Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work
Abstract
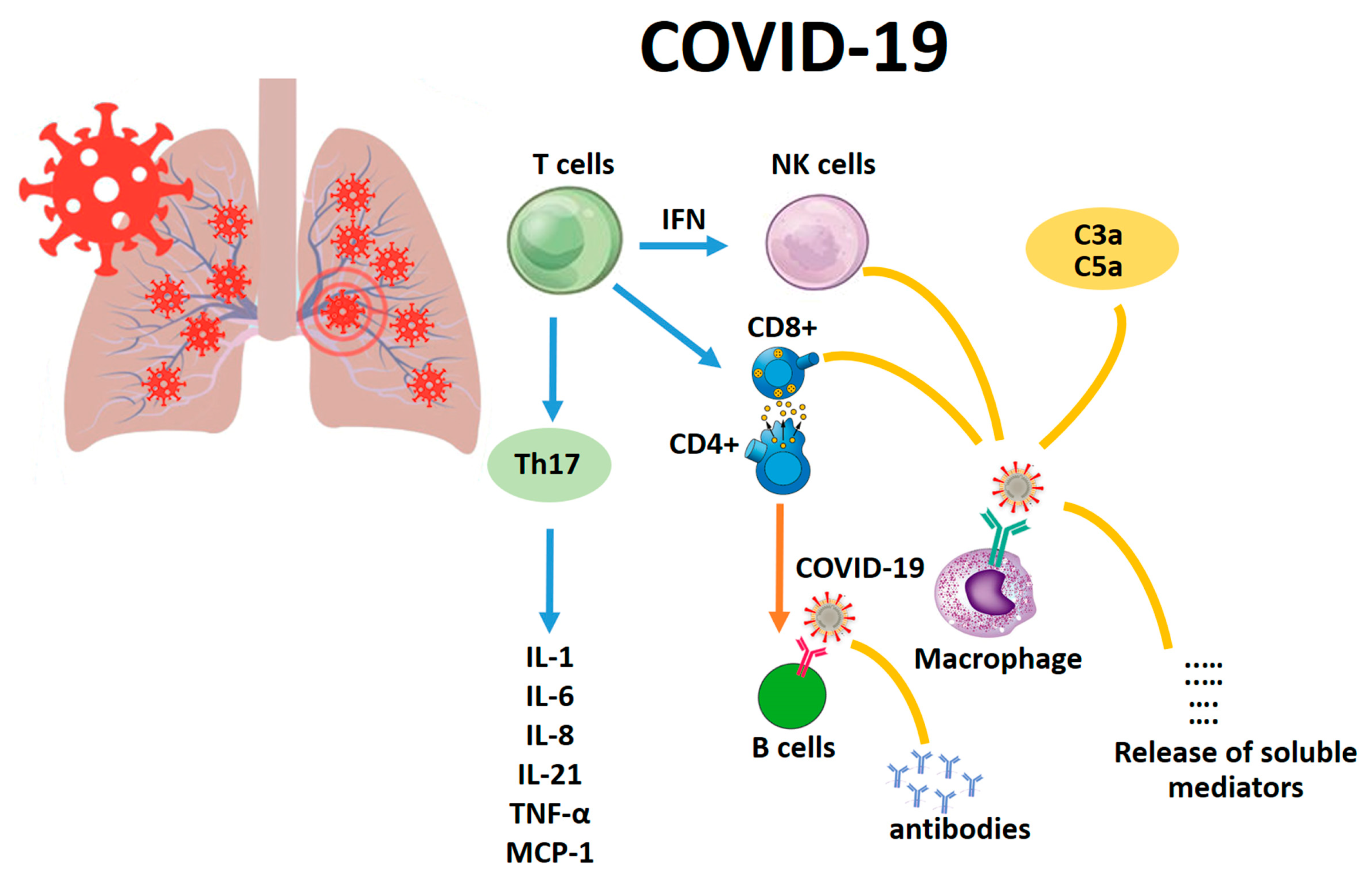
1. Background
2. The Pivotal Role of IL-6 and TNF-α in Lung Infections
3. Adiponectin Function in Lung Infections
4. ω-3 PUFAs and Lung Infections
5. Other Dietary Constituents and Lung Infections
6. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banerjee, A.; Kulcsar, K.; Misra, V.; Frieman, M.; Mossman, K. Bats and coronaviruses. Viruses 2019. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols; The Pirbright Institute: Surrey, UK, 2015. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Fan, R.; Wen, B.; Zhang, J.; Cao, X.; Wang, C.; Song, Z.; Li, S.; Li, X.; et al. Coronavirus Infections in the Central Nervous System and Respiratory Tract Show Distinct Features in Hospitalized Children. Intervirology 2017. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes on Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 2020. [Google Scholar] [CrossRef]
- Tse, G.M.K.; To, K.F.; Chan, P.K.S.; Lo, A.W.I.; Ng, K.C.; Wu, A.; Lee, N.; Wong, H.C.; Mak, S.M.; Chan, K.F.; et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J. Clin. Pathol. 2004. [Google Scholar] [CrossRef]
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Addi, A.B.; Lefort, A.; Hua, X.; Libert, F.; Communi, D.; Ledent, C.; Macours, P.; Tilley, S.L.; Boeynaems, J.M.; Robaye, B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: Involvement of the A2B receptor. Eur. J. Immunol. 2008. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, Immunity, And disease. Cold Spring Harb. Perspect. Biol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Monda, V.; Carotenuto, M.; Precenzano, F.; Iacono, D.; Messina, A.; Salerno, M.; Sessa, F.; Lanzara, V.; Messina, G.; Quatrosi, G.; et al. Neuropeptides’ Hypothalamic Regulation of Sleep Control in Children Affected by Functional Non-Retentive Fecal Incontinence. Brain Sci. 2020. [Google Scholar] [CrossRef]
- Robb, C.T.; Regan, K.H.; Dorward, D.A.; Rossi, A.G. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 2016. [Google Scholar] [CrossRef]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F.; et al. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Seo, S.H.; Webster, R.G. Tumor Necrosis Factor Alpha Exerts Powerful Anti-Influenza Virus Effects in Lung Epithelial Cells. J. Virol. 2002. [Google Scholar] [CrossRef]
- Lundblad, L.K.A.; Thompson-Figueroa, J.; Leclair, T.; Sullivan, M.J.; Poynter, M.E.; Irvin, C.G.; Bates, J.H.T. Tumor necrosis factor-α overexpression in lung disease: A single cause behind a complex phenotype. Am. J. Respir. Crit. Care Med. 2005, 171, 1363–1370. [Google Scholar] [CrossRef]
- Qiao, L.Y.; Goldberg, J.L.; Russell, J.C.; Xiao Jian, S. Identification of enhanced serine kinase activity in insulin resistance. J. Biol. Chem. 1999, 274, 10625–10632. [Google Scholar] [CrossRef] [PubMed]
- Wisse, B.E. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Bafunno, V.; Divella, C.; Sessa, F.; Tiscia, G.L.; Castellano, G.; Gesualdo, L.; Margaglione, M.; Montinaro, V. De novo homozygous mutation of the C1 inhibitor gene in a patient with hereditary angioedema. J. Allergy Clin. Immunol. 2013, 132, 6. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). MedRxiv 2020. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 913. [Google Scholar] [CrossRef]
