Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination
Abstract
:1. Introduction
1.1. Myelinating Cells of the Central Nervous System
1.2. Myelination
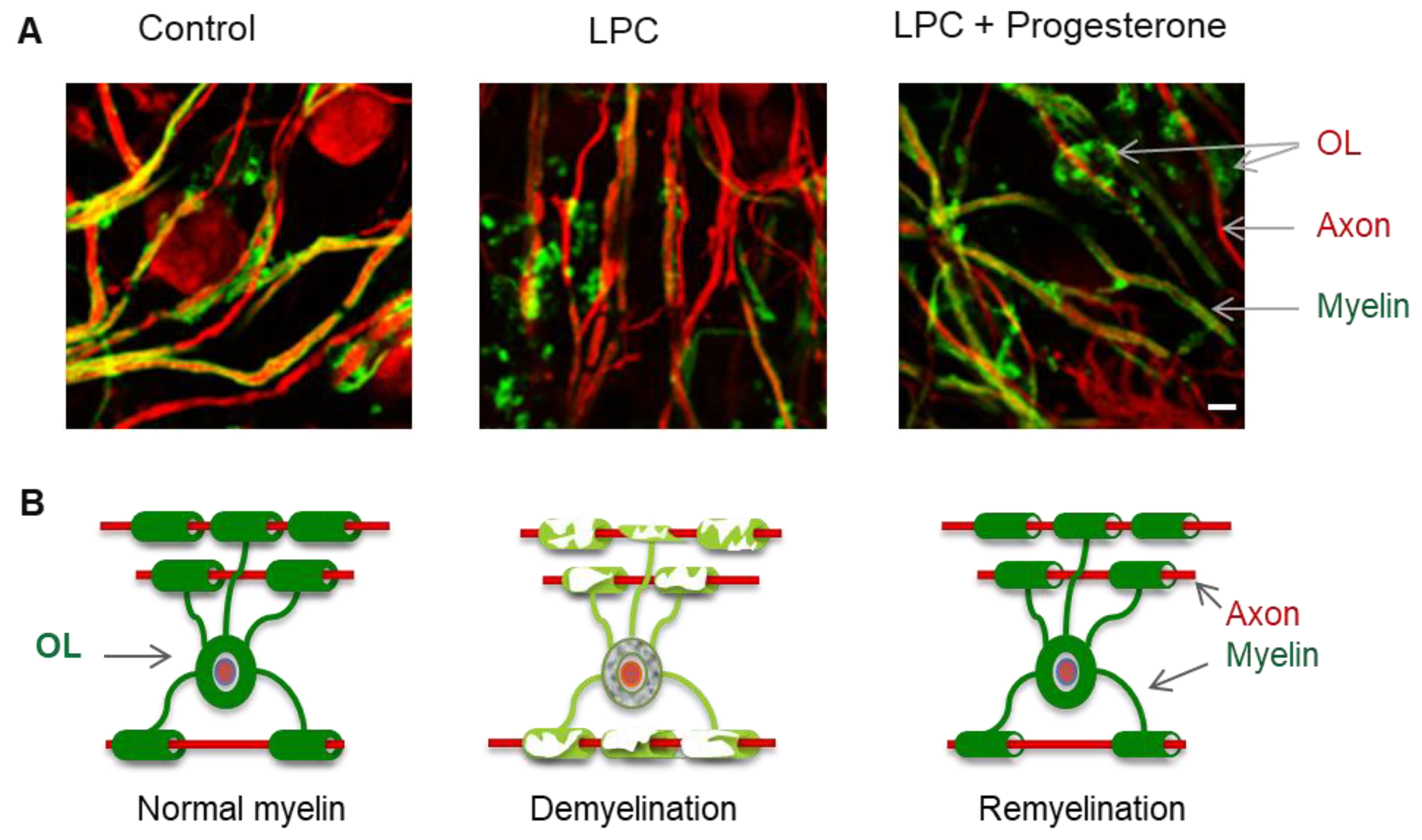
1.3. Remyelination
2. Steroid Hormones and Myelination/Remyelination
2.1. Steroid Hormones and Their Nuclear Receptor Signaling Mechanisms
2.2. Steroid Hormones and Myelin
2.3. Roles of Progesterone and Progestins in Myelination/Remyelination
2.3.1. In Vitro Studies
2.3.2. In Vivo Studies
2.4. Roles of Androgens in Myelination/Remyelination
2.4.1. Androgen Receptor’s Functions
2.4.2. In Vitro and In Vivo Translational Studies and Clinical Trials
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A2B5 | Cell surface ganglioside epitope |
| AR | Androgen Receptor |
| ARE | Androgen Response Elements |
| BBB | blood-brain barrier |
| 5α-DHP | 5α-dihydroprogesterone |
| 5α-DHT | 5α-dihydrotestosterone |
| 3α,5α-THP | 3α,5α-tetrahydroprogesterone: allopregnanolone |
| CAIS | Complete Androgen Insensitivity Syndrome |
| CC1 | Clone that recognizes the N-terminal of the protein product of the APC (adenomatous polyposis coli), and binds Quaking 7 (an RNA-binding protein). |
| CNPase | 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase |
| CNS | Central nervous system |
| EAE | Experimental Autoimmune Encephalomyelitis |
| E2 | Estradiol |
| GABA | γ-aminobutyric acid |
| GalC | Galactocerebroside |
| GR | Glucocorticoid Receptors |
| HMG-CoA | 3-hydroxy-3-methyl-glutaryl-coenzyme A |
| HSP | Heat-Shock Protein |
| HRE | Hormone Response Element |
| LPC | Lysophosphatidylcholine (Lysolecithin) |
| MAG | Myelin-associated glycoprotein |
| MBP | Myelin basic protein |
| MENT | 7α-methyl-19-nortestosterone |
| MOG | Myelin oligodendrocyte glycoprotein |
| MPA | Medroxyprogesterone acetate |
| MS | Multiple Sclerosis |
| NG2 | Sulfated proteoglycan |
| NK | Natural Killer |
| NKx2.2 | Homeodomain transcription factor |
| NP | Neural Precursors |
| O1&O4 | Oligodendrocyte Markers 1&4 |
| OL | Oligodendrocyte |
| Olig1&2 | Basic helix-loop-helix (bHLH) transcription factors 1&2 |
| OPCs | Oligodendrocyte Progenitor Cells |
| P450scc | P450 side chain cleavage (CYP11A1) |
| PDGFRα | Platelet Derived Growth Factor Receptor alpha |
| PGRMC1 | Membrane-associated progesterone receptor membrane component 1 |
| PLP/DM20 | Proteolipid protein and its alternatively spliced isoform DM-20 |
| PM | Plasma Membrane |
| PR | Progesterone Receptor |
| PRIMS | Pregnancy in Multiple Sclerosis |
| SCI | Spinal Cord Injury |
| SR | Steroid Receptor |
| STZ | Streptozotocin |
| Tfm | Testicular feminization mutation |
References
- Bunge, R.P. Glial cells and the central myelin sheath. Physiol. Rev. 1968, 48, 197–251. [Google Scholar] [CrossRef]
- Miller, R.H. Regulation of oligodendrocyte development in the vertebrate CNS. Prog. Neurobiol. 2002, 67, 451–467. [Google Scholar] [CrossRef]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Kuypers, N.J. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timsit, S.; Martinez, S.; Allinquant, B.; Peyron, F.; Puelles, L.; Zalc, B. Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J. Neurosci. 1995, 15, 1012–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois-Dalcq, M.; Behar, T.; Hudson, L.; Lazzarini, R.A. Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J. Cell Biol. 1986, 102, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Harlow, D.E.; Saul, K.E.; Culp, C.M.; Vesely, E.M.; Macklin, W.B. Expression of proteolipid protein gene in spinal cord stem cells and early oligodendrocyte progenitor cells is dispensable for normal cell migration and myelination. J. Neurosci. 2014, 34, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef]
