Abstract
Ovarian cancer (OvCA) accounts for one of the leading causes of death from gynecologic malignancy. Despite progress in therapy improvements in OvCA, most patients develop a recurrence after first-line treatments, dependent on the tumor and non-tumor complexity/heterogeneity of the neoplasm and its surrounding tumor microenvironment (TME). The TME has gained greater attention in the design of specific therapies within the new era of immunotherapy. It is now clear that the immune contexture in OvCA, here referred as tumor immune microenvironment (TIME), acts as a crucial orchestrator of OvCA progression, thus representing a necessary target for combined therapies. Currently, several advancements of antitumor immune responses in OvCA are based on the characterization of tumor-infiltrating lymphocytes, which have been shown to correlate with a significantly improved clinical outcome. Here, we reviewed the literature on selected TIME components of OvCA, such as macrophages, neutrophils, γδ T lymphocytes, and natural killer (NK) cells; these cells can have a role in either supporting or limiting OvCA, depending on the TIME stimuli. We also reviewed and discussed the major (immune)-therapeutic approaches currently employed to target and/or potentiate macrophages, neutrophils, γδ T lymphocytes, and NK cells in the OvCA context.
1. Overview on Ovarian cancer
Ovarian cancer (OvCA) is one of the most common gynecologic malignancies [1], and it is characterized by relatively high incidence, poor prognosis, and a very high mortality rate [2]. A large number of patients can be successfully treated by conventional therapeutic strategies before the cancer spreads beyond the ovaries in patients diagnosed at International Federation of Gynecology and Obstetrics (FIGO) stage I. The survival rate significantly decreases after OvCA has metastasized to pelvic organs (stage II), across the pelvic cavity to abdominal organs (stage III), or beyond the peritoneal cavity to distant parenchymal organs (stage IV) [3]. The poor survival rate in OvCA is associated with diagnosis at late stage due to delayed onset of symptoms and lack of proper screening [1]. Indeed, surgery is effective in most cases of early stage (FIGO stages I–IIA) with a 5-year survival rate of around 90%, but more than 70% of patients are diagnosed with advanced disease (FIGO stages III–IV) presenting malignant ascites which is an indicator of poor prognosis.
Approximately 90% of all OvCA cases are of epithelial cell origin and, according to their nature could be classified in distinct subtypes: high- and low-grade serous, endometrioid, clear cell, mucinous carcinomas, malignant Brenner tumors, and mixed histology [4]. High-grade serous OvCA (HGSOC), often diagnosed in stages III (51%) and IV (29%) when the spread to the peritoneum has already occurred, exhibits the highest frequency and aggressiveness [5]. HGSOC has been associated with frequent somatic genetic mutations of the tumor suppressor protein p53 (TP53) [6], accounting for over 95% of cases. Notably, p53 mutations have been correlated with enhanced proinflammatory chemokine levels and inflammatory tumor microenvironment (TME) [7]. Germline mutations are involved in more than one-fifth of OvCA cases, and about 65–85% of hereditary ovarian tumors are related to highly penetrant DNA repair-associated genes like BRCA1 and BRCA2 [8]. Other tumor suppressor genes and oncogenes, including the mismatch repair (MMR) genes in Lynch syndrome and other DNA repair genes (i.e., BARD1, CHEK2, RAD51C, RAD51D, PALB2, and BRIP1) are also known to be involved in the mechanism of hereditary ovarian tumorigenesis [9].
Standard treatments for OvCA-diagnosed patients include surgery and chemotherapy (co-treatment with carboplatin and paclitaxel). Currently targeted therapies under investigation include antiangiogenic agents, poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors, hormone receptor modulators, and immune checkpoint inhibitors [10]. It has been reported that combination therapy with antiangiogenic antibody bevacizumab and standard chemotherapy does not give a substantial difference in the overall survival compared to chemotherapy alone [11]. While the exploitation of neoadjuvant chemotherapy is an even more expanding option, treatment of HGSOC remains a clinical challenge [12].
Recurrence of remission post-surgery and/or chemotherapy is a major feature of OvCA, as a consequence of the induction of multidrug resistance. Genetic and epigenetic mutations leading to extrusion or inactivation of cytotoxic drugs, impaired apoptosis, and enhanced induction of repair mechanisms are major orchestrators of this process, all together contributing to the poor prognosis of OvCA. Thus, novel therapeutic strategies and biomarkers are urgently needed.
2. OvCA Tumor Immune Microenvironment (TIME)
Besides malignant transformed cells, tumors are composed of normal cells including epithelial cells, fibroblasts, muscle cells, and inflammatory immune cells, altogether generating the TIME [13,14,15]. Within this environment and upon tumor-driven stimuli, cancer can generate a tumor-permissive soil by reprogramming cells of the hosts that acquire tumor-supporting phenotypes and functions [13,14,15].
Evidence has demonstrated that OvCA possesses specific metastatic tropism to the omentum, characterized by highly vascularized immune cell structures called milky spots, playing a pivotal role in the creation of the metastatic TME in the intraperitoneal cavity. OvCA peritoneal metastasis is distinctive because of the extraordinary inflammatory and immunosuppressive milieu of the intraperitoneal cavity, associated with accumulation of malignant ascites [16,17]. Research to date has not yet determined the cellular dynamics that establish the premetastatic niche in OvCA and the omentum.
However, it is now recognized that the metastatic step to the omentum and peritoneum is sustained by a TME enriched with pro-tumor soluble factors, migrated cancer cells, anergic and pro-tumor inflammatory/immune cells, and other host cells, supporting tumor cell proliferation, progression, chemoresistance, and immune evasion [18].
Apart from genetic and epigenetic alterations, OvCA is characterized by a unique peritoneal TME that instructs the complex network between tumor cells and immune cells of the hosts [18,19].
After the “genomic era”, a relevant shift to the host cells as target, first with endothelial cells and angiogenesis and recently with impaired immune response as a principal hallmark of many tumors, occurred. This knowledge has launched numerous clinical trials testing immunotherapy, starting with melanoma, lung, colon cancer and also OvCA [20].
Several cells of both innate and adaptive immunity, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), γδ T cells, and natural killer (NK) cells, directly or indirectly (via soluble interactions) shape the peritoneal TME, creating a permissive environment for tumor development and a favorable metastatic soil [18,19].
One of the main reasons for disease progression and treatment failure is the establishment of a complex immune suppression network that effectively neutralizes antitumor activity of TAMs, TANs, γδ T cells, and NK cells. To make matters worse, TAMs, TANs, and NK cells acquire pro-tumor activities by increasing vascular endothelial growth factor (VEGF) and matrix metalloproteinase (MMP) expression, sustaining tumor vascularization metastasis [21,22,23].
Based on this knowledge, it is clear the central role of innate and adaptive immunity in orchestrating tumor cell fate. Indeed, cancer immunotherapy has emerged as one of the most promising approaches in oncology, assuring the (re)activation of the host defenses, a major strategic role in fighting cancers.
A wide literature is available on immune suppressive effector cells within the TIME, such as MDSCs and T regulatory (Treg) cells [24,25,26], but here we will focus on selected players of innate immunity. In this review, we discuss on macrophages, neutrophils, γδ T cells, and NK cells as relevant TIME-drivers in OvCA progression and as targets for immune therapy.
3. Macrophages
Circulating monocytes are recruited to the tumor site by different factors, such as CCL2 (MCP-1), macrophage colony stimulating factor-1 (M-CSF/CSF-1), and metabolites of 5-lipoxygenase [27], and differentiate in response to the microenvironment stimuli into classically activated M1 or alternative activated M2 phenotypes. The M1 macrophages are induced by interferon γ (IFNγ) and lipo poly saccharide (LPS) and exhibit antitumor activity, whereas Th2 signals, such as IL-4 and IL-13, polarize monocytes to the M2 subtype that promote tumor development secreting a variety of growth factors as well as angiogenic and immunosuppressive cytokines [28]. The recruitment of macrophages represents one of the crucial events for the ovarian cancer progression and metastasis [29]. Given the heterogeneity of TAM subsets, the role of these cells in OvCA is still widely discussed. Different studies have shown that a high density of M2-polarized macrophages, particularly the CD163+ subset, is associated with poor prognosis and worse overall survival in epithelial ovarian cancer (EOC) whereas high M1/M2 macrophages ratio predicted better prognosis [30,31,32]. A recent cohort study conducted on 140 patients with both different histotypes of primary OvCA and ovarian metastasis from other sites demonstrated that primary HGSOC correlates with a prevalence of M1-like TAMs, a higher M1/M2 macrophages ratio, and longer overall survival (OS) than other tumor subtypes [33]. On the other hand, low-grade serous OvCA exhibited a lower density of CD68+ M1-like macrophages and an M2-skewed (CD163+) phenotype [34].
High levels of CD163+ macrophages are also associated with high levels of IL-6 and IL-10 and short survival in HGSOC patients [35]. These data indicate that the differential macrophage polarization may influence the development of different OvCA subtypes, suggesting the relevance of TAM as a prognostic and predictive factor for OvCA. Although the factors responsible for TAM polarization is not completely understood, it is widely accepted that TAM differentiation involved complex crosstalk between tumor cells and macrophages. Co-culture experiments of OvCA cell lines and macrophages revealed much about the interactions between these two cell types.
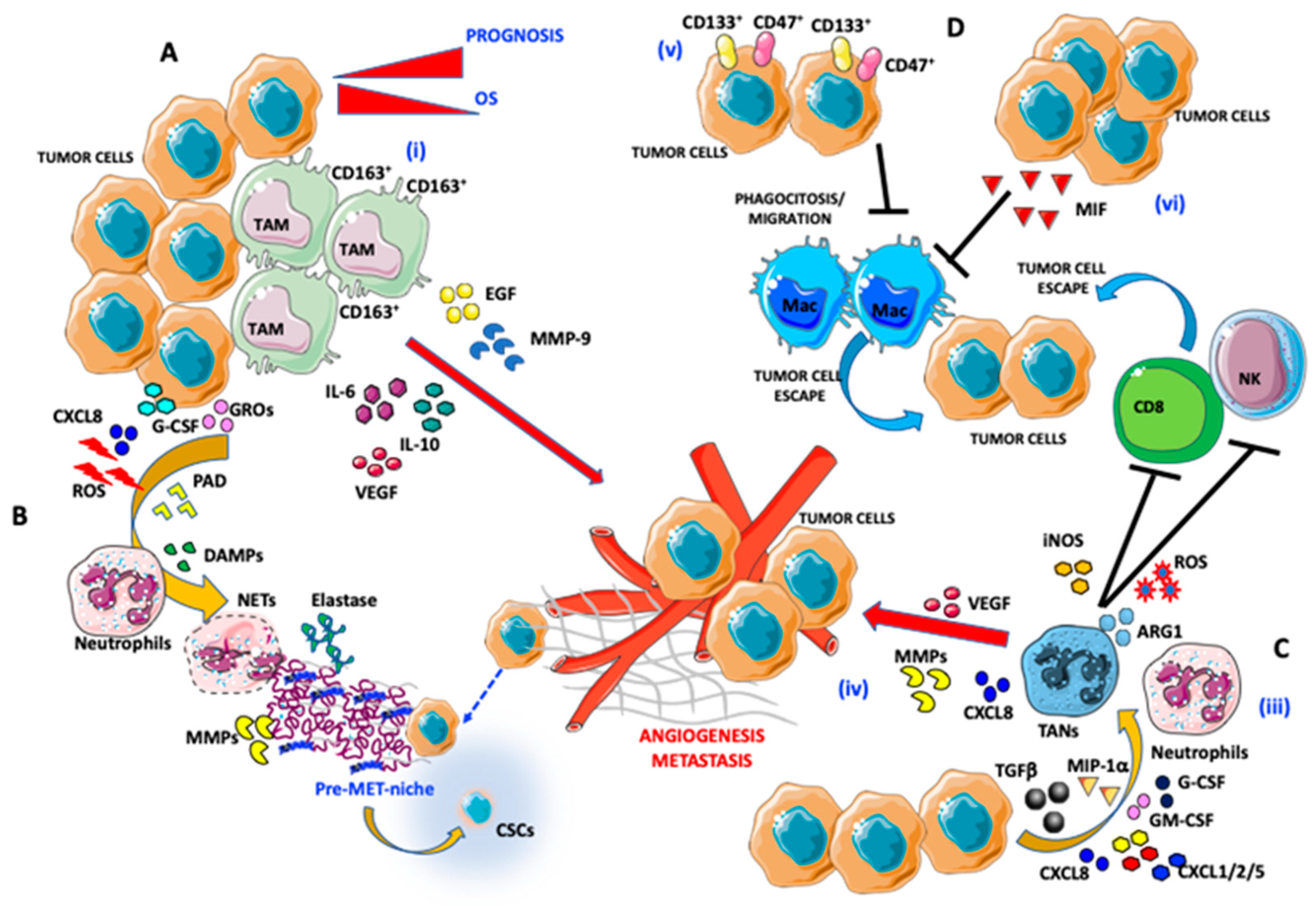
Within the TME, M2-like TAMs promote cancer cell proliferation through the activation of the MMP9/HB-EGF pathway and epidermal growth factor (EGF) production, leading to the stimulation of VEGF signaling that supports tumor metastasis [36] (Figure 1).