- Li, M.; Kim, D.H.; Tsenovoy, P.L.; Peterson, S.J.; Rezzani, R.; Rodella, L.F.; Aronow, W.S.; Ikehara, S.; Abraham, N.G. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008, 57, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Hara, K.; Kamura, Y.; Honoki, H.; Fujisaka, S.; Ishiki, M.; Usui, I.; Yagi, K.; Fukushima, Y.; Takano, A.; et al. Ratio of low molecular weight serum adiponectin to the total adiponectin value is associated with type 2 diabetes through its relation to increasing insulin resistance. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Toussirot, E.; Binda, D.; Gueugnon, C.; Dumoulin, G. Adiponectin in autoimmune diseases. Curr. Med. Chem. 2012, 19, 5474–5480. [Google Scholar] [CrossRef]
- Maeda, N.; Takahashi, M.; Funahashi, T.; Kihara, S.; Nishizawa, H.; Kishida, K.; Nagaretani, H.; Matsuda, M.; Komuro, R.; Ouchi, N.; et al. PPARγ Ligands Increase Expression and Plasma Concentrations of Adiponectin, an Adipose-Derived Protein. Diabetes 2001, 50, 2094–2099. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.H.; Oldewage-Theron, W.; Shen, C.L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef]
- Perrotta, F.; Nigro, E.; Mollica, M.; Costigliola, A.; D’agnano, V.; Daniele, A.; Bianco, A.; Guerra, G. Pulmonary hypertension and obesity: Focus on adiponectin. Int. J. Mol. Sci. 2019, 20, 912. [Google Scholar] [CrossRef]
- Monda, V.; Salerno, M.; Fiorenzo, M.; Villano, I.; Viggiano, A.; Sessa, F.; Triggiani, A.I.; Cibelli, G.; Valenzano, A.; Marsala, G.; et al. Role of sex hormones in the control of vegetative and metabolic functions of middle-aged women. Front. Physiol. 2017, 8, 773. [Google Scholar] [CrossRef]
- Bianco, A.; Mazzarella, G.; Turchiarelli, V.; Nigro, E.; Corbi, G.; Scudiero, O.; Sofia, M.; Daniele, A. Adiponectin: An attractive marker for metabolic disorders in chronic obstructive pulmonary disease (COPD). Nutrients 2013, 14, 4115–4125. [Google Scholar] [CrossRef]
- Desruisseaux, M.S.; Trujillo, M.E.; Tanowitz, H.B.; Scherer, P.E. Adipocyte, adipose tissue, and infectious disease. Infect. Immun. 2007, 75, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Nigro, E.; Monaco, M.L.; Matera, M.G.; Scudiero, O.; Mazzarella, G.; Daniele, A. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm. Pharmacol. Ther. 2017, 43, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, R.; Maldonato, M.N.; Messina, A.; Cozzolino, P.; Monda, M.; Cerroni, F.; Romano, P.; Salerno, M.; Maltese, A.; Roccella, M.; et al. Orexin system: Network multi-tasking. Acta Med. Mediterr. 2018, 34, 349–356. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Daniele, A.; De Rosa, A.; Nigro, E.; Scudiero, O.; Capasso, M.; Masullo, M.; De Laurentiis, G.; Oriani, G.; Sofia, M.; Bianco, A. Adiponectin oligomerization state and adiponectin receptors airway expression in chronic obstructive pulmonary disease. Int. J. Biochem. Cell Biol. 2012, 44, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, M. Different effects of adiponectin isoforms in human monocytic cells. J. Leukoc. Biol. 2006, 79, 803–808. [Google Scholar] [CrossRef]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Talkington, D.F. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect. Immun. 2002, 70, 3649–3655. [Google Scholar] [CrossRef]
- Sessa, F.; Franco, S.; Picciocchi, E.; Geraci, D.; Chisari, M.G.; Marsala, G.; Polito, A.N.; Sorrentino, M.; Tripi, G.; Salerno, M.; et al. Addictions substance free during lifespan. Acta Med. Mediterr. 2018. [Google Scholar] [CrossRef]
- Mclaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ajuwon, K.M.; Spurlock, M.E. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Chiba, S.; Tatsukawa, H.; Yasuda, T.; Noguchi, H.; Seike, M.; Yoshimatsu, H. Adiponectin protects LPS-induced liver injury through modulation of TNF-α in KK-Ay obese mice. Hepatology 2004, 40, 177–184. [Google Scholar] [CrossRef]
- Polito, R.; Nigro, E.; Elce, A.; Monaco, M.L.; Iacotucci, P.; Carnovale, V.; Comegna, M.; Gelzo, M.; Zarrilli, F.; Corso, G.; et al. Adiponectin Expression Is Modulated by Long-Term Physical Activity in Adult Patients Affected by Cystic Fibrosis. Mediat. Inflamm. 2019, 2019, 934. [Google Scholar] [CrossRef]
- Garcia, P.; Sood, A. Adiponectin in Pulmonary Disease and Critically Ill Patients. Curr. Med. Chem. 2012. [Google Scholar] [CrossRef]
- Wulster-Radcliffe, M.C.; Ajuwon, K.M.; Wang, J.; Christian, J.A.; Spurlock, M.E. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 2004, 316, 924–929. [Google Scholar] [CrossRef]
- Venkatesh, B.; Hickman, I.; Nisbet, J.; Cohen, J.; Prins, J. Changes in serum adiponectin concentrations in critical illness: A preliminary investigation. Crit. Care 2009. [Google Scholar] [CrossRef]
- Shore, S.A.; Terry, R.D.; Flynt, L.; Xu, A.; Hug, C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J. Allergy Clin. Immunol. 2006, 118, 389–395. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Caglar, K.; Gok, D.E.; Eyileten, T.; Yenicesu, M.; Acikel, C.; Bingol, N.; Kilic, S.; Oguz, Y.; et al. Peroxisome proliferator-activated receptor γ (PPAR-γ) agonist increases plasma adiponectin levels in type 2 diabetic patients with proteinuria. Endocrine 2004, 25, 207–214. [Google Scholar] [CrossRef]
- Kanda, Y.; Matsuda, M.; Tawaramoto, K.; Kawasaki, F.; Hashiramoto, M.; Matsuki, M.; Kaku, K. Effects of sulfonylurea drugs on adiponectin production from 3T3-L1 adipocytes: Implication of different mechanism from pioglitazone. Diabetes Res. Clin. Pract. 2008, 81, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Salerno, M.; Sessa, F.; Bernardini, R.; Valenzano, A.; Marsala, G.; Zammit, C.; Avola, R.; Carotenuto, M.; Messina, G.; et al. Functional Changes of Orexinergic Reaction to Psychoactive Substances. Mol. Neurobiol. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Villano, I.; Nicolosi, D.; Longhitano, L.; Loreto, C.; Lovino, A.; Sessa, F.; Polito, A.N.; Monda, V.; Chieffi, S.; et al. Modafinil and orexin system: Interactions and medico-legal considerations. Front. Biosci. Landmark 2019, 24, 564–575. [Google Scholar] [CrossRef]
- Hiuge, A.; Tenenbaum, A.; Maeda, N.; Benderly, M.; Kumada, M.; Fisman, E.Z.; Tanne, D.; Matas, Z.; Hibuse, T.; Fujita, K.; et al. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 635–641. [Google Scholar] [CrossRef]
- Koh, K.K.; Quon, M.J.; Lim, S.; Lee, Y.; Sakuma, I.; Lee, Y.H.; Han, S.H.; Shin, E.K. Effects of fenofibrate therapy on circulating adipocytokines in patients with primary hypertriglyceridemia. Atherosclerosis 2011, 214, 144–147. [Google Scholar] [CrossRef]
- Furuhashi, M.; Ura, N.; Higashiura, K.; Murakami, H.; Tanaka, M.; Moniwa, N.; Yoshida, D.; Shimamoto, K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 2003, 42, 76–81. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Caglar, K.; Celik, T.; Yenicesu, M.; Eyileten, T.; Acikel, C.; Oguz, Y.; Yavuz, I.; Vural, A. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology 2007, 12, 147–153. [Google Scholar] [CrossRef]