- Tekki-Kessaris, N.; Woodruff, R.; Hall, A.C.; Gaffield, W.; Kimura, S.; Stiles, C.D.; Rowitch, D.H.; Richardson, W.D. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 2001, 128, 2545–2554. [Google Scholar]
- Vallstedt, A.; Klos, J.M.; Ericson, J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 2005, 45, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotter, J.; Karram, K.; Nishiyama, A. NG2 cells: Properties, progeny and origin. Brain Res. Rev. 2010, 63, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, M.R.L.; Polito, A.; Levine, J.M.; Reynolds, R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Hamada, M.S.; Kole, M.H.P. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 2015, 35, 7272–7286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andica, C.; Hagiwara, A.; Kamagata, K.; Yokoyama, K.; Shimoji, K.; Saito, A.; Takenaka, Y.; Nakazawa, M.; Hori, M.; Cohen-Adad, J.; et al. Gray Matter Alterations in Early and Late Relapsing-Remitting Multiple Sclerosis Evaluated with Synthetic Quantitative Magnetic Resonance Imaging. Sci. Rep. 2019, 9, 8147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackman, N.; Ishii, A.; Bansal, R. Oligodendrocyte development and myelin biogenesis: Parsing out the roles of glycosphingolipids. Physiology 2009, 24, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoumari, A.M.; Baulieu, E.E.; Schumacher, M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience 2005, 135, 47–58. [Google Scholar] [CrossRef]
- Raff, M.C.; Lillien, L.E.; Richardson, W.D.; Burne, J.F.; Noble, M.D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 1988, 333, 562–565. [Google Scholar] [CrossRef]
- Cellerino, A.; Carroll, P.; Thoenen, H.; Barde, Y.A. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol. Cell. Neurosci. 1997, 9, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Menichella, D.M.; Goodenough, D.A.; Sirkowski, E.; Scherer, S.S.; Paul, D.L. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003, 23, 5963–5973. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Schmid, R.; Sendnter, M.; Raff, M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 1993, 118, 283–295. [Google Scholar] [PubMed]
- Nicaise, C.; Marneffe, C.; Bouchat, J.; Gilloteaux, J. Osmotic Demyelination: From an Oligodendrocyte to an Astrocyte Perspective. IJMS 2019, 20, 1124. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.S.; Abdullah, S.L.; Brown, A.; Arulpragasam, A.; Crocker, S.J. How factors secreted from astrocytes impact myelin repair. J. Neurosci. Res. 2011, 89, 13–21. [Google Scholar] [CrossRef]
- Kacem, K.; Lacombe, P.; Seylaz, J.; Bonvento, G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 1998, 23, 1–10. [Google Scholar] [CrossRef]
- O’brien, J.S. Stability of the myelin membrane. Science 1965, 147, 1099–1107. [Google Scholar] [CrossRef]
- Franklin, R.J.; Crang, A.J.; Blakemore, W.F. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J. Neurocytol. 1991, 20, 420–430. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Gilson, J.M.; Crang, A.J. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Exp. Neurol. 2003, 184, 955–963. [Google Scholar] [CrossRef]
