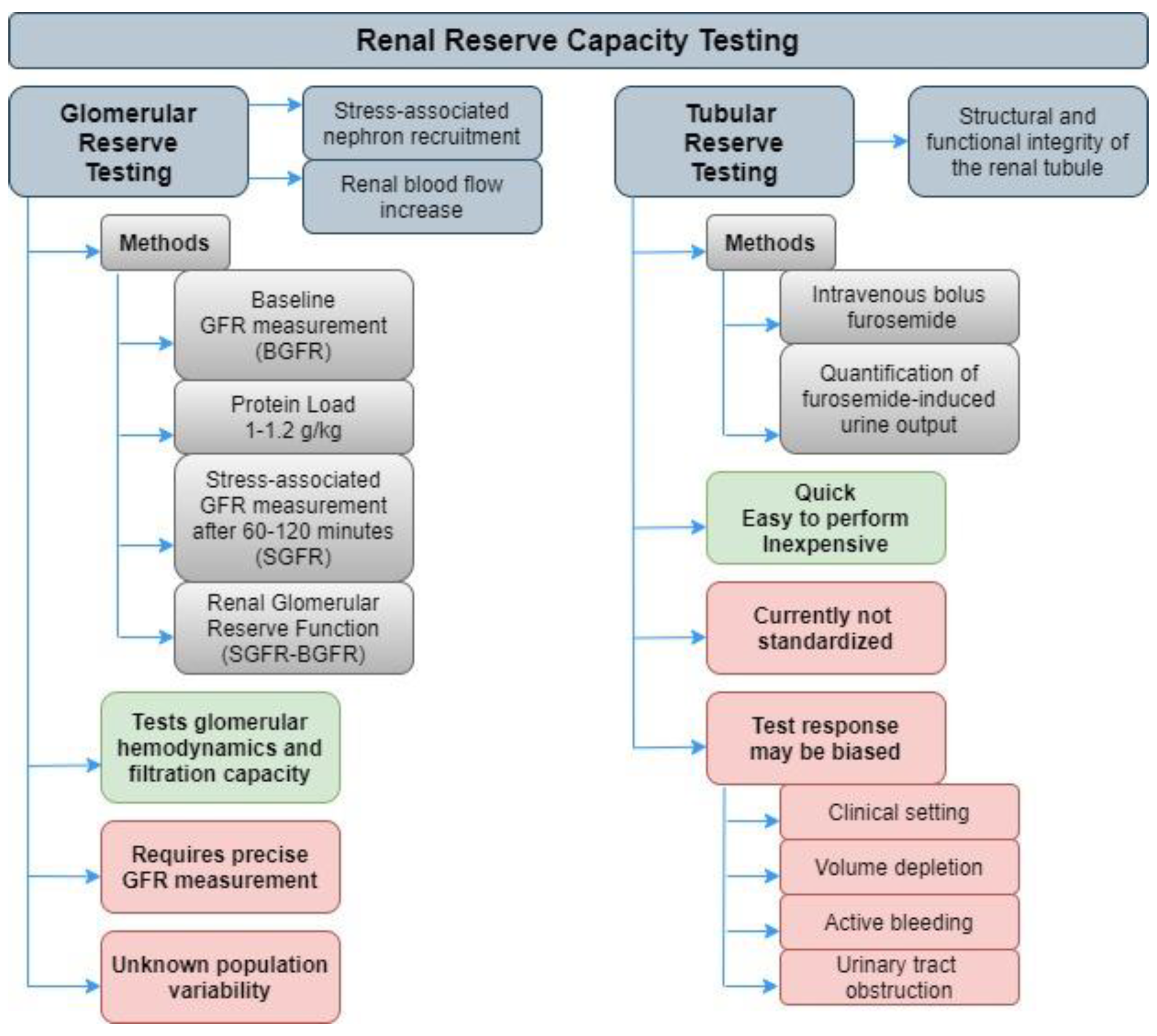
1. Introduction
2. Kidney Tubular Stress Test Assessment
3. Furosemide Stress Test
4. Critical Care
5. Kidney Transplantation
6. Other Clinical Settings (CKD)
7. Current Limitations and Future Challenges
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AKIN | acute kidney injury network |
| AUC | area under the curve |
| CKD | chronic kidney disease |
| CRRT | continuous renal replacement therapy |
| DGF | delayed graft function |
| FEM | furosemide excreted mass |
| FR | furosemide response |
| FST | furosemide stress test |
| IL-18 | interleukin 18 |
| KIM-1 | kidney injury molecule 1 |
| L-FABP | L-type fatty acid binding protein |
| MC | multi-center |
| NA | not applicable |
| NGAL | neutrophil gelatinase-associated lipocalin |
| P | prospective |
| R | retrospective |
| RRT | renal replacement therapy |
| SC | single-center |
| TIMP-2 | tissue inhibitor of metalloproteinases 2 |
| UO | urine output |
References
- Hoste, E.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Monjas, P.; Bustamante-Munguira, J.; Lorenzo, M.; Heredia-Rodríguez, M.; Fierro, I.; Gómez-Sánchez, E.; Hernandez, A.; Álvarez, F.J.; Bermejo-Martin, J.F.; Gómez-Pesquera, E.; et al. Predicting cardiac surgery–associated acute kidney injury: The CRATE score. J. Crit. Care 2016, 31, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Agapito, F.J.; Jorge, S.; Lopes, J.A. Acute kidney injury definition and diagnosis: A narrative review. J. Clin. Med. 2018, 7, 307. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Thongprayoon, C.; Cheungpasitporn, W.; Kashani, K.B. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 2016, 8, E305–E311. [Google Scholar] [CrossRef]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum creatinine: Not so simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Delanaye, P.; Mariat, C.; Cavalier, E.; Maillard, N.; Krzesinski, J.-M.; White, C.A. Trimethoprim, creatinine and creatinine-based equations. Nephron 2011, 119, 187–194. [Google Scholar] [CrossRef]
- Van Acker, B.; Koopman, M.; Arisz, L.; Koomen, G.; de Waart, D. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 1992, 340, 1326–1329. [Google Scholar] [CrossRef]
- Parikh, C.R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Rogers, N.; Greene, T.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Bragadottir, G.; Redfors, B.; Ricksten, S.-E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury - true GFR versus urinary creatinine clearance and estimating equations. Crit. Care 2013, 17, R108. [Google Scholar] [CrossRef]
- Chawla, L.S.; Ronco, C. Renal stress testing in the assessment of kidney disease. Kidney Int. Rep. 2016, 1, 57–63. [Google Scholar] [CrossRef]
- Siew, E.D.; Ware, L.B.; Ikizler, T.A. Biological markers of acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 810–820. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney Injury Molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.; Maranduca, M.; Floria, M.; Rezus, C. The predictive role of the biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) cisplatin-induced nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, Y.; Sun, M.; Zhu, J.H. Urinary neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, N-acetyl-beta-D-glucosaminidase levels and mortality risk in septic patients with acute kidney injury. Arch. Med. Sci. 2018, 14, 1381–1386. [Google Scholar] [CrossRef]