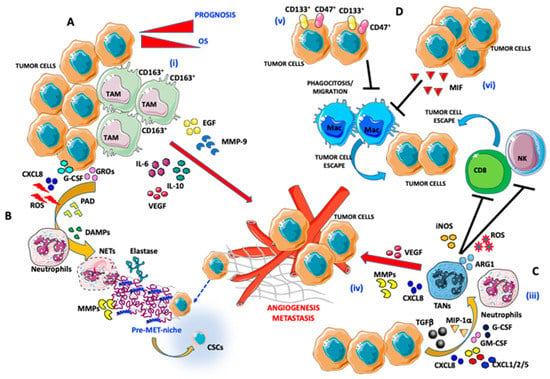
Figure 1.
Pro-angiogenic and pro-metastatic tumor immune microenvironment (TIME) landscape in ovarian cancer: focus on macrophages and neutrophils. (A) Interaction between tumor-associated macrophages (TAMs) and ovarian cancer (OvCA) cells: (i) CD163+ TAMs, by secreting epidermal growth factor (EGF), IL-6, IL-10, vascular endothelial growth factor (VEGF), and matrix metalloproteinase (MMP)-9 trigger angiogenesis and metastatic dissemination. (B,C) Interaction between tumor-associated neutrophils (TANs) and OvCA cells: (ii) OvCA cells by releasing CXCL8, g-colony stimulating factor (CSF), growth-regulated oncogenes (GROs), and reactive oxygen species (ROS) cooperate with peptidyl arginine deiminase (PAD) and damage-associated molecular patterns (DAMPs) in inducing neutrophil extracellular trap (NET) formation by neutrophils. NET induction is associated with elastase and MMP release that instruct a tumor permissive metastatic niche formation that includes the cancer stem cell (CSC) components and tumor-escape. (iii) Neutrophils are polarized into TANs through transforming growth factor β (TGFβ), macrophage inflammatory protein (MIP)-1a, CXCL1/2/5/8, GM-CSF, and G-CSF secretion by OvCA cells. (iv) Induction of TAN results in promotion of angiogenesis (via VEGF and CXCL8 production) and natural killer (NK) cells and CD8 anergy in an iNOS/ARG1/ROS-dependent manner. (D) OvCA/TAM interaction in the TIME supporting tumor cell escape: (v) CD133- and CD47-expressing OvCA cells act on macrophages by blocking their migration in tumor tissues and phagocytosis. (vi) Macrophage migration inhibitory factor (MIF) released by OvCA cells also blocks efficient phagocytosis by macrophages.
In addition, co-culture of stage III human ovarian adenocarcinoma cells (IGROV1) and polarized M2 macrophages resulted in enhanced invasiveness of tumor cells, increasing the activity of NF-kB-p65 and c-Jun [37]. Since the blockade of two NF-kB and JNK II-dependent factors, such as extracellular matrix metalloproteinase inducer (EMMPRIN) and macrophage migration inhibitory factor (MIF), reduced the invasiveness of the tumor cell lines, the authors suggested their involvement in this context. Particularly, IGROV1 upregulation of EMMPRIN appeared to induce MMP9 and MMP2 expression in macrophages. Similarly, MIF promotes tumor cell invasiveness, inducing macrophage secretion of MMP [37]. In line with these results, co-culture of the monocytic cell line (THP-1) and BRCA2-mutated ovarian adenocarcinoma cell line (PEO-1) showed that the tumor-derived matrix metalloproteinase-stimulating factor (MMPSF) among with autocrine monocyte-derived TNFα stimulate the production of pro-MMP-9 [38]. In addition, TAMs can induce OvCA invasion, upregulating scavenger receptor SR-A [39]. Indeed, macrophages from SR-A-/- mice co-cultured with the mouse ovarian epithelial papillary serous adenocarcinoma cells (ID8) showed a reduced ability to promote tumor cell invasion. The inhibition of SR-A by 4F peptide, an apolipoprotein A-1 mimetic, was able to counteract tumor growth in an in vitro assay as well as in a mouse model of OvCA, providing support for the SR-A involvement [40]. Together with the previous antitumor effect of subcutaneous and oral administration of 4F in C57BL/6 mice [40], these data suggest a novel potential therapeutic target.
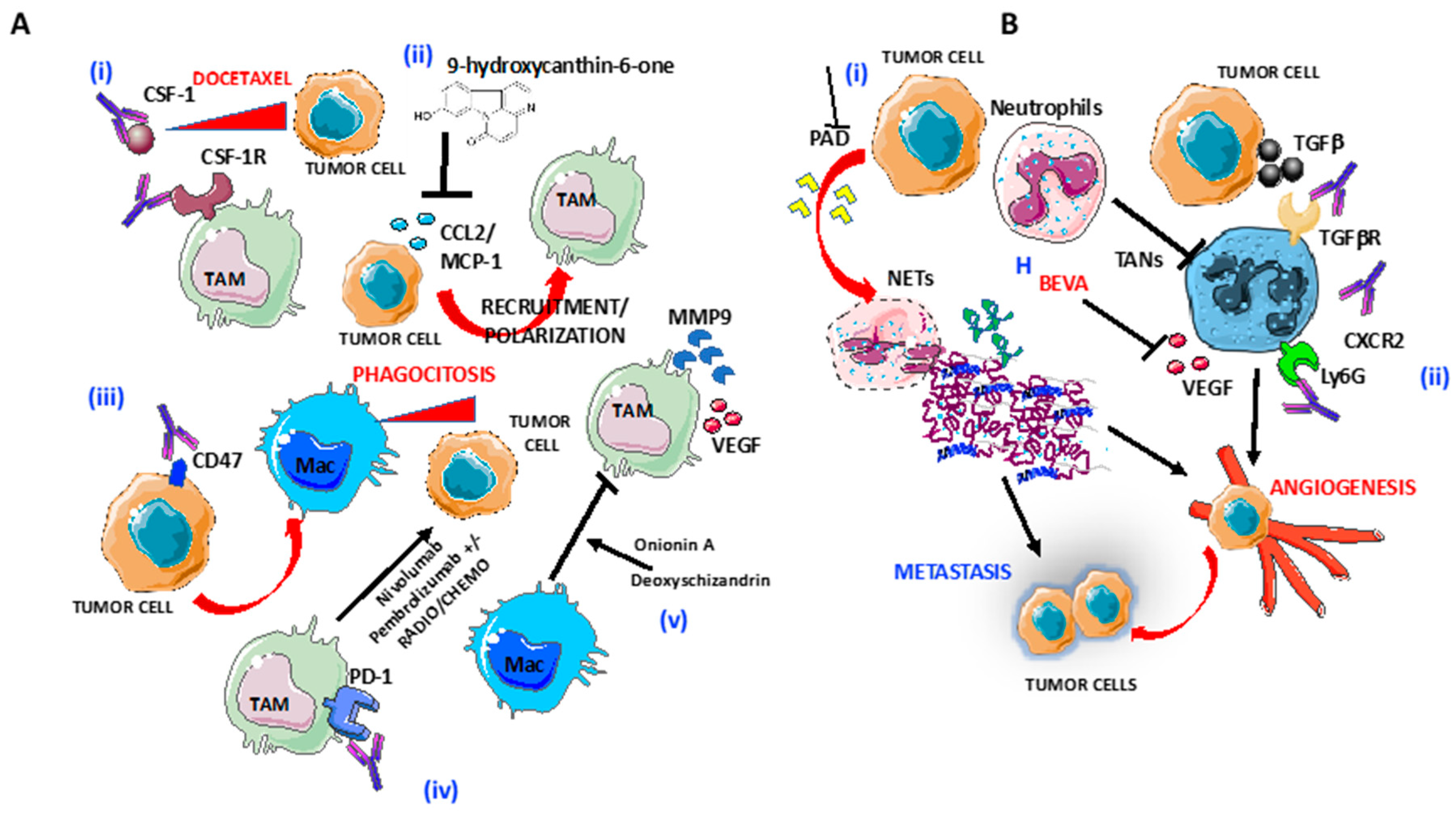
Based on their role in tumorigenesis, TAM depletion emerged as a promising target for OvCA treatment. Germano and colleagues demonstrated that the DNA binder agent trabectedin, approved for the treatment of platinum-sensitive OvCA, causes a significant and selective reduction of tumor macrophages in both the syngeneic (ID8) and xenograft (IGROV) models of OvCA [41]. In the same animal models, the G5-methotrexate nanoparticles targeting folate receptor-2 (FOLR-2) was proposed as the alternative way to deplete TAM and showed greater effectiveness than cisplatin [42]. Currently, many other different approaches acting on TAM have been developed and some are available for clinical evaluation. One of the strategies accounts for the inhibition of macrophage recruitment at the tumor site. For this reason, some clinical trials involving the CSF-1R and CCR2 pathways are ongoing. The reduction of infiltrating macrophages due to the blockade of CSF-1 and its receptor (CSF-1R) was shown to enhance the anti-tumor effect of docetaxel in a mouse model of epithelial OvCA [43] (Figure 2). In another syngeneic mouse model of late-stage EOC, selective inhibition of CSF-1R significantly reduced ascites accumulation and M2 macrophages infiltration, thus reversing the correlated vascular dysfunction [44]. Likewise, the reduction of CCL2 expression by the natural product 9-hydroxycanthin-6-one has been reported to inhibit macrophage recruitment, as well as to counteract the ovarian conditioned medium-driven M2-like phenotype [45] (Figure 2A). Indeed, limitation of M2 polarization represents a second strategy to target TAM. A gene expression analysis showed that the antitumor agent paclitaxel, used for the treatment of several solid tumors, including OvCA, can reinstruct the M2 signature of TAM toward the M1 phenotype [46]. Since, in TLR4 KO mice, paclitaxel-induced M1 polarization was abolished, this effect seems to involve TLR4 activation [46]. Further, as described above for the scavenger receptor SR-A (CD204), M2 macrophages upregulate the mannose receptor (CD206). Human antibody against CD206 has been demonstrated to prevent in vitro TAM polarization by blocking the crosstalk between the HGSOC cell line OVCAR5 and differentiated CD206low/high macrophages via tumor-released mesothelin [47]. TAM repolarization may be also induced by natural compounds, such as Onionin A, from onions and Deoxyschizandrin, from berries, that can abrogate pro-tumor activation of M2 macrophages and can reduce the production of the tumor-promoting factors (MMP9, CCL5, and VEGF) [48] (Figure 2A).