- Talbott, J.F.; Cao, Q.; Enzmann, G.U.; Benton, R.L.; Achim, V.; Cheng, X.X.; Mills, M.D.; Rao, M.S.; Whittemore, S.R. Schwann cell-like differentiation by adult oligodendrocyte precursor cells following engraftment into the demyelinated spinal cord is BMP-dependent. Glia 2006, 54, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Bielecki, B.; Mattern, C.; Ghoumari, A.M.; Javaid, S.; Smietanka, K.; Abi Ghanem, C.; Mhaouty-Kodja, S.; Ghandour, M.S.; Baulieu, E.-E.; Franklin, R.J.M.; et al. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc. Natl. Acad. Sci. USA 2016, 113, 14829–14834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traiffort, E.; Kassoussi, A.; Zahaf, A.; Laouarem, Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front. Cell Neurosci. 2020, 14, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, M.J.; Stein, R.B. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983, 259, 41–56. [Google Scholar] [CrossRef]
- Hussain, R.; Macklin, W.B. Integrin-Linked Kinase (ILK) Deletion Disrupts Oligodendrocyte Development by Altering Cell Cycle. J. Neurosci. 2017, 37, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.M.; Oakley, T.; Goldstone, J.V.; Dugas, J.C.; Brady, S.T.; Gow, A. Myelin sheaths are formed with proteins that originated in vertebrate lineages. Neuron Glia Biol. 2008, 4, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Zalc, B.; Goujet, D.; Colman, D. The origin of the myelination program in vertebrates. Curr. Biol. 2008, 18, R511–R512. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Jung, P. Simulation analysis of intermodal sodium channel function. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008, 78, 061916. [Google Scholar] [CrossRef]
- Huxley, C. Myelin disorders. Neuropathol. Appl. Neurobiol. 1998, 24, 87–90. [Google Scholar] [CrossRef]
- Boullerne, A.I. The history of myelin. Exp. Neurol. 2016, 283, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Waxman, S.G.; Ritchie, J.M. Molecular dissection of the myelinated axon. Ann. Neurol. 1993, 33, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.-A.; Werner, H.B. Myelination of the Nervous System: Mechanisms and Functions. Ann. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Bosio, A. Myelin glycolipids and their functions. Curr. Opin. Neurobiol. 1997, 7, 654–661. [Google Scholar] [CrossRef]
- Kahn, D.W.; Morell, P. Phosphatidic Acid and Phosphoinositide Turnover in Myelin and Its Stimulation by Acetylcholine. J Neurochem. 1988, 50, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Castelvetri, L.C.; Simons, M. Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 2015, 1851, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.E.; Warrington, A.E.; Bansal, R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993, 3, 191–197. [Google Scholar] [CrossRef]
- Trapp, B.D.; Bö, L.; Mörk, S.; Chang, A. Pathogenesis of tissue injury in MS lesions. J. Neuroimmunol. 1999, 98, 49–56. [Google Scholar] [CrossRef]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Dutta, R.; Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007, 68, S22–S31. [Google Scholar] [CrossRef]
- Fancy, S.P.J.; Chan, J.R.; Baranzini, S.E.; Franklin, R.J.M.; Rowitch, D.H. Myelin regeneration: A recapitulation of development? Annu. Rev. Neurosci. 2011, 34, 21–43. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N.; Peles, E. The Nodes of Ranvier: Molecular Assembly and Maintenance. Cold Spring Harb. Perspect. Biol. 2015, 8, a020495. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.F. Pattern of remyelination in the CNS. Nature 1974, 249, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Reynolds, R. Activation and Proliferation of Endogenous Oligodendrocyte Precursor Cells during Ethidium Bromide-Induced Demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef]
- Targett, M.P.; Sussman, J.; Scolding, N.; O’Leary, M.T.; Compston, D.A.; Blakemore, W.F. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol. Appl. Neurobiol. 1996, 22, 199–206. [Google Scholar] [CrossRef]