- Bertozzi, G.; Salerno, M.; Pomara, C.; Sessa, F. Neuropsychiatric and Behavioral Involvement in AAS Abusers. A Literature Review. Medicina 2019, 55, 396. [Google Scholar] [CrossRef]
- Iwai, M.; Chen, R.; Imura, Y.; Horiuchi, M. TAK-536, a New AT1 Receptor Blocker, Improves Glucose Intolerance and Adipocyte Differentiation. Am. J. Hypertens. 2007, 20, 579–586. [Google Scholar] [CrossRef][Green Version]
- Schupp, M.; Janke, J.; Clasen, R.; Unger, T.; Kintscher, U. Angiotensin Type 1 Receptor Blockers Induce Peroxisome Proliferator-Activated Receptor-γ Activity. Circulation 2004, 109, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Kwang, K.K.; Quon, M.J.; Sang, J.L.; Seung, H.H.; Jeong, Y.A.; Kim, J.A.; Chung, W.J.; Lee, Y.; Eak, K.S. Efonidipine simultaneously improves blood pressure, endothelial function, and metabolic parameters in nondiabetic patients with hypertension. Diabetes Care 2007, 36, 1605–1607. [Google Scholar] [CrossRef][Green Version]
- Nowak, Ł.; Adamczak, M.; Wiȩcek, A. Blockade of sympathetic nervous system activity by rilmenidine increases plasma adiponectin concentration in patients with essential hypertension. Am. J. Hypertens. 2005, 18, 1470–1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, K.K.; Quon, M.J.; Sakuma, I.; Lee, Y.; Lim, S.; Han, S.H.; Shin, E.K. Effects of simvastatin therapy on circulating adipocytokines in patients with hypercholesterolemia. Int. J. Cardiol. 2011, 146, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.G.; Bressan, J.; Alfenas, R.C.G. Effect of the diet components on adiponectin levels. Nutr. Hosp. 2010. [Google Scholar] [CrossRef]
- Parolini, C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar. Drugs 2019, 27, 374. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Sözener, Z.Ç.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 990. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of acute inflammation and the role of resolvins in immunity, thrombosis, and vascular biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Sharma, S.; Chhibber, S.; Mohan, H.; Sharma, S. Dietary supplementation with omega-3 polyunsaturated fatty acids ameliorates acute pneumonia induced by Klebsiella pneumoniae in BALB/c mice. Can. J. Microbiol. 2013. [Google Scholar] [CrossRef]
- Jones, G.J.B.; Roper, R.L. The effects of diets enriched in omega-3 polyunsaturated fatty acids on systemic vaccinia virus infection. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Shapiro, H. Enteral omega-3 in acute respiratory distress syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bo, L.; Liu, W.; Lu, X.; Jin, F. Enteral immunomodulatory diet (omega-3 fatty acid, γ-linolenic acid and antioxidant supplementation) for acute lung injury and acute respiratory distress syndrome: An updated systematic review and meta-analysis. Nutrients 2015, 7, 5572–5585. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Cusack, R.; Burgess, V.A.; Grocott, M.P.W.; Calder, P.C. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst. Rev. 2019, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- García De Acilu, M.; Leal, S.; Caralt, B.; Roca, O.; Sabater, J.; Masclans, J.R. The Role of Omega-3 Polyunsaturated Fatty Acids in the Treatment of Patients with Acute Respiratory Distress Syndrome: A Clinical Review. Biomed. Res. Int. 2015, 2015, 750. [Google Scholar] [CrossRef]