- Murray, P.T.; Wettersten, N.; Van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Müeller, G.A.; et al. Utility of urine neutrophil gelatinase-associated lipocalin for worsening renal function during hospitalization for acute heart failure: Primary findings of the urine N-gal acute kidney injury N-gal evaluation of symptomatic heart failure study (AKINESIS). J. Card. Fail. 2019, 25, 654–665. [Google Scholar] [CrossRef]
- Park, M.Y.; Lee, Y.W.; Choi, S.J.; Kim, J.K.; Hwang, S. Urinary cystatin C levels as a diagnostic and prognostic biomarker in patients with acute kidney injury. Nephrology 2013, 18, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Westhuyzen, J.; Endre, Z.H.; Reece, G.; Reith, D.M.; Saltissi, D.; Morgan, T.J. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol. Dial. Transplant. 2003, 18, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Heise, D.; Rentsch, K.; Braeuer, A.; Friedrich, M.; Quintel, M. Comparison of urinary neutrophil glucosaminidase-associated lipocalin, cystatin C, and ?1-microglobulin for early detection of acute renal injury after cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2011, 39, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Yanishi, M.; Kinoshita, H.; Mishima, T.; Taniguchi, H.; Yoshida, K.; Komai, Y.; Yasuda, K.; Watanabe, M.; Sugi, M.; Matsuda, T. Urinary l-type fatty acid-binding protein is a predictor of early renal function after partial nephrectomy. Ren. Fail. 2016, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Noiri, E.; Sugaya, T. Urinary L-type fatty acid-binding protein as a new renal biomarker in critical care. Curr. Opin. Crit. Care 2010, 16, 545–549. [Google Scholar] [CrossRef]
- Tang, K.W.A.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef]
- Parikh, C.R.; Mansour, S.G. Perspective on clinical application of biomarkers in AKI. J. Am. Soc. Nephrol. 2017, 28, 1677–1685. [Google Scholar] [CrossRef]
- McMahon, B.A.; Koyner, J.L. Risk stratification for acute kidney injury: Are biomarkers enough? Adv. Chronic Kidney Dis. 2016, 23, 167–178. [Google Scholar] [CrossRef]
- Liu, B.-C.; Tang, T.-T.; Lv, L.-L.; Lan, H.-Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Yard, B.A.; Daha, M.R.; Kooymans-Couthino, M.; Bruijn, J.A.; Paape, M.E.; Schrama, E.; Van Es, L.A.; Van Der Woude, F.J. IL-1α stimulated TNFα production by cultured human proximal tubular epithelial cells. Kidney Int. 1992, 42, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-H.; Zeng, R.; Weinmann-Menke, J.; Valerius, M.T.; Wada, Y.; Ajay, A.K.; Colonna, M.; Kelley, V.R. IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J. Clin. Investig. 2015, 125, 3198–3214. [Google Scholar] [CrossRef] [PubMed]