- Keirstead, H.S.; Blakemore, W.E. Identification of Post-mitotic Oligodendrocytes Incapable of Remyelination within the Demyelinated Adult Spinal Cord. J. Neuropathol. Exp. Neurol. 1997, 56, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Crawford, A.H.; Tripathi, R.B.; Foerster, S.; McKenzie, I.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D.; Franklin, R.J.M. Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination. Am. J. Pathol. 2016, 186, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [Green Version]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Witt, K.A.; Sandoval, K.E. Steroids and the blood-brain barrier: Therapeutic implications. Adv. Pharmacol. 2014, 71, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Vaudry, H. Biosynthesis of neurosteroids and regulation of their synthesis. Int. Rev. Neurobiol. 2001, 46, 33–78. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997, 52, 1–32. [Google Scholar]
- Jurevics, H.; Morell, P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J. Neurochem. 1995, 64, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Duhamel-Clerin, E.; Villarroya, H.; Mehtali, M.; Lapie, P.; Besnard, F.; Gumpel, M.; Lachapelle, F. Cellular expression of an HMGCR promoter-CAT fusion gene in transgenic mouse brain: Evidence for a developmental regulation in oligodendrocytes. Glia 1994, 11, 35–46. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Celotti, F.; Ballabio, M.; Poletti, A.; Castano, P.; Martini, L. Testosterone 5 alpha-reductase activity in the rat brain is highly concentrated in white matter structures and in purified myelin sheaths of axons. J. Steroid Biochem. 1988, 31, 173–179. [Google Scholar] [CrossRef]
- Poletti, A.; Celotti, F.; Melcangi, R.C.; Ballabio, M.; Martini, L. Kinetic properties of the 5 alpha-reductase of testosterone in the purified myelin, in the subcortical white matter and in the cerebral cortex of the male rat brain. J. Steroid Biochem. 1990, 35, 97–101. [Google Scholar] [CrossRef]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Kumar, R.; McEwan, I.J. Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation. Endocr. Rev. 2012, 33, 271–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkikas, D.; Tsampoula, M.; Politis, P.K. Nuclear receptors in neural stem/progenitor cell homeostasis. Cell. Mol. Life Sci. 2017, 74, 4097–4120. [Google Scholar] [CrossRef] [PubMed]
- Mhaouty-Kodja, S. Role of the androgen receptor in the central nervous system. Mol. Cell. Endocrinol. 2018, 465, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective: The Nuclear Receptor Superfamily. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Su, C.; Ng, S. Non-genomic mechanisms of progesterone action in the brain. Front. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, S.L.; Intlekofer, K.A.; Moura-Conlon, P.J.; Brewer, D.N.; Del Pino Sans, J.; Lopez, J.A. Nonclassical progesterone signaling molecules in the nervous system. J. Neuroendocrinol. 2013, 25, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Skerrett, R.; Malm, T.; Landreth, G. Nuclear receptors in neurodegenerative diseases. Neurobiol. Dis. 2014, 72, 104–116. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-L.; Bakhru, P.; Conley, B.; Nelson, J.S.; Free, M.; Martin, A.; Starmer, J.; Wilson, E.M.; Su, M.A. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 2016, 7, 11350. [Google Scholar] [CrossRef]
- Picillo, M.; Nicoletti, A.; Fetoni, V.; Garavaglia, B.; Barone, P.; Pellecchia, M.T. The relevance of gender in Parkinson’s disease: A review. J. Neurol. 2017, 264, 1583–1607. [Google Scholar] [CrossRef]
- Mazure, C.M.; Swendsen, J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016, 15, 451–452. [Google Scholar] [CrossRef] [Green Version]