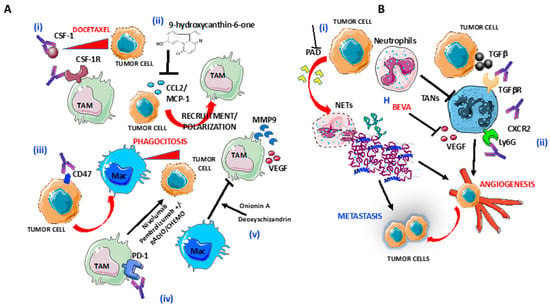
Figure 2.
Targeting of macrophages and neutrophils in OvCA: (A) Several strategies to target tumor-associated macrophages (TAMs) are shown: (i) blocking of CSF-1R by anti-CSF1 mAbs induce increased responsiveness of tumor cells to Docetaxel; (ii) blocking of CCL2 release by 9-hydroxycanthin-6-one inhibit TAMs recruitment and polarization; (iii) selective blocking of CD47 on tumor cells macrophage infiltration and phagocytosis of ovarian tumor cells; (iv) anti-PD1 Nivolumab and Pembrolizumab in combination with radiotherapy and chemotherapy enhance tumor cells lysis; and (v) Blocking of M2-like/TAM polarization by as Onionin A, from onions and Deoxyschizandrin, from berries. (B) Several strategies to target tumor-associated neutrophils (TANs) are shown: (i) blocking of peptidyl-arginine deaminase (PAD) release by tumor cells inhibits neutrophils extracellular trap formation and (ii) preventing TAN polarization by blocking Ly6G and CXCR2 in combination with checkpoint inhibitors, TGFβR and VEGF, through Bevacizumab.
Another macrophage-oriented treatment approach consists of the retrieval of the existing antitumor response, acting on immune checkpoint molecules such as PD-L1 and B7-H4. The programmed cell death-1 (PD-1) is a cell surface receptor that binds two PD-1 ligands (PD-L1 and PD-L2) and negatively regulates T cell activation, suppressing the immune response [49]. Many tumors, including OvCA, can escape host immunity through the PD-1 pathway [50]. Recently, it has been demonstrated that TAMs express PD-L1 in primary epithelial OvCA and metastatic HGSOC [51]. Whereas, in primary epithelial OvCA, PD-L1 is predominantly expressed on immune cells, in recurrent epithelial OvCA, the expression extends to both immune and cancer cells [52,53]. Interestingly, analysis of TME in ovarian platinum-sensitive recurrent cancer revealed higher “classically activated” M1-like macrophage density and higher CD25+ Treg cells than in primary tumor, which correlate with long overall survival. Despite the role of PD-L1 as a negative regulator of antitumor response, the data suggested that the dynamics between TAM PD-L1 expression and cytolytic and regulatory T cell subsets consist in a mechanism of adaptive resistance, resulting in an immunological stalemate. In such condition, long-termed immunotherapy may be required [51].
Similar to PD-L1, B7-H4 is highly expressed on the surface of TAMs in high-grade OvCA [54]. Its overexpression correlates with the presence of IL-6 and IL-10 in TME, and it is inhibited by GM-CSF and IL-4 [55]. B7-H4+ macrophages counteract T cell-proliferation, cytokine production, and cytotoxicity, suggesting its involvement in the tumor immune escape mechanism [55]. Furthermore, another crucial role is played by CD47, an integrin highly expressed by OvCA cells, which correlates with poor prognosis in OvCA. CD47, interacting with thrombospondin-1 (TSP-1) and signal regulatory protein alpha (SIRPα), negatively regulates macrophage activity [56]. Anti-CD47 mAb treatment was shown to increase in vitro macrophage phagocytosis, to inhibit tumor formation, and to enhance macrophage infiltration in xenograft model of ovarian clear cell adenocarcinoma (SKOV-3) (Figure 2A). In addition, CD47 was highly expressed on the CD133+ tumor-initiating population and was able to trigger their phagocytosis [57]. Therefore, given their role as suppressive regulators of the immune response in OvCA, blocking B7-H4 and CD47 molecules or depleting this suppressor cell subtype of TAM have been proposed as promising targets to prevent metastasis and recurrence of OvCA. Thus, the strategy to exploit TAM by repolarization and deletion by killing or blocking of recruitment in combination with immune checkpoint inhibitors represents an attractive target to switch macrophages response in favor of ovarian cell death.
4. Neutrophils
Neutrophils represent the first line of defense of the innate immunity against insults from pathogens [58]. Mobilization of neutrophils from the bone marrow depends on a series of stimulating factors and cytokines, including IL-23, IL-17, G-CSF, and CXC chemokine receptors [59]. The detection of neutrophils within the TME is an indirect parameter of inflammation, which is a hallmark of cancer [60]. However, in the cancer scenario, neutrophils have been considered as inert bystanders due to their very short circulating lifespan [61,62]. Evidence now shows that TME extends the neutrophil lifespan and manipulates their differentiation state, generating diverse phenotypic and functional subsets: antitumor “N1-neutrophils” and pro-tumor “N2-like/tumor-associated neutrophils” (TANs). TAN activities in cancer are complex and context dependent [63]. Like TAMs, TANs can exert antitumor or pro-tumor functions [64,65], depending on the tumor microenvironment and related stimuli [63,64,66]. Gene expression analysis revealed TAN-specific signatures associated with tumor resistance to radiotherapy and predicted poor patient survival in 25 malignancies [67]. CXCR1 and CXCR2 are the major neutrophil receptors that mediate neutrophil chemotaxis to the TME in response to G-CSF, GM-CSF, CXCL1, CXCL2, CXCL5, CXCL8, and MIP-1α [61,68] (Figure 1). Neutrophils promote tumor initiation, growth, proliferation, and metastatic spreading through release of growth factors, myeloperoxidases ROS [61,69], VEGF, and MMP9 [70] (Figure 1). Finally, neutrophils facilitate tumor proliferation by enhancing immunosuppression and by attenuating the immune system response. Increased production of ROS, inducible nitric oxide synthase (iNOS), or arginase 1 (ARG1) released by neutrophils suppress CD8+ T lymphocyte antitumor activities [66] and can reduce NK cell function [71], thereby facilitating the extravasation of tumor cells and promoting tumor growth and metastasis (Figure 1).
In OvCA, neutrophils are critical players and have been proposed as new potential biomarkers of disease outcome or as therapeutic targets [72]. Meta-analysis studies on systemic inflammatory markers suggest that neutrophil–lymphocyte ratio (NLR) can potentially serve as a prognostic biomarker in ovarian cancer patients [73,74].
An elevated NLR is a predictive factor both for early stage [75] and for poor overall survival in advanced stage of OvCA [30,73,76]. These findings suggest a role of neutrophil influx into the premetastatic niche during early-stage ovarian tumors. Results from recent studies highlight new insight about neutrophils as a “mobile fertilizer” [77] that establish the premetastatic omental niche via neutrophil extracellular traps (NETs). NETs have been found in the omentum of ovarian tumor-bearing mice before metastasis and of patients with early-stage OvCA [78]. Thus, NETs represent an early response by neutrophils to the presence of OvCA, with a key role in the generation of the “fertile” and permissive niche for the subsequent colonization of ovarian circulating cancer cells [77,78]. Accordingly, blockade of peptidyl-arginine deiminase 4 (PAD4), an essential enzyme for NET generation, decreased omental metastasis in orthotopic OvCA models [78] (Figure 2B). NETosis depends on the generation of ROS, CXCL-8, G-CSF, growth-regulated oncogene (GRO) α, or GROβ factors released by early-stage ovarian tumors that stimulate the influx of neutrophils into the premetastatic omental niche [78] (Figure 1). Neutrophil elastases and MPPs of NETs favor the induction of an immunosuppressive microenvironment and reactivate the tumorigenic program in dormant/cancer stem cells [62] (Figure 1). Mitochondrial damage-associated molecular patterns (DAMPs) also may activate neutrophil and generate NETs, thus facilitating metastasis and obstruct antitumor immunity in OvCA [79] (Figure 1).
Neutrophils can modulate and suppress the activity of T cells in the OvCA microenvironment [80,81] and can facilitate the immune escape of OvCA cells by upregulating the expression of PD-L1 [80] (Figure 1). Another recent study confirmed that NLR ratio positively correlates with increased Th17/IL-17 levels and expression of PD-L1 in ovarian carcinoma [82].
Since neutrophils act as pro-tumor immune cells in many human cancers, strategies intended to reduce their activity have been explored and some have entered clinical evaluation. Therapies targeting CXCLs/CXCR2 axis [83], and G-CSF [84,85], that may reduce the migration and the number of neutrophils in tumors areas, have been evaluated in several preclinical studies and clinical trials. Genetic approaches targeting CXCR2 or employment of pharmaceutical CXCR2 inhibitor SB225002 showed promising results in impeding OvCA peritoneal metastases in SKOV-3 xenograft mouse models [86].
The use of CXCR2 inhibitors reduced both neutrophil recruitment and NETosis, enhancing the survival of mouse models of pancreatic cancer. [87]. Similarly, blockades of IL-23 and IL-17 production which in turn regulates granulopoiesis through G-CSF represent a valid approach to control neutrophil production in vivo [88,89,90].
TANs are driven by TGFβ to acquire a polarized N2-like/TAN phenotype; thus, employing TGFβ receptor blockers could be a strategy to shift from N2 to the acquisition of N1 antitumor phenotype and activities [66] (Figure 2B). Some studies have reported an increased effect of combining CXCR2 inhibitors or anti-Ly6G and checkpoint inhibitors that boost the immune system against tumor growth and dissemination [91] (Figure 2B).
Since resistance to antiangiogenic agents has been linked with neutrophil stimulation, combining antiangiogenic drugs with neutrophil targeting agents may sensitize resistant tumors to therapy with antibodies against VEGF [92]. On the other hand, bevacizumab enhanced clinical outcome in OvCA patients with a high NLR [93] (Figure 2B).
Given the dual role of neutrophils in cancer, the consequences of depleting tumor-promoting and antitumor neutrophils are not fully understood. Enhancing our understanding of the role of neutrophils in OvCA is crucial to identify novel prognostic markers and to develop effective therapeutic strategies.
5. γδ T Lymphocytes
The involvement of the immune system in the control of OvCA has been shown, and therapeutic options are based on regulating the ovarian TME to stimulate antitumor effector cells [94,95,96,97]. Among antitumor lymphocytes, besides NK cells and cytotoxic T cells (CTL), γδ T lymphocytes can eliminate OvCA cells [94,95,96,97]. The γδ T lymphocytes can be roughly subdivided into two major subsets: Vδ1 subtype is mainly present in tissues, while Vδ2 is the major subset of γδ T cells in the peripheral blood [89,94,95,97]. Both these two cell subsets share some relevant activating receptors such as NKG2D and DNAM-1 [98] that can recognize counter-ligands on ovarian target cells leading to tumor cell killing. Several inhibitory receptors can play a role in regulating γδ T cell killing such as Killer Ig-like inhibitory receptors and C-type lectin inhibitory receptors [95,97,98,99]. Thus, a strict balance between positive and negative signals delivered during the interaction of γδ T cells and OvCA cells eventually leads to the elimination of target cells.