- Disteldorf, E.M.; Krebs, C.; Paust, H.-J.; Turner, J.-E.; Nouailles, G.; Tittel, A.; Meyer-Schwesinger, C.; Stege, G.; Brix, S.R.; Velden, J.; et al. CXCL5 Drives neutrophil recruitment in TH17-Mediated GN. J. Am. Soc. Nephrol. 2014, 26, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Lan, R.; Singha, P.K.; Gilchrist, A.; Weinreb, P.H.; Violette, S.M.; Weinberg, J.M.; Saikumar, P.; Venkatachalam, M.A. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Galphaq-mediated Rho and alphavbeta6 integrin-dependent activation of TGF-beta. Am. J. Pathol. 2012, 181, 1236–1249. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, M.; Fang, L.; Jiang, L.; Wen, P.; Dai, C.; Zhang, C.Y.; Yang, J. miR-21–containing microvesicles from injured tubular epithelial cells promote tubular phenotype transition by targeting PTEN protein. Am. J. Pathol. 2013, 183, 1183–1196. [Google Scholar] [CrossRef]
- Strutz, F. EMT and proteinuria as progression factors. Kidney Int. 2009, 75, 475–481. [Google Scholar] [CrossRef]
- Cosentino, C.C.; Skrypnyk, N.I.; Brilli, L.L.; Chiba, T.; Novitskaya, T.; Woods, C.; West, J.; Korotchenko, V.N.; McDermott, L.; Day, B.W.; et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 2013, 24, 943–953. [Google Scholar] [CrossRef]
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Physiol. 2014, 306, F970–F980. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Ponto, L.L.; Schoenwald, R.D. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part II). Clin. Pharmacokinet. 1990, 18, 460–471. [Google Scholar] [PubMed]
- Mariano, F.; Mella, A.; Vincenti, M.; Biancone, L. Furosemide as a functional marker of acute kidney injury in ICU patients: A new role for an old drug. J. Nephrol. 2019, 32, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.B.; Ogg, C.S.; Cameron, J.S. High dose frusemide in acute renal failure: A controlled trial. Clin. Nephrol. 1981, 15, 90–96. [Google Scholar]
- Schmidt, C.; Hocherl, K.; Schweda, F.; Kurtz, A.; Bucher, M. Regulation of renal sodium transporters during severe inflammation. J. Am. Soc. Nephrol. 2007, 18, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Kunin, M.; Holtzman, E.J.; Melnikov, S.; Dinour, D. Urinary organic anion transporter protein profiles in AKI. Nephrol. Dial. Transplant. 2011, 27, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.M.; Brown, R.S.; Shoemaker, W.C. Early prediction of acute renal failure and recovery. Crit. Care Med. 1973, 1, 179. [Google Scholar] [CrossRef]
- Chawla, L.S.; Davison, D.; Brasha-Mitchell, E.; Koyner, J.L.; Arthur, J.; Shaw, A.; Tumlin, J.; Trevino, S.A.; Kimmel, P.L.; Seneff, M.G. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit. Care 2013, 17, R207. [Google Scholar] [CrossRef]
- Koyner, J.L.; Davison, D.L.; Brasha-Mitchell, E.; Chalikonda, D.M.; Arthur, J.; Shaw, A.; Tumlin, J.A.; Trevino, S.A.; Bennett, M.R.; Kimmel, P.L.; et al. Furosemide stress test and biomarkers for the prediction of aki severity. J. Am. Soc. Nephrol. 2015, 26, 2023–2031. [Google Scholar] [CrossRef]
- Van der Voort, P.H.J.; Boerma, E.C.; Pickkers, P. The furosemide stress test to predict renal function after continuous renal replacement therapy. Crit. Care 2014, 18, 429. [Google Scholar] [CrossRef]
- Matsuura, R.; Komaru, Y.; Miyamoto, Y.; Yoshida, T.; Yoshimoto, K.; Isshiki, R.; Mayumi, K.; Yamashita, T.; Hamasaki, Y.; Nangaku, M.; et al. Response to different furosemide doses predicts AKI progression in ICU patients with elevated plasma NGAL levels. Ann. Intensiv. Care 2018, 8, 8. [Google Scholar] [CrossRef]