- Alvarado, A.; Arce, I. Antioxidants in respiratory diseases: Basic science research and therapeutic alternatives. Clin. Res. Trials 2016. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Muzykantov, V.R. Antioxidant strategies in respiratory medicine. Treat. Respir. Med. 2006, 5, 47–78. [Google Scholar] [CrossRef]
- Galvão, A.M.; de Andrade, A.D.; de Souza, M.B.; da Silva, K.E.R.; de Andrade Bezerra, A.; de Melo, J.F.; de Morais, N.G.; da Costa, T.B.; de Castro, C.M.M.B. Antioxidant supplementation for the treatment of acute lung injury: A meta-analysis. Rev. Bras. Ter. Intensiva 2011, 23. [Google Scholar] [CrossRef]
- Carr, A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Cai, X.; Shang, Y. Epigallocatechin-3-gallate inhibits inflammation and epithelial-mesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma. Int. J. Mol. Med. 2018, 41, 818–828. [Google Scholar] [CrossRef]
- Ling, L.J.; Lu, Y.; Zhang, Y.Y.; Zhu, H.Y.; Tu, P.; Li, H.; Chen, D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 150. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kreda, S.M.; Davis, C.W.; Rose, M.C. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb. Perspect. Med. 2012, 2, 589. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Capote, K.; McCormack, F.X.; Possmayer, F. Pulmonary surfactant protein-A (SP-A) restores the surface properties of surfactant after oxidation by a mechanism that requires the Cys6 interchain disulfide bond and the phospholipid binding domain. J. Biol. Chem. 2003, 278, 20461–20474. [Google Scholar] [CrossRef]
- Weismann, D.; Binder, C.J. The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2465–2475. [Google Scholar] [CrossRef]
- Shirey, K.A.; Lai, W.; Scott, A.J.; Lipsky, M.; Mistry, P.; Pletneva, L.M.; Karp, C.L.; McAlees, J.; Gioannini, T.L.; Weiss, J.; et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Wagner, A.C.; Navab, M.; Anantharamaiah, G.M.; Hui, E.K.W.; Nayak, D.P.; Fogelman, A.M. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 2004, 110, 3252–3258. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.C.; Wang, H.; et al. Identification of Oxidative Stress and Toll-like Receptor 4 Signaling as a Key Pathway of Acute Lung Injury. Cell 2008, 113, 235–249. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Fessler, M.B.; Gowdy, K.M. Role for phospholipid acyl chains and cholesterol in pulmonary infections and inflammation. J. Leukoc. Biol. 2016. [Google Scholar] [CrossRef]
- Fessler, M.B.; Summer, R.S. Surfactant lipids at the host-environment interface metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 624–635. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Caron, E.; Desseyn, J.L.; Sergent, L.; Bartke, N.; Husson, M.O.; Duhamel, A.; Gottrand, F. Impact of fish oils on the outcomes of a mouse model of acute Pseudomonas aeruginosa pulmonary infection. Br. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tiesset, H.; Bernard, H.; Bartke, N.; Beermann, C.; Flachaire, E.; Desseyn, J.-L.; Gottrand, F.; Husson, M.-O. (n-3) Long-Chain PUFA Differentially Affect Resistance to Pseudomonas aeruginosa Infection of Male and Female cftr–/–Mice. J. Nutr. 2011, 141, 1101–1107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Rotzinger, D.C.; Beigelman-Aubry, C.; von Garnier, C.; Qanadli, S.D. Pulmonary embolism in patients with COVID-19: Time to change the paradigm of computed tomography. Thromb. Res. 2020, 190, 58–59. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Imai, Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 496–502. [Google Scholar] [CrossRef]