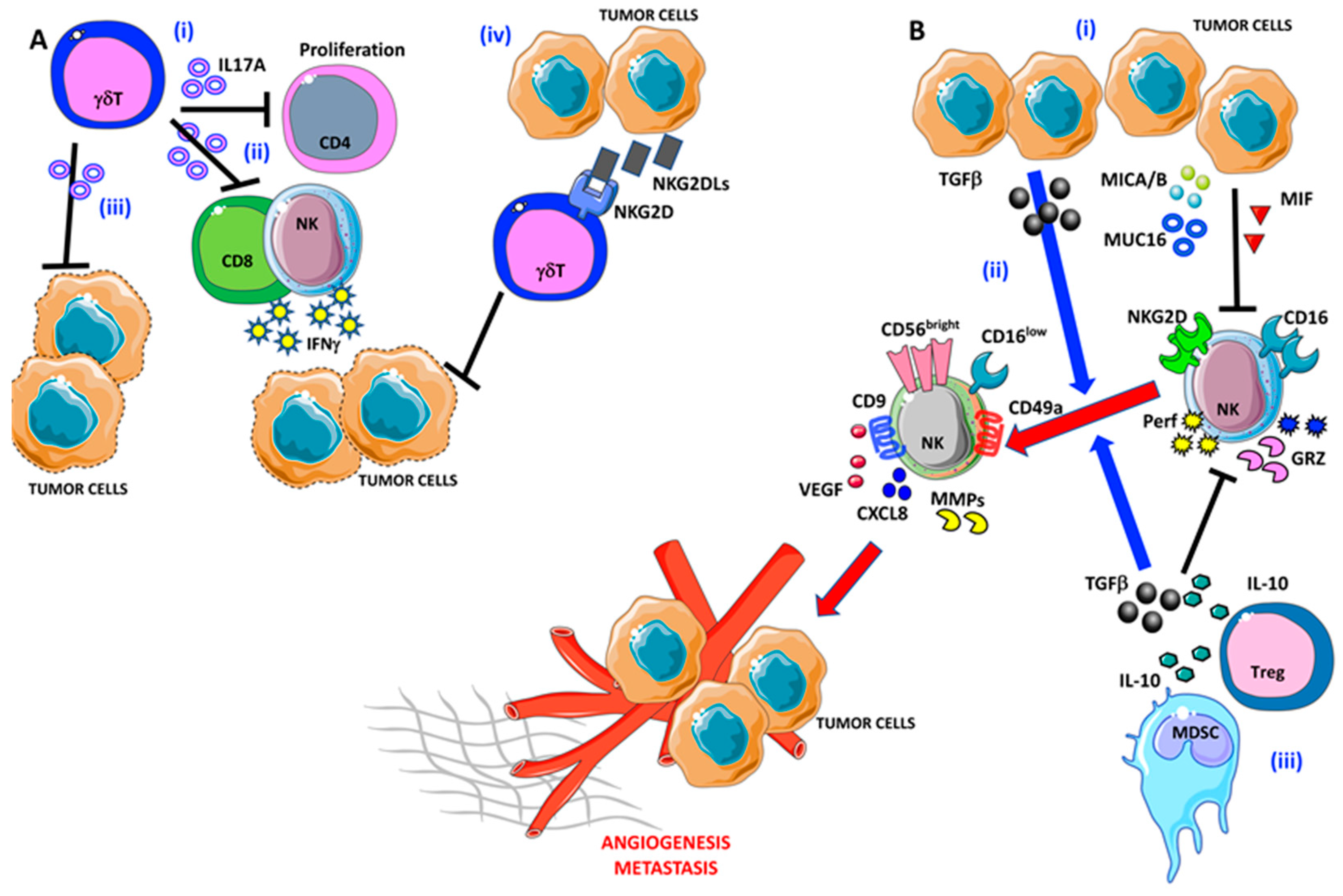
Epithelial ovarian cancers typically express abundant NKG2DL, which are strongly associated with negative disease outcomes [100,101,102] (Figure 3A).
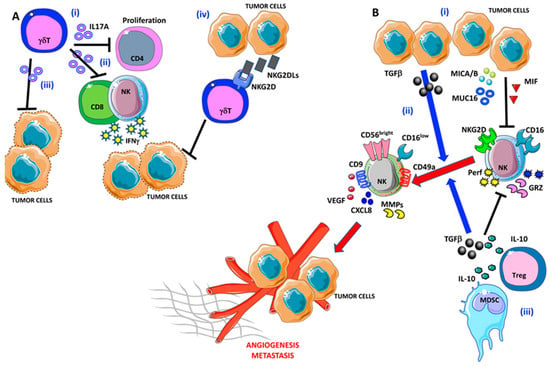
Figure 3.
Pro-angiogenic and pro-metastatic TIME landscape in ovarian cancer: focus on γδ T and NK cells. (A) (i) γδ T cells are able to block CD4 T cell proliferation and (ii) interferon γ (IFNγ) release by NK cells and CD8+ T cells via IL-17A. (iii) IL-17A-producing γδ T cells are not efficient in tumor cell lysis. Moreover, OvCA cells are able to produce large amounts of NKG2DLs that interfere with tumor cell elimination by γδ T cells (iv). (B) (i) OvCA released several factors that are involved in NK cell polarization, in particular TGFβ, MICA/B, MUC16, and MIF that induce NK cell anergy by decreasing their ability to produce perforin and granzymes. (ii) Besides, TGFβ participates in the pro-angiogenic switch of NK cells that acquire the decidual-like phenotype and functions. (iii) Finally, myeloid-derived suppressor cells (MDSCs) cooperate with the OvCA cells to NK cell anergy/acquisition of pro-angiogenic feature via TGFβ and IL-10.
This unexpected effect can be dependent on the immunosuppressive microenvironment supported by the continuous presence in the extracellular milieu of soluble NKG2DLs, which function as decoy receptors binding the NKG2D molecules on antitumor effector lymphocytes such as γδ T cells, besides NK cells and cytolytic CD8+ T cells [103,104]. Alternatively, the expression of NKG2DL can give an advantage to tumor cell growth and expansion [105]. More interestingly, the expression of NKG2D molecules on OvCA cells together with NKG2DL can trigger autonomous stimulation of cancer cells as shown in breast cancer cell lines [105,106]. Thus, NKG2D+ ovarian cells can have a stronger ability of self-renewing sphere formation in vitro and tumor generation in vivo in immunodeficient mice than NKG2D negative tumor cells [106].
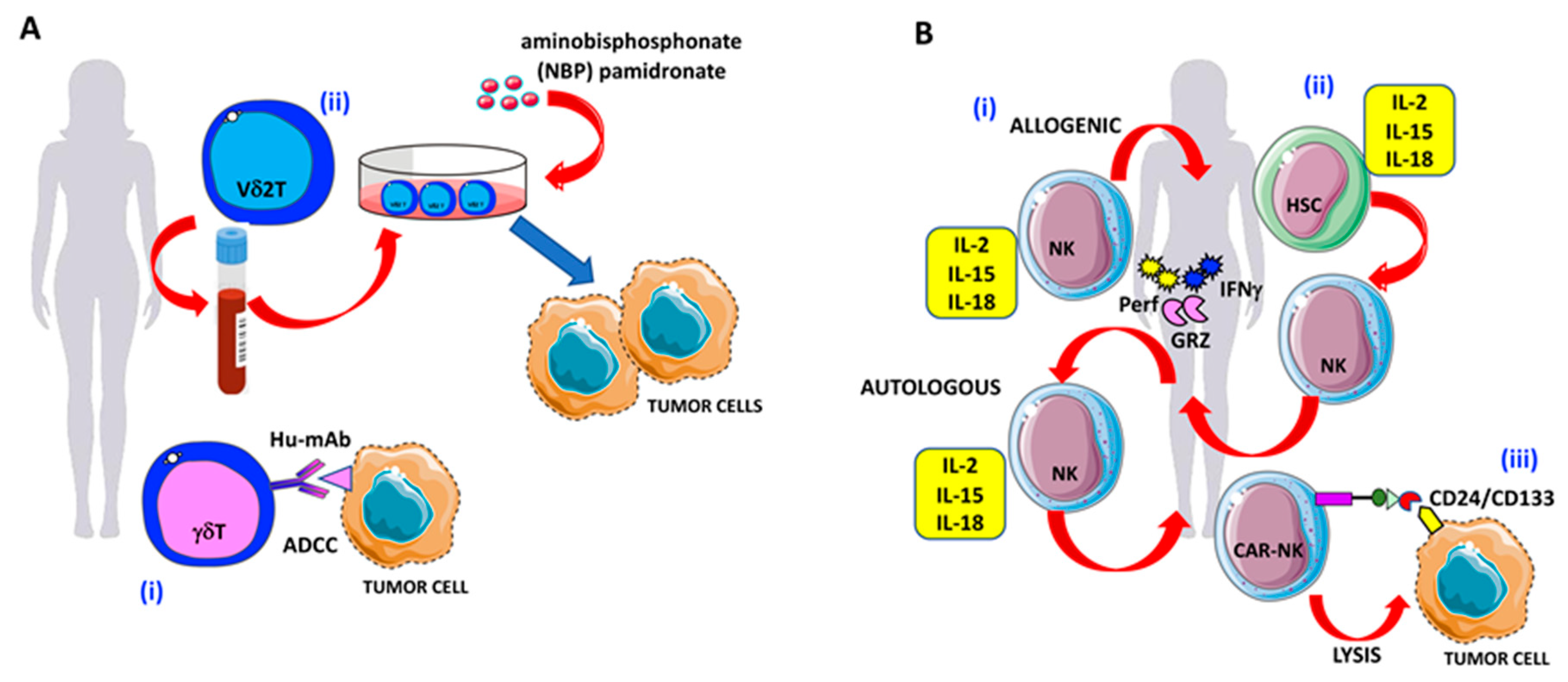
The γδ T cell-mediated killing can be exploited also using humanized monoclonal antibodies (Hu-mAb) able to elicit the antibody-dependent cellular cytotoxicity (ADCC) (Figure 4A) exerted by immune cells bearing the FcγRI/II/III. Notably, this mechanism is shared by γδ T cells and other effectors of the innate immunity, including NK cells and monocytes/macrophages [95,97,99,107,108]. Thus, γδ T cells can eliminate OvCA cells both through specific activating receptors and by using Hu-mAb if the antigen recognized by these antibodies is expressed on cancer cells (Figure 4A).
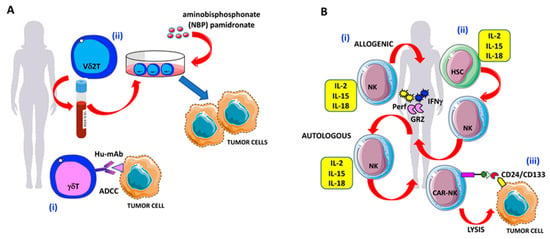
Figure 4.
γδ T and NK cell-based therapies against OvCA cells: (A) Strategies to enhance γδ T cell antitumor activities in OvCA. (i) Triggering of γδ T cell-mediated antibody-dependent cellular cytotoxicity (ADCC) by specific hu-mAbs. (ii) Expansion of Vδ2 T cells derived from the peripheral blood in the presence of the amino-bisphosphonates (N-BPs) such as pamidronate to increase OvCA cell elimination. (B) Strategies to enhance NK cell antitumor activities in OvCA. (i) Adoptive transferred NK cells, primed with IL-2, IL-15, and IL-18 to increase antitumor activities against OvCA via the production of granzymes, perforin, and IFNγ. (ii) Generation of IFNγ-, granzyme-, and perforin-producing NK cells by the administration of cytokine cocktails (IL-2, IL-15, and IL-18) to bone marrow or peripheral blood or cord blood-derived hematopoietic stem cells (HSCs); (iii) CD24/CD133 chimeric antigen receptor (CAR)-engineered NK cells with increased cytotoxic activity against CD24-positive OvCA cell lines (SKOV3 and OVCAR3).