- Gaignard, P.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Role of Sex Hormones on Brain Mitochondrial Function, with Special Reference to Aging and Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Age at disability milestones in multiple sclerosis. Brain 2006, 129, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, S.; van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender Differences in Multiple Sclerosis: Induction of Estrogen Signaling in Male and Progesterone Signaling in Female Lesions. J. Neuropathol. Exp. Neurol. 2014, 73, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, D.; Melis, M.; Fenu, G.; Giatti, S.; Romano, S.; Grimoldi, M.; Crippa, D.; Marrosu, M.G.; Cavaletti, G.; Melcangi, R.C. Neuroactive steroid levels in plasma and cerebrospinal fluid of male multiple sclerosis patients. J. Neurochem. 2014, 130, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Boghozian, R.; McKenzie, B.A.; Saito, L.B.; Mehta, N.; Branton, W.G.; Lu, J.; Baker, G.B.; Noorbakhsh, F.; Power, C. Suppressed oligodendrocyte steroidogenesis in multiple sclerosis: Implications for regulation of neuroinflammation: BOGHOZIAN et al. Glia 2017, 65, 1590–1606. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Ellestad, K.K.; Maingat, F.; Warren, K.G.; Han, M.H.; Steinman, L.; Baker, G.B.; Power, C. Impaired neurosteroid synthesis in multiple sclerosis. Brain 2011, 134, 2703–2721. [Google Scholar] [CrossRef] [Green Version]
- Simard, J.; Gingras, S. Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol. Cell. Endocrinol. 2001, 171, 25–40. [Google Scholar] [CrossRef]
- Avila, M.; Bansal, A.; Culberson, J.; Peiris, A.N. The Role of Sex Hormones in Multiple Sclerosis. Eur. Neurol. 2018, 80, 93–99. [Google Scholar] [CrossRef]
- Kanceva, R.; Stárka, L.; Kancheva, L.; Hill, M.; Veliková, M.; Havrdová, E. Increased Serum Levels of C21 Steroids in Female Patients with Multiple Sclerosis. Physiol. Res. 2015, 64, 8. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Hussain, R.; Gago, N.; Oudinet, J.-P.; Mattern, C.; Ghoumari, A.M. Progesterone Synthesis in the Nervous System: Implications for Myelination and Myelin Repair. Front. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef]
- Melcangi, R.C.; Giatti, S.; Calabrese, D.; Pesaresi, M.; Cermenati, G.; Mitro, N.; Viviani, B.; Garcia-Segura, L.M.; Caruso, D. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog. Neurobiol. 2014, 113, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Harney, S.C.; Belelli, D.; Peters, J.A. Neurosteroid modulation of recombinant and synaptic GABAA receptors. Int. Rev. Neurobiol. 2001, 46, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLusky, N.J.; McEwen, B.S. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature 1978, 274, 276–278. [Google Scholar] [CrossRef]
- Kastner, P.; Bocquel, M.T.; Turcotte, B.; Garnier, J.M.; Horwitz, K.B.; Chambon, P.; Gronemeyer, H. Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. J. Biol. Chem. 1990, 265, 12163–12167. [Google Scholar]
- Hirata, S.; Shoda, T.; Kato, J.; Hoshi, K. The novel exon, exon T, of the human progesterone receptor gene and the genomic organization of the gene. J. Steroid Biochem. Mol. Biol. 2002, 80, 365–367. [Google Scholar] [CrossRef]
- Sitruk-Ware, R. Non-clinical studies of progesterone. Climacteric 2018, 21, 315–320. [Google Scholar] [CrossRef]