- Hecker, M.; Linder, T.; Ott, J.; Walmrath, H.D.; Lohmeyer, J.; Vadász, I.; Marsh, L.M.; Herold, S.; Reichert, M.; Buchbinder, A.; et al. Immunomodulation by lipid emulsions in pulmonary inflammation: A randomized controlled trial. Crit. Care 2015, 19, 226. [Google Scholar] [CrossRef]
- Chan, K.H.; Yeung, S.C.; Yao, T.J.; Ip, M.S.M.; Cheung, A.H.K.; Chan-Yeung, M.M.W.; Mak, J.C.W.; Lam, W.K.; Yew, W.W.; Wong, P.C.; et al. Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2010, 14, 1193–2000. [Google Scholar]
- Fragopoulou, E.; Panagiotakos, D.B.; Pitsavos, C.; Tampourlou, M.; Chrysohoou, C.; Nomikos, T.; Antonopoulou, S.; Stefanadis, C. The association between adherence to the Mediterranean diet and adiponectin levels among healthy adults: The ATTICA study. J. Nutr. Biochem. 2010, 21, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, M.; Rzepka, E.; Chudek, J.; Wiȩcek, A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin. Endocrinol. 2005, 62, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mirco, N.; Andrea, C.; Angelo, G.; Pietro, B.; Federico, L.; Michele, P.; Giuseppe, G.; Daniele, B.; Francesco, F.; Richard, N.; et al. At the Epicenter of the Covid-19 Pandemic and Humanitarian Crises in Italy: Changing Perspectives on Preparation and Mitigation. Catal. Non-Issue Content 2020, 1, 80. [Google Scholar] [CrossRef]
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55, 20. [Google Scholar] [CrossRef] [PubMed]

| Dietary Supplementation | Main Natural Sources | Potential Effects in COVID-19 Infection |
|---|---|---|
| Flavonoids | Red wine, oranges, red fruits and vegetables | Reduces inflammation and immune response, blocking nuclear NF-κB translocation |
| Polyphenols | Green tea, broccoli, apples | |
| Vitamin C | Oranges, lemons, mangoes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di Nunno, N.; Di Mizio, G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. https://doi.org/10.3390/ijms21093104
Messina G, Polito R, Monda V, Cipolloni L, Di Nunno N, Di Mizio G, Murabito P, Carotenuto M, Messina A, Pisanelli D, et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. International Journal of Molecular Sciences. 2020; 21(9):3104. https://doi.org/10.3390/ijms21093104
Chicago/Turabian StyleMessina, Giovanni, Rita Polito, Vincenzo Monda, Luigi Cipolloni, Nunzio Di Nunno, Giulio Di Mizio, Paolo Murabito, Marco Carotenuto, Antonietta Messina, Daniela Pisanelli, and et al. 2020. "Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work" International Journal of Molecular Sciences 21, no. 9: 3104. https://doi.org/10.3390/ijms21093104
APA StyleMessina, G., Polito, R., Monda, V., Cipolloni, L., Di Nunno, N., Di Mizio, G., Murabito, P., Carotenuto, M., Messina, A., Pisanelli, D., Valenzano, A., Cibelli, G., Scarinci, A., Monda, M., & Sessa, F. (2020). Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. International Journal of Molecular Sciences, 21(9), 3104. https://doi.org/10.3390/ijms21093104