Recently, it has been shown that γδ T lymphocytes in several solid tumors, such as colorectal, pancreatic, and breast cancers, can promote tumorigenesis and metastasis [109,110,111,112]. Similarly, it has been reported that γδ T cells present in OvCA specimens can produce IL-17A and can exert an inhibitory effect on cell proliferation of CD4+ naïve T cells together with low production of IFNγ and a reduced anti-OvCA cytolysis [113] (Figure 3A). Vδ2 T cells expanded from the peripheral blood (PB) can efficiently eliminate in vitro and in vivo murine models of OvCA cells (Figure 4A). This effect is further increased in the presence of pamidronate, a kind of amino-bisphosphonate (N-BPs) [114] (Figure 4A). This finding indicates that it is possible to trigger anti-OvCA effects using appropriate drugs and Vδ2 T cells. These data are in line with previous observations that liposome-encapsulated N-BPs could trigger the elimination of OvCA cells using PB-expanded Vδ2 T cells (Figure 4A). Importantly, this latter formulation of N-BPs can achieve a higher drug concentration than the free drug; the alteration of the pharmacokinetic properties of N-BPs can favor the delivery at the tumor site, reducing their tropism to bone [115,116].
6. Natural Killer cells
NK cells are effector lymphocytes of innate immunity, primarily involved in the recognition and elimination of virus-infected and malignant transforming cells [117,118,119]. NK effector functions include ADCC via CD16; production of perforin and granzymes (resulting in the induction of apoptosis on target cells); and release of antitumor cytokines, such as IFNγ and TNFα [117,118,119]. Two major NK cell subsets have been characterized within PB lymphocytes, according to the expression of the CD56 and CD16 surface antigens. CD56dimCD16+ NK cells (90–95% of total circulating NK cells) act mainly via perforin/granzymes and ADCC [117,118,119]. CD56brightCD16- NK cells (5–10% of total circulating NK cells) act via cytolytic cytokine production [117,118,119]. According to the tissue type and the physiopathological conditions, the frequency and distribution of these two NK cell subsets significantly vary, along with their functional impairment or over-activation [120,121,122,123,124,125].
Among the tissue adaptation of NK cell phenotypes, the decidua represents a very peculiar scenario. Apart from their immunotolerant behavior for the developing fetus, in the decidua (d), NK cells acquire the CD56brightCD16- phenotype, characterized by the release of high amounts of pro-angiogenic cytokines (such as VEGF, PlGF, and CXCL8) [86,87]. These decidual NK (dNK) cells account for over 50% of total lymphocytes found in this tissue, and they are necessary for the formation of the spiral arteries needed to assure the proper fetus/maternal interface [86,87].
Alteration of NK cell functions had been observed in preclinical models and patients with several cancers [117,120,121,126,127,128,129,130,131], including OvCA [132,133,134]. On the other hand, several studies supported the use of NK cell therapy in OvCA [132,133,134]. Altered NK cell phenotype and functions in OvCA arise as a consequence of OvCA-associated immunosuppressive cytokines and those produced by the surrounding microenvironment [135].
Macrophage migration inhibitory factor (MIF) overexpression has been found in OvCA (Figure 2B). MIF acts on NK cells by downregulating the expression levels for the NKG2D activation receptor and by increasing expression for the inhibitory checkpoint B7-H6; these features are associated with poor OvCA prognosis [136].
MDSCs [137,138] and Treg cells [139,140] are increased in OvCA TME and release large amounts of IL-10 and TGFβ (Figure 3B). TGFβ is largely produced in the TME, both by tumor, stroma, and immune cells [131,141,142,143]. Apart from immunosuppression, cytolytic NK cells exposed to TGFβ have been demonstrated to acquire the dNK-like, pro-angiogenic CD56brightCD16low subsets [143,144] (Figure 3B). Given the similarities between the decidua microenvironment and the TME, it is expected that OvCA, as a consequence of its “embryonic-like” features, will employ altered NK cells for tumor progression and angiogenesis.
Sun et al. demonstrated that NK cells from patients with OvCA can effectively lyse different OvCA cell lines, including the HGSOC cell lines OVCAR3 and CAOV-3, the clear cancer cell line SKOV3, and the ovarian endometrioid carcinoma cell line A2780, and can reduce their migratory activities [134]. In addition, immunotherapy with NK cells resulted in reduced metastasis and prolongs survival in an in vivo model where immune-deficient BALB/c-nude mice were implanted with patient-derived CAOV-3 cells [134]. Moreover, mice treated with NK cells also exhibited increased infiltration of CD4+ and CD8+ T cells along with larger IFNγ levels [134]. Poznansky et al. showed that expanded CD56superbrightCD16+ NK cells from OvCA patients are cytotoxic against autologous tumor in a patient-derived xenograft murine model [132].
Mucin 16 (MUC16/CA125) has been reported to be highly expressed in OvCA [145,146] and has been recognized as a biomarker to monitor epithelial OvCA and for the differential diagnosis of pelvic masses [147,148]. MUC16 supports tumor cell proliferation and dampens immune cell response. Large amounts of MUC16 have been also detected in the peritoneal fluids (PFs) of patients with OvCA [149,150]. Reduced expression of CD16 has been detected in NK cells from OvCA TME [151]. MUC16 has been found to inhibit CD16 expression in peritoneal fluids (Figure 3B). NK cells derived from healthy donors exhibit the same reduced expression of CD16 when exposed to peritoneal fluids of patients with OvCA that are enriched in MUC16. Interestingly, MUC16 is highly expressed during pregnancy. Similar to OvCA, MUC16 production is elevated in early pregnancy and contributes to the induction of the tolerogenic environment during decidualization acting on dNK cells [152,153]. This reinforces the hypothesis that the immune contexture between the TME and the pregnancy environment share several features both at the cellular and molecular levels, governing the fetus–maternal tolerance and immune evasion by OvCA.
The development of ascites is a major feature of OvCA. Ascites fluids are enriched in cytokines and growth factors that support tumor cell proliferation and anergy in immune cells [154,155]. CD3-CD56+CD16+ NK cells have been found at higher concentration in ascites fluids, as compared to those of peripheral blood of patients with OvCA [151]. Therefore, these NK cells exhibit decreased expression of CD16, along with reduced cell proliferation, cytokine release, and cytolytic activities.
Overproduction of TGFβ has been found in patients with OvCA [76,141,142]. TGFβ is also abundant in ascites fluids from OvCA patients [156]. TGFβ acts as a master switcher of immune suppression in NK cells [157], also by converting cytolytic CD56dimCD16+ ADCC competent NK cells into CD56brightCD16-/lowIFNγ-producing NK cells [158] (Figure 3B). TGFβ is also implicated in the induction of a pro-angiogenic NK cell phenotype in non-small cell lung cancer and colorectal cancer patients [120,121,126,127,128] (Figure 3B) and thus might contribute to NK cell angiogenic switch in OvCA.
The tumor-related MICA/B and B7-H6 ligands have been frequently found in the peritoneal fluids of patients with OvCA. MICA/B and B7-H6 ligand–NK cell interactions resulted in impaired NK cell functions of NK cells [104,156] due to downregulation of NKp30 and decreased ability to release perforin, granzyme B, and IFNγ [156] (Figure 3B).
Cytokines represent the first line of immunotherapy approaches to boost NK cell antitumor activities. Intraperitoneal administration of IL-2 has been reported to increase NK cell number and activation in vivo. Limitations for use of IL-2 for immunotherapy deal with the fact that IL-2 exhibits very heterogeneous variability of success due to the effect of the tumor burden, prior to therapy administration. Combination approaches represent the best strategies to both combine enhanced activities and to reduce the toxicities related to the use of high doses of selected agents. In this scenario, the use of low doses of IL-2 in combination with 13-cis-retinoic acid has been reported to improve clinical outcome in platinum-sensitive advanced OvCA [159]; indeed, this combination resulted in increased T cell and NK cell frequency [159].
Cancer therapy also impacts priming the immune cell response [160,161]. In this view, Mallmann-Gottschalk and collaborators recently showed that OvCA cells, pretreated with anti- epithelial growth factor receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs), acquired increased drug-sensitivity via NK cell-mediated ADCC. Conversely, reduced cytokine secretion was observed in NK cells following TKI sensitization, suggesting that NK cells are differentially modulated by anti-EGFR TKIs [77].
The use of high doses of IL-2 has been associated with elevated toxicities [162,163,164]. Indeed, immunosuppressive Treg cells exhibit higher affinity with IL-2. To overcome these major issues, the use of immune-cytokines, known as antibody–cytokine fusion proteins, has been employed [162,163,164].
IL-15 has potent effects on NK cell activation [165] (Figure 4B). While less toxic compared to IL-2, to reach clinical effectiveness, higher doses of IL-15 are necessary [166]. Therefore, IL-15 administration overcomes the problem of Treg cell expansion that has a major affinity for IL-2 [167]. Current efforts are focusing on the association of IL-15 with super-agonists that act as cargos for the IL-15–IL-15Ra-subunit in a more stable complex [168]. Diverse clinical trials are currently evaluating the IL-15-primed NK immunotherapeutic properties [169]. In this context, elevated activation of NK cells in OvCA ascites has been reported, following interactions with monomeric IL-15 or the IL-15 super-agonist fusion complex ALT-803 [170,171].
Adoptive transferred NK cells, following pre-activation with IL-2, IL-15, and IL-18, have been reported to display increased antitumor activities in OvCA in vitro and in vivo (Figure 4B), and they can also persist in OvCA ascites fluids. NK cells exposed to the IL-15 super-agonist ALT-803 increase their degranulation activities against OvCA cells in vitro, and ascites from OvCA patients can release higher amounts of IFNγ.
Another source of NK cells for immunotherapy is represented by adult cell population, namely hematopoietic stem cells (HSCs) from bone marrow (BM), PB, or cord blood (CB) [121,172,173,174], following ex vivo expansion, cytokine activation. and patient reinfusion (Figure 4B). Induced pluripotent stem cells (iPSCs) have been also explored as a source for NK cell production [175]. Li et al. recently demonstrated that iPSC-NK cells, endowed with a chimeric antigen receptor (CAR) containing the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain, and the CD3ζ signaling domain, significantly inhibited tumor growth and prolonged survival compared to PB-NK cells in a xenograft model of OvCA. Therefore, NK-CAR-iPSC-NK exhibited similar in vivo activity as T-CAR-expressing T cells, though with less toxicity [175].
Several studies confirmed that CAR-engineered immune cells are powerful tools for cancer immunotherapy, and a plethora of CAR have been produced [176,177,178]. This approach has been characterized by a T-cell centric view. Considering their innate killing activities, it is clear that NK cells may be better CAR drivers, and many studies have been recently oriented toward this direction [179,180]. CD24 is a very promising target, which is rarely expressed in normal human tissue and is predominantly found on hematologic cells, and antibody-based treatments against CD24 have been evaluated in preclinical studies [181,182,183].
Lastly, Klapdor et al. recently showed that the human NK-cell line NK-92 with the anti-CD24 CAR showed high cytotoxic activity against CD24-positive OvCA cell lines (SKOV3 and OVCAR3) (Figure 4B). This effect was restricted to CD24-expressing cells [184].
7. Conclusions
The lack of curative treatment and the high relapse rate for women with advanced OvCA still represent an urgent unmet clinical problem, highlighting a clear need for new therapeutic strategies. As for some other cancers, immunotherapy approaches have not been as successful as expected. Indeed, the OvCA TME displays unique features leading to immune suppression and tolerance; this impairs both the presence and the activity of immune system components including TAMs, neutrophils, γδ T cells, and NK cells. The design of novel effective therapies should take into account this immunosuppression and should boost up the antitumor activity of the immune system. It is clear that some TIME components have a dual role in the development and progression of OvCA. Further studies can aid to plan effective strategies for the eradication of cancer cells by targeting innate immunity effectors. A better knowledge of the molecular mechanisms regulating the interaction between cancer cells and different cell subsets of innate immune system within the OvCA TME may provide new targets to relieve the impairment of immune response. This approach can contribute to the discovery of effective immunotherapeutic strategies aimed to stimulate the innate arm of immune system in combination with standard therapies for OvCA.