- Gago, N.; Akwa, Y.; Sananès, N.; Guennoun, R.; Baulieu, E.E.; El-Etr, M.; Schumacher, M. Progesterone and the oligodendroglial lineage: Stage-dependent biosynthesis and metabolism. Glia 2001, 36, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Guennoun, R.; Ghoumari, A.; Massaad, C.; Robert, F.; El-Etr, M.; Akwa, Y.; Rajkowski, K.; Baulieu, E.-E. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr. Rev. 2007, 28, 387–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, R.; El-Etr, M.; Gaci, O.; Rakotomamonjy, J.; Macklin, W.B.; Kumar, N.; Sitruk-Ware, R.; Schumacher, M.; Ghoumari, A.M. Progesterone and Nestorone Facilitate Axon Remyelination: A Role for Progesterone Receptors. Endocrinology 2011, 152, 3820–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Etr, M.; Rame, M.; Boucher, C.; Ghoumari, A.M.; Kumar, N.; Liere, P.; Pianos, A.; Schumacher, M.; Sitruk-Ware, R. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex: Progesterone Receptor and Myelin Repair. Glia 2015, 63, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Garay, L.; Gonzalez Deniselle, M.C.; Sitruk-Ware, R.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Efficacy of the selective progesterone receptor agonist Nestorone for chronic experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2014, 276, 89–97. [Google Scholar] [CrossRef]
- Jung-Testas, I.; Schumacher, M.; Robel, P.; Baulieu, E.E. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell. Mol. Neurobiol. 1996, 16, 439–443. [Google Scholar] [CrossRef]
- Chan, J.R.; Phillips, L.J.; Glaser, M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc. Natl. Acad. Sci. USA 1998, 95, 10459–10464. [Google Scholar] [CrossRef] [Green Version]
- Ghoumari, A.M.; Ibanez, C.; El-Etr, M.; Leclerc, P.; Eychenne, B.; O’Malley, B.W.; Baulieu, E.E.; Schumacher, M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J. Neurochem. 2003, 86, 848–859. [Google Scholar] [CrossRef]
- Ghoumari, A.M.; Wehrlé, R.; Bernard, O.; Sotelo, C.; Dusart, I. Implication of Bcl-2 and Caspase-3 in age-related Purkinje cell death in murine organotypic culture: An in vitro model to study apoptosis. Eur. J. Neurosci. 2000, 12, 2935–2949. [Google Scholar] [CrossRef]
- Ghoumari, A.M.; Wehrlé, R.; De Zeeuw, C.I.; Sotelo, C.; Dusart, I. Inhibition of protein kinase C prevents Purkinje cell death but does not affect axonal regeneration. J. Neurosci. 2002, 22, 3531–3542. [Google Scholar] [CrossRef] [Green Version]
- Labombarda, F.; Gonzalez, S.; Gonzalez Deniselle, M.C.; Garay, L.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma 2006, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; González, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp. Neurol. 2011, 231, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Jure, I.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J. Steroid Biochem. Mol. Biol. 2015, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Jure, I.; De Nicola, A.F.; Labombarda, F. Progesterone effects on oligodendrocyte differentiation in injured spinal cord. Brain Res. 2019, 1708, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Gonzalez Deniselle, M.C.; Lima, A.; Roig, P.; De Nicola, A.F. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J. Steroid Biochem. Mol. Biol. 2007, 107, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Gonzalez Deniselle, M.C.; Meyer, M.; Costa, J.J.L.; Lima, A.; Roig, P.; De Nicola, A.F. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009, 1283, 177–185. [Google Scholar] [CrossRef]
- Ibanez, C.; Shields, S.A.; El-Etr, M.; Baulieu, E.-E.; Schumacher, M.; Franklin, R.J.M. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol. Appl. Neurobiol. 2004, 30, 80–89. [Google Scholar] [CrossRef]
- Acs, P.; Kipp, M.; Norkute, A.; Johann, S.; Clarner, T.; Braun, A.; Berente, Z.; Komoly, S.; Beyer, C. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia 2009, 57, 807–814. [Google Scholar] [CrossRef]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef]