- Lumlertgul, N.; Peerapornratana, S.; Trakarnvanich, T.; Pongsittisak, W.; Surasit, K.; Chuasuwan, A.; Tankee, P.; Tiranathanagul, K.; Praditpornsilpa, K.; Tungsanga, K.; et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit. Care 2018, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Rewa, O.G.; Bagshaw, S.; Wang, X.; Wald, R.; Smith, O.; Shapiro, J.; McMahon, B.; Liu, K.; Trevino, S.; Chawla, L.; et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: A multicenter, prospective, observational study. J. Crit. Care 2019, 52, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sakhuja, A.; Bandak, G.; Barreto, E.F.; Vallabhajosyula, S.; Jentzer, J.; Albright, R.; Kashani, K.B. Role of loop diuretic challenge in stage 3 acute kidney injury. Mayo Clin. Proc. 2019, 94, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Borasino, S.; Wall, K.M.; Crawford, J.H.; Hock, K.M.; Cleveland, D.C.; Rahman, F.; Martin, K.D.; Alten, J.A. Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr. Crit. Care Med. 2018, 19, 310–317. [Google Scholar] [CrossRef]
- McMahon, B.A.; Koyner, J.L.; Novick, T.; Menez, S.; Moran, R.A.; Lonze, B.E.; Desai, N.; Alasfar, S.; Borja, M.; Merritt, W.T.; et al. The prognostic value of the furosemide stress test in predicting delayed graft function following deceased donor kidney transplantation. Biomarkers 2017, 23, 61–69. [Google Scholar] [CrossRef]
- Udomkarnjananun, S.; Townamchai, N.; Iampenkhae, K.; Petchlorlian, A.; Srisawat, N.; Katavetin, P.; Sutherasan, M.; Santingamkun, A.; Praditpornsilpa, K.; Eiam-Ong, S.; et al. Furosemide stress test as a predicting biomarker for delayed graft function in kidney transplantation. Nephron 2019, 141, 236–248. [Google Scholar] [CrossRef]
- Rivero, J.; Rodríguez, F.; Soto, V.; Macedo, E.; Chawla, L.S.; Mehta, R.L.; Vaingankar, S.; Garimella, P.S.; Garza, C.; Madero, M. Furosemide stress test and interstitial fibrosis in kidney biopsies in chronic kidney disease. BMC Nephrol. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Cook, N.R. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007, 115, 928–935. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coca, A.; Aller, C.; Reinaldo Sánchez, J.; Valencia, A.L.; Bustamante-Munguira, E.; Bustamante-Munguira, J. Role of the Furosemide Stress Test in Renal Injury Prognosis. Int. J. Mol. Sci. 2020, 21, 3086. https://doi.org/10.3390/ijms21093086
Coca A, Aller C, Reinaldo Sánchez J, Valencia AL, Bustamante-Munguira E, Bustamante-Munguira J. Role of the Furosemide Stress Test in Renal Injury Prognosis. International Journal of Molecular Sciences. 2020; 21(9):3086. https://doi.org/10.3390/ijms21093086
Chicago/Turabian StyleCoca, Armando, Carmen Aller, Jimmy Reinaldo Sánchez, Ana Lucía Valencia, Elena Bustamante-Munguira, and Juan Bustamante-Munguira. 2020. "Role of the Furosemide Stress Test in Renal Injury Prognosis" International Journal of Molecular Sciences 21, no. 9: 3086. https://doi.org/10.3390/ijms21093086
APA StyleCoca, A., Aller, C., Reinaldo Sánchez, J., Valencia, A. L., Bustamante-Munguira, E., & Bustamante-Munguira, J. (2020). Role of the Furosemide Stress Test in Renal Injury Prognosis. International Journal of Molecular Sciences, 21(9), 3086. https://doi.org/10.3390/ijms21093086