Author Contributions
D.B., A.B. (Annalisa Bosi), M.G., M.R., D.M.N., A.P., A.B. (Antonino Bruno), and L.M. wrote the manuscript. A.B. (Antonino Bruno) prepared the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Insubria intramural grant, Fondo di Ateneo per la Ricerca FAR 2018 and FAR 2019 to L.M. and by the Italian Ministry of University and Research PRIN 2017 grant 2017NTK4HY to D.M.N and the 5xmille 2015 and 2016 and Ricerca Corrente from Italian Ministry of Health, to A.P. The research leading to these results has received funding from AIRC under MFAG 2019-ID 22818-PI to A.B. This work has been supported by Italian Ministry of Health Ricerca Corrente—IRCCS MultiMedica. D.B. is funded by an “Assegno di Ricerca”, by the Italian Ministry of University and Research, University of Insubria. M.G. is a participant to the PhD course in Life Sciences and Biotechnology at the University of Insubria, Varese, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ADCC | Antibody Dependent Cellular Cytotoxicity |
| ARG | Arginase |
| BM | Bone Marrow |
| CAR-NK cells | Chimeric Antigen Receptor Natural Killer cells |
| CB | Cord Blood cells |
| CCL3/MIP-1α | Macrophage Inflammatory Protein-1α |
| CSF | Colony Stimulator Factor |
| CTLs | Cytotoxic T Cells |
| DNAM-1 | DNAX Accessory Molecule-1 |
| EGF | Epidermal Growth Factor |
| EOC | Epithelial Ovarian Cancer |
| FIGO | International Federation of Gynecology and Obstetrics |
| GROα/β | Growth-Regulated Oncogeneα/β |
| HB-EGF | Heparin-Binding EGF-like Growth Factor |
| HGSOC | High-Grade Serous Ovarian Cancer |
| HSCs | Hematopoietic Stem Cells |
| Hu-mAb | Humanized monoclonal antibodies |
| IFNγ | Interferon γ |
| iNOS | Inducible Nitric Oxide Synthase |
| iPSC | Induced Pluripotent Stem Cell |
| LPS | Lipo Poly Saccharide |
| MCP-1/CCL2 | Monocyte Chemoattractant Protein-1 |
| M-CSF-1 | Macrophage Colony Stimulating Factor-1 |
| mDAMPs | Mitochondrial Damage-Associated Molecular Patterns |
| MDSCs | Myeloid Derived Suppressor Cells |
| MIC A/B | MHC class I chain-related protein A and B |
| MIF | Macrophage migration Inhibitory Factor |
| MMPs | Matrix Metallo Proteinases |
| N-BPs | Aminobisphosphonate |
| NETs | Neutrophil Extracellular Traps |
| NK | Natural Killer |
| NKG2D | Natural Killer Group 2 member D |
| NKG2DL | Natural Killer Group 2 member D Ligand |
| NLR | Neutrophil–Lymphocyte Ratio |
| OvCA | Ovarian Cancer |
| PAD4 | Peptidyl Arginine Deiminase 4 |
| PARP | Poly (Adenosine diphosphate-Ribose) Polymerase |
| PB | Peripheral Blood cells |
| PD-1 | Programmed cell Death-1 |
| PD-L1 | Programmed cell Death Ligand-1 |
| PFs | Peritoneal Fluids |
| PlGF | Placental Growth Factor |
| ROS | Reactive Oxygen Species |
| SIRPα | Signal Regulatory Protein α |
| TAM | Tumor-Associated Macrophages |
| TANs | Tumor-Associated Neutrophils |
| TME | Tumor Microenvironment |
| TIME | Tumor Immune Microenvironment |
| TGFβ | Transforming Growth Factor β |
| TKIs | Tyrosine Kinase Inhibitors |
| TNFα | Tumor Necrosis Factor α |
| TSP-1 | ThromboSPondin-1 |
| VEGF | Vascular Endothelial Growth Factor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Subtypes of Ovarian Cancer and Ovarian Cancer Screening. Diagnostics (Basel) 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Kohn, E.C. The new classifications of ovarian, fallopian tube, and primary peritoneal cancer and their clinical implications. Ann. Oncol. 2017, 28, viii8–viii12. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Lee, E.S.; Wilson, A.J.; Beeghly-Fadiel, A.; Whalen, M.M.; Son, D.S. Chemokine Network and Overall Survival in TP53 Wild-Type and Mutant Ovarian Cancer. Immune Netw. 2018, 18, e29. [Google Scholar] [CrossRef]
- Lynch, H.T.; Snyder, C.; Casey, M.J. Hereditary ovarian and breast cancer: What have we learned? Ann. Oncol. 2013, 24 (Suppl. S8), viii83–viii95. [Google Scholar] [CrossRef]
- Toss, A.; Tomasello, C.; Razzaboni, E.; Contu, G.; Grandi, G.; Cagnacci, A.; Schilder, R.J.; Cortesi, L. Hereditary ovarian cancer: Not only BRCA 1 and 2 genes. BioMed Res. Int. 2015, 2015, 341723. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, M.; Redondo, A.; Heredia-Soto, V.; Herranz, J.; Berjon, A.; Hernandez, A.; Miguel-Martin, M.; Crespo, R.; Barriuso, J.; Cruz, P.; et al. Predicting Response to Standard First-line Treatment in High-grade Serous Ovarian Carcinoma by Angiogenesis-related Genes. Anticancer Res. 2018, 38, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Muller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef]
- Leinster, D.A.; Kulbe, H.; Everitt, G.; Thompson, R.; Perretti, M.; Gavins, F.N.; Cooper, D.; Gould, D.; Ennis, D.P.; Lockley, M.; et al. The peritoneal tumour microenvironment of high-grade serous ovarian cancer. J. Pathol. 2012, 227, 136–145. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef]
- Curtis, M.; Mukherjee, A.; Lengyel, E. The Tumor Microenvironment Takes Center Stage in Ovarian Cancer Metastasis. Trends Cancer 2018, 4, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Drakes, M.L.; Stiff, P.J. Regulation of Ovarian Cancer Prognosis by Immune Cells in the Tumor Microenvironment. Cancers (Basel) 2018, 10, 302. [Google Scholar] [CrossRef]
- Hansen, J.M.; Coleman, R.L.; Sood, A.K. Targeting the tumour microenvironment in ovarian cancer. Eur. J. Cancer 2016, 56, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Okla, K.; Wertel, I.; Polak, G.; Surowka, J.; Wawruszak, A.; Kotarski, J. Tumor-Associated Macrophages and Myeloid-Derived Suppressor Cells as Immunosuppressive Mechanism in Ovarian Cancer Patients: Progress and Challenges. Int. Rev. Immunol. 2016, 35, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Cannon, M.J.; Nakagawa, M. Regulatory T Cells in Gynecologic Cancer. MOJ Immunol. 2018, 6, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, A.E.; Abrams, S.I.; Moysich, K.B. A Call for Epidemiological Research on Myeloid-Derived Suppressor Cells in Ovarian Cancer: A Review of the Existing Immunological Evidence and Suggestions for Moving Forward. Front. Immunol. 2019, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, H.; Li, M.; Li, B.; Gao, W.; Shao, Q.; Fan, B.; Zhao, F.; Wang, Q.; Xie, Q.; et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene 2015, 34, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Liu, L.; Gong, C.Y.; Shi, H.S.; Zeng, Y.H.; Wang, X.Z.; Zhao, Y.W.; Wei, Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [PubMed]
- Maccio, A.; Gramignano, G.; Cherchi, M.C.; Tanca, L.; Melis, L.; Madeddu, C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci. Rep. 2020, 10, 6096. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, A.; Zannoni, G.F.; Buttarelli, M.; Martinelli, E.; Mascilini, F.; Petrillo, M.; Ferrandina, G.; Scambia, G.; Gallo, D. Ovarian low and high grade serous carcinomas: Hidden divergent features in the tumor microenvironment. Oncotarget 2016, 7, 68033–68043. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schworer, A.M.; Wagner, U.; Muller-Brusselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Carroll, M.J.; Kapur, A.; Felder, M.; Patankar, M.S.; Kreeger, P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget 2016, 7, 86608–86620. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Kulbe, H.; Li, N.F.; Leinster, D.A.; Charles, K.; Klemm, F.; Pukrop, T.; Binder, C.; Balkwill, F.R. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol. 2005, 175, 1197–1205. [Google Scholar] [CrossRef]
- Leber, T.M.; Balkwill, F.R. Regulation of monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble factor (MMPSF). Br. J. Cancer 1998, 78, 724–732. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Burke, F.; Kulbe, H.; Li, N.F.; Pluddemann, A.; Charles, K.; Gordon, S.; Balkwill, F.R. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 2006, 176, 5023–5032. [Google Scholar] [CrossRef]
- Neyen, C.; Pluddemann, A.; Mukhopadhyay, S.; Maniati, E.; Bossard, M.; Gordon, S.; Hagemann, T. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J. Immunol. 2013, 190, 3798–3805. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.A.; Yang, K.; Zong, H.; Lim, J.Y.; Cole, A.; Yang, D.; Baker, J.; Goonewardena, S.N.; Buckanovich, R.J. Therapeutic Impact of Nanoparticle Therapy Targeting Tumor-Associated Macrophages. Mol. Cancer Ther. 2018, 17, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Meng, T. Depletion of tumor-associated macrophages enhances the anti-tumor effect of docetaxel in a murine epithelial ovarian cancer. Immunobiology 2019, 224, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Moughon, D.L.; He, H.; Schokrpur, S.; Jiang, Z.K.; Yaqoob, M.; David, J.; Lin, C.; Iruela-Arispe, M.L.; Dorigo, O.; Wu, L. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015, 75, 4742–4752. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.M.; Ahn, J.H.; Lee, K.T.; Jang, D.S.; Choi, J.H. 9-Hydroxycanthin-6-one isolated from stem bark of Ailanthus altissima induces ovarian cancer cell apoptosis and inhibits the activation of tumor-associated macrophages. Chem. Biol. Interact. 2018, 280, 99–108. [Google Scholar] [CrossRef]
- Wanderley, C.W.; Colon, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Dangaj, D.; Abbott, K.L.; Mookerjee, A.; Zhao, A.; Kirby, P.S.; Sandaltzopoulos, R.; Powell, D.J., Jr.; Lamaziere, A.; Siegel, D.L.; Wolf, C.; et al. Mannose receptor (MR) engagement by mesothelin GPI anchor polarizes tumor-associated macrophages and is blocked by anti-MR human recombinant antibody. PLoS ONE 2011, 6, e28386. [Google Scholar] [CrossRef]
- Lee, K.; Ahn, J.H.; Lee, K.T.; Jang, D.S.; Choi, J.H. Deoxyschizandrin, Isolated from Schisandra Berries, Induces Cell Cycle Arrest in Ovarian Cancer Cells and Inhibits the Protumoural Activation of Tumour-Associated Macrophages. Nutrients 2018, 10, 91. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, X.; Xu, P.; Li, R.; Fu, Y.; Dong, S.; Zhangsun, D.; Wu, Y.; Luo, S. d-Amino Acid Substitution of alpha-Conotoxin RgIA Identifies its Critical Residues and Improves the Enzymatic Stability. Mar. Drugs 2019, 17, 142. [Google Scholar] [CrossRef]
- Gottlieb, C.E.; Mills, A.M.; Cross, J.V.; Ring, K.L. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: A comparison of matched primary and metastatic tumors. Gynecol. Oncol. 2017, 144, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, K.U.; Kim, A.; Lee, S.J.; Lee, J.H.; Suh, D.S.; Kwon, B.S.; Hwang, C. PD-L1 expression on stromal tumor-infiltrating lymphocytes is a favorable prognostic factor in ovarian serous carcinoma. J. Ovarian Res. 2019, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, B.; Zhang, Z.; Zhang, Y.; Yang, R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin. Immunol. 2008, 129, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Zhu, G.; Myers, L.; Mottram, P.; Cheng, P.; Chen, L.; Coukos, G.; Zou, W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007, 67, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Zou, L.; Rodriguez, P.; Zhu, G.; Wei, S.; Mottram, P.; Brumlik, M.; Cheng, P.; Curiel, T.; Myers, L.; et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006, 203, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.N. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr. Opin. Immunol. 2009, 21, 47–52. [Google Scholar] [CrossRef]