- Pesaresi, M.; Giatti, S.; Calabrese, D.; Maschi, O.; Caruso, D.; Melcangi, R.C. Dihydroprogesterone increases the gene expression of myelin basic protein in spinal cord of diabetic rats. J. Mol. Neurosci. 2010, 42, 135–139. [Google Scholar] [CrossRef]
- Pike, C.J.; Nguyen, T.-V.V.; Ramsden, M.; Yao, M.; Murphy, M.P.; Rosario, E.R. Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 2008, 53, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traish, A.; Bolanos, J.; Nair, S.; Saad, F.; Morgentaler, A. Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J. Clin. Med. 2018, 7, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, T.; Tirador, K.A.; Miyamoto, H. Androgen receptor activation: A prospective therapeutic target for bladder cancer? Expert Opin. Ther. Targets 2017, 21, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Joseph, D.R.; Sullivan, P.M.; Willard, H.F.; French, F.S.; Wilson, E.M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 1988, 240, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sakari, M.; Okada, M.; Yokoyama, A.; Takahashi, S.; Kouzmenko, A.; Kato, S. The androgen receptor in health and disease. Annu. Rev. Physiol. 2013, 75, 201–224. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Lavrovsky, Y.; Ahn, S.C.; Song, C.S.; Chatterjee, B.; Roy, A.K. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol. Endocrinol. 2000, 14, 1162–1174. [Google Scholar] [CrossRef]
- Jenster, G.; Trapman, J.; Brinkmann, A.O. Nuclear import of the human androgen receptor. Biochem. J. 1993, 293, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Casella, R.; Maduro, M.R.; Lipshultz, L.I.; Lamb, D.J. Significance of the polyglutamine tract polymorphism in the androgen receptor. Urology 2001, 58, 651–656. [Google Scholar] [CrossRef]
- Quigley, C.A.; De Bellis, A.; Marschke, K.B.; el-Awady, M.K.; Wilson, E.M.; French, F.S. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocr. Rev. 1995, 16, 271–321. [Google Scholar] [CrossRef]
- Ben Jemaa, A.; Sallami, S.; Céraline, J.; Oueslati, R. A novel regulation of PSMA and PSA expression by Q640X AR in 22Rv1 and LNCaP prostate cancer cells. Cell Biol. Int. 2013, 37, 464–470. [Google Scholar] [CrossRef]
- Lyon, M.F.; Hawkes, S.G. X-linked gene for testicular feminization in the mouse. Nature 1970, 227, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- DonCarlos, L.L.; Sarkey, S.; Lorenz, B.; Azcoitia, I.; Garcia-Ovejero, D.; Huppenbauer, C.; Garcia-Segura, L.-M. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience 2006, 138, 801–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, S.K.; Kritzer, M.F. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J. Neurobiol. 1999, 40, 446–457. [Google Scholar] [CrossRef]
- Hussain, R.; Ghoumari, A.M.; Bielecki, B.; Steibel, J.; Boehm, N.; Liere, P.; Macklin, W.B.; Kumar, N.; Habert, R.; Mhaouty-Kodja, S.; et al. The neural androgen receptor: A therapeutic target for myelin repair in chronic demyelination. Brain 2013, 136, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Cerghet, M.; Skoff, R.P.; Bessert, D.; Zhang, Z.; Mullins, C.; Ghandour, M.S. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J. Neurosci. 2006, 26, 1439–1447. [Google Scholar] [CrossRef] [Green Version]
- Abi Ghanem, C.; Degerny, C.; Hussain, R.; Liere, P.; Pianos, A.; Tourpin, S.; Habert, R.; Macklin, W.B.; Schumacher, M.; Ghoumari, A.M. Long-lasting masculinizing effects of postnatal androgens on myelin governed by the brain androgen receptor. PLoS Genet. 2017, 13, e1007049. [Google Scholar] [CrossRef] [Green Version]
- Palaszynski, K.M.; Loo, K.K.; Ashouri, J.F.; Liu, H.; Voskuhl, R.R. Androgens are protective in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. J. Neuroimmunol. 2004, 146, 144–152. [Google Scholar] [CrossRef]
- Brown, M.A.; Weinberg, R.B. Mast Cells and Innate Lymphoid Cells: Underappreciated Players in CNS Autoimmune Demyelinating Disease. Front. Immunol. 2018, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Bebo, B.F.; Schuster, J.C.; Vandenbark, A.A.; Offner, H. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J. Neurosci. Res. 1998, 52, 420–426. [Google Scholar] [CrossRef]
- Dalal, M.; Kim, S.; Voskuhl, R.R. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 1997, 159, 3–6. [Google Scholar]
- Liva, S.M.; Voskuhl, R.R. Testosterone Acts Directly on CD4 + T Lymphocytes to Increase IL-10 Production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giatti, S.; Rigolio, R.; Romano, S.; Mitro, N.; Viviani, B.; Cavaletti, G.; Caruso, D.; Garcia-Segura, L.M.; Melcangi, R.C. Dihydrotestosterone as a Protective Agent in Chronic Experimental Autoimmune Encephalomyelitis. Neuroendocrinology 2015, 101, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Bove, R.; Musallam, A.; Healy, B.; Raghavan, K.; Glanz, B.; Bakshi, R.; Weiner, H.; De Jager, P.; Miller, K.; Chitnis, T. Low testosterone is associated with disability in men with multiple sclerosis. Mult. Scler. J. 2014, 20, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Bove, R.; Chitnis, T. Sexual disparities in the incidence and course of MS. Clin. Immunol. 2013, 149, 201–210. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Giesser, B.S.; Tandon, V.; Klutch, R.; Steiner, B.; Drain, A.E.; Shattuck, D.W.; Hull, L.; Wang, H.-J.; Elashoff, R.M.; et al. Testosterone Treatment in Multiple Sclerosis: A Pilot Study. Arch. Neurol. 2007, 64, 683. [Google Scholar] [CrossRef] [Green Version]
- Gold, S.M.; Chalifoux, S.; Giesser, B.S.; Voskuhl, R.R. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflamm. 2008, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Kurth, F.; Luders, E.; Sicotte, N.L.; Gaser, C.; Giesser, B.S.; Swerdloff, R.S.; Montag, M.J.; Voskuhl, R.R.; Mackenzie-Graham, A. Neuroprotective effects of testosterone treatment in men with multiple sclerosis. NeuroImage Clin. 2014, 4, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Collongues, N.; Patte-Mensah, C.; De Seze, J.; Mensah-Nyagan, A.-G.; Derfuss, T. Testosterone and estrogen in multiple sclerosis: From pathophysiology to therapeutics. Expert Rev. Neurother. 2018, 18, 515–522. [Google Scholar] [CrossRef]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8 (Suppl. 1), 3–63. [Google Scholar] [CrossRef]
- Borst, S.E.; Yarrow, J.F. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1035–E1042. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoumari, A.M.; Abi Ghanem, C.; Asbelaoui, N.; Schumacher, M.; Hussain, R. Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. Int. J. Mol. Sci. 2020, 21, 3163. https://doi.org/10.3390/ijms21093163
Ghoumari AM, Abi Ghanem C, Asbelaoui N, Schumacher M, Hussain R. Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. International Journal of Molecular Sciences. 2020; 21(9):3163. https://doi.org/10.3390/ijms21093163
Chicago/Turabian StyleGhoumari, Abdel Mouman, Charly Abi Ghanem, Narimène Asbelaoui, Michael Schumacher, and Rashad Hussain. 2020. "Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination" International Journal of Molecular Sciences 21, no. 9: 3163. https://doi.org/10.3390/ijms21093163
APA StyleGhoumari, A. M., Abi Ghanem, C., Asbelaoui, N., Schumacher, M., & Hussain, R. (2020). Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. International Journal of Molecular Sciences, 21(9), 3163. https://doi.org/10.3390/ijms21093163