- Liu, R.; Wei, H.; Gao, P.; Yu, H.; Wang, K.; Fu, Z.; Ju, B.; Zhao, M.; Dong, S.; Li, Z.; et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget 2017, 8, 39021–39032. [Google Scholar] [CrossRef]
- McKinstry, W.J.; Li, C.L.; Rasko, J.E.; Nicola, N.A.; Johnson, G.R.; Metcalf, D. Cytokine receptor expression on hematopoietic stem and progenitor cells. Blood 1997, 89, 65–71. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Swierczak, A.; Mouchemore, K.A.; Hamilton, J.A.; Anderson, R.L. Neutrophils: Important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015, 34, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498. [Google Scholar] [CrossRef]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Jamieson, T.; Clarke, M.; Steele, C.W.; Samuel, M.S.; Neumann, J.; Jung, A.; Huels, D.; Olson, M.F.; Das, S.; Nibbs, R.J.; et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Investig. 2012, 122, 3127–3144. [Google Scholar] [CrossRef]
- Antonio, N.; Bonnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Shojaei, F.; Singh, M.; Thompson, J.D.; Ferrara, N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc. Natl. Acad. Sci. USA 2008, 105, 2640–2645. [Google Scholar] [CrossRef]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.T.; Zhou, L.; Zeng, W.J.; Ma, Q.Q.; Wang, W.; Zhong, M.; Yu, Y.H. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cell. Physiol. Biochem. 2017, 41, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hong, L.; Zuo, M.Z.; He, Z. Prognostic significance of neutrophil to lymphocyte ratio in ovarian cancer: Evidence from 4,910 patients. Oncotarget 2017, 8, 68938–68949. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhou, S.; Liu, Y.; Zhai, L.; Sun, X. Prognostic value of systemic inflammatory markers in ovarian Cancer: A PRISMA-compliant meta-analysis and systematic review. BMC Cancer 2018, 18, 443. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshikawa, N.; Shirakawa, A.; Niimi, K.; Suzuki, S.; Kajiyama, H.; Kikkawa, F. Prognostic value of neutrophil-to-lymphocyte ratio in early-stage ovarian clear-cell carcinoma. J. Gynecol. Oncol. 2019, 30, e85. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.L.; Leung, C.S.; Wong, K.K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef]
- Mallmann-Gottschalk, N.; Sax, Y.; Kimmig, R.; Lang, S.; Brandau, S. EGFR-Specific Tyrosine Kinase Inhibitor Modifies NK Cell-Mediated Antitumoral Activity against Ovarian Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4693. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- Singel, K.L.; Grzankowski, K.S.; Khan, A.; Grimm, M.J.; D’Auria, A.C.; Morrell, K.; Eng, K.H.; Hylander, B.; Mayor, P.C.; Emmons, T.R.; et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br. J. Cancer 2019, 120, 207–217. [Google Scholar] [CrossRef]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long non-coding RNA HOTTIP enhances IL-6 expression to potentiate immune escape of ovarian cancer cells by upregulating the expression of PD-L1 in neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef]
- Singel, K.L.; Emmons, T.R.; Khan, A.N.H.; Mayor, P.C.; Shen, S.; Wong, J.T.; Morrell, K.; Eng, K.H.; Mark, J.; Bankert, R.B.; et al. Mature neutrophils suppress T cell immunity in ovarian cancer microenvironment. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Aotsuka, A.; Matsumoto, Y.; Arimoto, T.; Kawata, A.; Ogishima, J.; Taguchi, A.; Tanikawa, M.; Sone, K.; Mori-Uchino, M.; Tsuruga, T.; et al. Interleukin-17 is associated with expression of programmed cell death 1 ligand 1 in ovarian carcinoma. Cancer Sci. 2019, 110, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.A.; Le Tourneau, C.; Toulmonde, M.; Cannarile, M.A.; Ries, C.; Brillouet, A.; Muller, C.; Jegg, A.M.; et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015, 16, 949–956. [Google Scholar] [CrossRef]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.; Tang, H.W.; Cai, P.C.; Leung, T.H.; Ngu, S.F.; Chan, K.K.; Xu, D.; Yang, H.; Ngan, H.Y.; Chan, D.W. GRO-alpha and IL-8 enhance ovarian cancer metastatic potential via the CXCR2-mediated TAK1/NFkappaB signaling cascade. Theranostics 2018, 8, 1270–1285. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.G.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z.; et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef]
- Benevides, L.; da Fonseca, D.M.; Donate, P.B.; Tiezzi, D.G.; De Carvalho, D.D.; de Andrade, J.M.; Martins, G.A.; Silva, J.S. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer Res. 2015, 75, 3788–3799. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014, 6, 237ra267. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.S.; Wu, X.; Zhuang, G.; Ngu, H.; Kasman, I.; Zhang, J.; Vernes, J.M.; Jiang, Z.; Meng, Y.G.; Peale, F.V.; et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013, 19, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Farolfi, A.; Petrone, M.; Scarpi, E.; Galla, V.; Greco, F.; Casanova, C.; Longo, L.; Cormio, G.; Orditura, M.; Bologna, A.; et al. Inflammatory Indexes as Prognostic and Predictive Factors in Ovarian Cancer Treated with Chemotherapy Alone or Together with Bevacizumab. A Multicenter, Retrospective Analysis by the MITO Group (MITO 24). Target. Oncol. 2018, 13, 469–479. [Google Scholar] [CrossRef]
- Bhat, J.; Kabelitz, D. gammadelta T cells and epigenetic drugs: A useful merger in cancer immunotherapy? Oncoimmunology 2015, 4, e1006088. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.A.; Reader, J.; Roque, D.M. Review of Immune Therapies Targeting Ovarian Cancer. Curr. Treat. Options Oncol. 2018, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Zocchi, M.R. gammadelta T Lymphocytes as a First Line of Immune Defense: Old and New Ways of Antigen Recognition and Implications for Cancer Immunotherapy. Front. Immunol. 2014, 5, 575. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Bjorkstrom, N.K.; Norell, H.; Bryceson, Y.; van Hall, T.; Baumann, B.C.; Hanson, M.; Schedvins, K.; Kiessling, R.; Ljunggren, H.G.; et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007, 67, 1317–1325. [Google Scholar] [CrossRef]
- Guillamon, C.F.; Martinez-Sanchez, M.V.; Gimeno, L.; Mrowiec, A.; Martinez-Garcia, J.; Server-Pastor, G.; Martinez-Escribano, J.; Torroba, A.; Ferri, B.; Abellan, D.; et al. NK Cell Education in Tumor Immune Surveillance: DNAM-1/KIR Receptor Ratios as Predictive Biomarkers for Solid Tumor Outcome. Cancer Immunol. Res. 2018, 6, 1537–1547. [Google Scholar] [CrossRef]
- McGilvray, R.W.; Eagle, R.A.; Rolland, P.; Jafferji, I.; Trowsdale, J.; Durrant, L.G. ULBP2 and RAET1E NKG2D ligands are independent predictors of poor prognosis in ovarian cancer patients. Int. J. Cancer 2010, 127, 1412–1420. [Google Scholar] [CrossRef]
- Wu, J.D.; Higgins, L.M.; Steinle, A.; Cosman, D.; Haugk, K.; Plymate, S.R. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J. Clin. Investig. 2004, 114, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mandai, M.; Hamanishi, J.; Matsumura, N.; Suzuki, A.; Yagi, H.; Yamaguchi, K.; Baba, T.; Fujii, S.; Konishi, I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: High expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol. Immunother. 2009, 58, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Caballero-Benitez, A.; Gewe, M.M.; Jenkins, I.C.; Drescher, C.W.; Strong, R.K.; Spies, T.; Groh, V. Control of Tumor Initiation by NKG2D Naturally Expressed on Ovarian Cancer Cells. Neoplasia 2017, 19, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.C.; Dai, Z.; Mann, H.H.; Reeves, R.S.; Margineantu, D.H.; Gooley, T.A.; Groh, V.; Spies, T. Expression, signaling proficiency, and stimulatory function of the NKG2D lymphocyte receptor in human cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4081–4086. [Google Scholar] [CrossRef]
- Cai, X.; Dai, Z.; Reeves, R.S.; Caballero-Benitez, A.; Duran, K.L.; Delrow, J.J.; Porter, P.L.; Spies, T.; Groh, V. Autonomous stimulation of cancer cell plasticity by the human NKG2D lymphocyte receptor coexpressed with its ligands on cancer cells. PLoS ONE 2014, 9, e108942. [Google Scholar] [CrossRef]
- Cua, S.; Tan, H.L.; Fong, W.J.; Chin, A.; Lau, A.; Ding, V.; Song, Z.; Yang, Y.; Choo, A. Targeting of embryonic annexin A2 expressed on ovarian and breast cancer by the novel monoclonal antibody 2448. Oncotarget 2018, 9, 13206–13221. [Google Scholar] [CrossRef]
- Oberg, H.H.; Kellner, C.; Gonnermann, D.; Sebens, S.; Bauerschlag, D.; Gramatzki, M.; Kabelitz, D.; Peipp, M.; Wesch, D. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing gammadelta T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front. Immunol. 2018, 9, 814. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Serre, K.; Norell, H. gammadelta T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Suto, A.; Kudo, D.; Yoshida, E.; Nagase, H.; Suto, S.; Mimura, J.; Itoh, K.; Hakamada, K. Increase of Tumor Infiltrating gammadelta T-cells in Pancreatic Ductal Adenocarcinoma Through Remodeling of the Extracellular Matrix by a Hyaluronan Synthesis Suppressor, 4-Methylumbelliferone. Pancreas 2019, 48, 292–298. [Google Scholar] [CrossRef]
- Ye, J.; Ma, C.; Wang, F.; Hsueh, E.C.; Toth, K.; Huang, Y.; Mo, W.; Liu, S.; Han, B.; Varvares, M.A.; et al. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res. 2013, 73, 6137–6148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (gammadelta) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, W.; Xu, R.; Wu, M.; Zhang, X.; Huang, P.; Wang, F.; Pan, S. Distribution and functions of gammadelta T cells infiltrated in the ovarian cancer microenvironment. J. Transl. Med. 2019, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.L.; Miao, C.H.; Liao, Y.J.; Chen, Y.J.; Yeh, C.Y.; Liu, C.L. Ex Vivo Expanded Human Vgamma9Vdelta2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2019, 20, 1139. [Google Scholar] [CrossRef]
- Coukos, G.; Tanyi, J.; Kandalaft, L.E. Opportunities in immunotherapy of ovarian cancer. Ann. Oncol. 2016, 27 (Suppl. S1), i11–i15. [Google Scholar] [CrossRef]
- Parente-Pereira, A.C.; Shmeeda, H.; Whilding, L.M.; Zambirinis, C.P.; Foster, J.; van der Stegen, S.J.; Beatson, R.; Zabinski, T.; Brewig, N.; Sosabowski, J.K.; et al. Adoptive immunotherapy of epithelial ovarian cancer with Vgamma9Vdelta2 T cells, potentiated by liposomal alendronic acid. J. Immunol. 2014, 193, 5557–5566. [Google Scholar] [CrossRef]
- Becknell, B.; Caligiuri, M.A. Natural killer cells in innate immunity and cancer. J. Immunother. 2008, 31, 685–692. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis from Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers (Basel) 2019, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Fogel, L.A.; Yokoyama, W.M.; French, A.R. Natural killer cells in human autoimmune disorders. Arthritis Res. Ther. 2013, 15, 216. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol. Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Bassani, B.; Tremolati, M.; Gini, E.; Farronato, G.; Bruno, A. Natural Killer Cells in the Orchestration of Chronic Inflammatory Diseases. J. Immunol. Res. 2017, 2017, 4218254. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, Y.; Gao, B. Natural killer cells in liver disease. Hepatology 2013, 57, 1654–1662. [Google Scholar] [CrossRef]
- Bosi, A.; Zanellato, S.; Bassani, B.; Albini, A.; Musco, A.; Cattoni, M.; Desio, M.; Nardecchia, E.; D’Urso, D.G.; Imperatori, A.; et al. Natural Killer Cells from Malignant Pleural Effusion Are Endowed with a Decidual-Like Proangiogenic Polarization. J. Immunol. Res. 2018, 2018, 2438598. [Google Scholar] [CrossRef]
- Bruno, A.; Bassani, B.; D’Urso, D.G.; Pitaku, I.; Cassinotti, E.; Pelosi, G.; Boni, L.; Dominioni, L.; Noonan, D.M.; Mortara, L.; et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. 2018. [Google Scholar] [CrossRef]
- Bruno, A.; Ferlazzo, G.; Albini, A.; Noonan, D.M. A think tank of TINK/TANKs: Tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. J. Natl. Cancer Inst. 2014, 106, dju200. [Google Scholar] [CrossRef]
- Bruno, A.; Pagani, A.; Pulze, L.; Albini, A.; Dallaglio, K.; Noonan, D.M.; Mortara, L. Orchestration of angiogenesis by immune cells. Front. Oncol. 2014, 4, 131. [Google Scholar] [CrossRef]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef]
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Nham, T.; Chew, M.V.; Lee, A.J.; Hammill, J.A.; Fan, I.Y.; Butcher, M.; Bramson, J.L.; Lee, D.A.; Hirte, H.W.; et al. Expanded CD56(superbright)CD16(+) NK Cells from Ovarian Cancer Patients Are Cytotoxic against Autologous Tumor in a Patient-Derived Xenograft Murine Model. Cancer Immunol. Res. 2018, 6, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers (Basel) 2018, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yao, Z.; Zhao, Z.; Xiao, H.; Xia, M.; Zhu, X.; Jiang, X.; Sun, C. Natural killer cells inhibit metastasis of ovarian carcinoma cells and show therapeutic effects in a murine model of ovarian cancer. Exp. Ther. Med. 2018, 16, 1071–1078. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Karadimou, A.; Dimopoulos, M.A.; Bamias, A. Immune response in ovarian cancer: How is the immune system involved in prognosis and therapy: Potential for treatment utilization. Clin. Dev. Immunol. 2010, 2010, 791603. [Google Scholar] [CrossRef]
- Patankar, M.S.; Jing, Y.; Morrison, J.C.; Belisle, J.A.; Lattanzio, F.A.; Deng, Y.; Wong, N.K.; Morris, H.R.; Dell, A.; Clark, G.F. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol. Oncol. 2005, 99, 704–713. [Google Scholar] [CrossRef]
- Baert, T.; Vankerckhoven, A.; Riva, M.; Van Hoylandt, A.; Thirion, G.; Holger, G.; Mathivet, T.; Vergote, I.; Coosemans, A. Myeloid Derived Suppressor Cells: Key Drivers of Immunosuppression in Ovarian Cancer. Front. Immunol. 2019, 10, 1273. [Google Scholar] [CrossRef]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Barnett, B.; Kryczek, I.; Cheng, P.; Zou, W.; Curiel, T.J. Regulatory T cells in ovarian cancer: Biology and therapeutic potential. Am. J. Reprod. Immunol. 2005, 54, 369–377. [Google Scholar] [CrossRef]
- Toker, A.; Nguyen, L.T.; Stone, S.C.; Yang, S.Y.C.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Ghazarian, D.; Al-Habeeb, A.; Easson, A.; et al. Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from that in Melanoma. Clin. Cancer Res. 2018, 24, 5685–5696. [Google Scholar] [CrossRef]
- Alsina-Sanchis, E.; Figueras, A.; Lahiguera, A.; Gil-Martin, M.; Pardo, B.; Piulats, J.M.; Marti, L.; Ponce, J.; Matias-Guiu, X.; Vidal, A.; et al. TGFbeta Controls Ovarian Cancer Cell Proliferation. Int. J. Mol. Sci. 2017, 18, 1658. [Google Scholar] [CrossRef] [PubMed]
- Alsina-Sanchis, E.; Figueras, A.; Lahiguera, A.; Vidal, A.; Casanovas, O.; Graupera, M.; Villanueva, A.; Vinals, F. The TGFbeta pathway stimulates ovarian cancer cell proliferation by increasing IGF1R levels. Int. J. Cancer 2016, 139, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFbeta in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S. Transforming of the Tumor Microenvironment: Implications for TGF-beta Inhibition in the Context of Immune-Checkpoint Therapy. J. Oncol. 2018, 2018, 9732939. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Wang, X.; Zhu, Q.; Wu, X.; Wang, X. MUC16 impacts tumor proliferation and migration through cytoplasmic translocation of P120-catenin in epithelial ovarian cancer cells: An original research. BMC Cancer 2019, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Bonfrer, J.M.; Kulpa, J.; Rustin, G.J.; Soletormos, G.; Torre, G.C.; Tuxen, M.K.; Zwirner, M. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int. J. Gynecol. Cancer 2005, 15, 679–691. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Stenman, U.H.; Lilja, H.; Brunner, N.; Chan, D.W.; Babaian, R.; Bast, R.C., Jr.; Dowell, B.; Esteva, F.J.; et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin. Chem. 2008, 54, e11–e79. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef]
- Harlozinska, A.; Sedlaczek, P.; Van Dalen, A.; Rozdolski, K.; Einarsson, R. TPS and CA 125 levels in serum, cyst fluid and ascites of patients with epithelial ovarian neoplasms. Anticancer Res. 1997, 17, 4473–4478. [Google Scholar]
- Lai, P.; Rabinowich, H.; Crowley-Nowick, P.A.; Bell, M.C.; Mantovani, G.; Whiteside, T.L. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 1996, 2, 161–173. [Google Scholar] [PubMed]
- Felder, M.; Kapur, A.; Rakhmilevich, A.L.; Qu, X.; Sondel, P.M.; Gillies, S.D.; Connor, J.; Patankar, M.S. MUC16 suppresses human and murine innate immune responses. Gynecol. Oncol. 2019, 152, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.; Kapur, A.; Felder, M.; Belisle, J.A.; Trautman, C.; Gubbels, J.A.; Connor, J.P.; Patankar, M.S. The mucin MUC16 (CA125) binds to NK cells and monocytes from peripheral blood of women with healthy pregnancy and preeclampsia. Am. J. Reprod. Immunol. 2012, 68, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.B.; May, C.; Hill, M.; Campbell, S.; Shaw, P.; Marks, A. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J. Clin. Investig. 1990, 86, 851–855. [Google Scholar] [CrossRef]
- Mills, G.B.; May, C.; McGill, M.; Roifman, C.M.; Mellors, A. A putative new growth factor in ascitic fluid from ovarian cancer patients: Identification, characterization, and mechanism of action. Cancer Res. 1988, 48, 1066–1071. [Google Scholar]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology 2015, 4, e1001224. [Google Scholar] [CrossRef]
- Allan, D.S.; Rybalov, B.; Awong, G.; Zuniga-Pflucker, J.C.; Kopcow, H.D.; Carlyle, J.R.; Strominger, J.L. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur. J. Immunol. 2010, 40, 2289–2295. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef]
- Recchia, F.; Di Orio, F.; Candeloro, G.; Guerriero, G.; Piazze, J.; Rea, S. Maintenance immunotherapy in recurrent ovarian cancer: Long term follow-up of a phase II study. Gynecol. Oncol. 2010, 116, 202–207. [Google Scholar] [CrossRef]
- Medler, T.R.; Cotechini, T.; Coussens, L.M. Immune response to cancer therapy: Mounting an effective antitumor response and mechanisms of resistance. Trends Cancer 2015, 1, 66–75. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kepp, O.; Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ai, X.; Wu, C.; Wang, H.; Zeng, G.; Yang, P.; Liu, G. A novel human IL-2 mutein with minimal systemic toxicity exerts greater antitumor efficacy than wild-type IL-2. Cell Death Dis. 2018, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Mortara, L.; Balza, E.; Bruno, A.; Poggi, A.; Orecchia, P.; Carnemolla, B. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Front. Immunol. 2018, 9, 2905. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ren, Z.; Yang, K.; Liu, Z.; Cao, S.; Deng, S.; Xu, L.; Liang, Y.; Guo, J.; Bian, Y.; et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat. Commun. 2019, 10, 3874. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yi, P.; Dong, W.; Nalin, A.P.; Zhang, J.; Zhu, Z.; Chen, L.; Benson, D.M.; Mundy-Bosse, B.L.; et al. The IL-15-AKT-XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat. Immunol. 2019, 20, 10–17. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef]
- Leclercq, G.; Debacker, V.; de Smedt, M.; Plum, J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J. Exp. Med. 1996, 184, 325–336. [Google Scholar] [CrossRef]
- Guo, Y.; Luan, L.; Patil, N.K.; Wang, J.; Bohannon, J.K.; Rabacal, W.; Fensterheim, B.A.; Hernandez, A.; Sherwood, E.R. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. J. Immunol. 2017, 198, 1320–1333. [Google Scholar] [CrossRef]
- Childs, R.W.; Carlsten, M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: The force awakens. Nat. Rev. Drug Discov. 2015, 14, 487–498. [Google Scholar] [CrossRef]
- Felices, M.; Chu, S.; Kodal, B.; Bendzick, L.; Ryan, C.; Lenvik, A.J.; Boylan, K.L.M.; Wong, H.C.; Skubitz, A.P.N.; Miller, J.S.; et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol. 2017, 145, 453–461. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Maas, R.J.; van der Meer, J.; Cany, J.; van der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; Massuger, L.; et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, Y.; Cho, W.C.; Zhu, H. The rise of human stem cell-derived natural killer cells for cancer immunotherapy. Expert Opin. Biol. Ther. 2019, 19, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tang, S.Y.; Toh, L.L.; Wang, S. Generation of “Off-the-Shelf” Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 1796–1812. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192. [Google Scholar] [CrossRef]
- Abate-Daga, D.; Davila, M.L. CAR models: Next-generation CAR modifications for enhanced T-cell function. Mol. Ther. Oncolytics 2016, 3, 16014. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Zhou, W.L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal. Transduct. Target. Ther. 2019, 4, 35. [Google Scholar] [CrossRef]
- Tokarew, N.; Ogonek, J.; Endres, S.; von Bergwelt-Baildon, M.; Kobold, S. Teaching an old dog new tricks: Next-generation CAR T cells. Br. J. Cancer 2019, 120, 26–37. [Google Scholar] [CrossRef]
- Leslie, M. New cancer-fighting cells enter trials. Science 2018, 361, 1056–1057. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Maliar, A.; Servais, C.; Waks, T.; Chmielewski, M.; Lavy, R.; Altevogt, P.; Abken, H.; Eshhar, Z. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 2012, 143, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Salnikov, A.V.; Bretz, N.P.; Perne, C.; Hazin, J.; Keller, S.; Fogel, M.; Herr, I.; Schlange, T.; Moldenhauer, G.; Altevogt, P. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br. J. Cancer 2013, 108, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, T.; Jiang, J.; Wang, Y.; Ma, Z.; Li, Z.; Han, Y.; Pan, M.; Cai, J.; Wang, M.; et al. Engineering a high-affinity humanized anti-CD24 antibody to target hepatocellular carcinoma by a novel CDR grafting design. Oncotarget 2017, 8, 51238–51252. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Wang, S.; Morgan, M.; Dork, T.; Hacker, U.; Hillemanns, P.; Buning, H.; Schambach, A. Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 660. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).