Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium
Abstract
1. Introduction
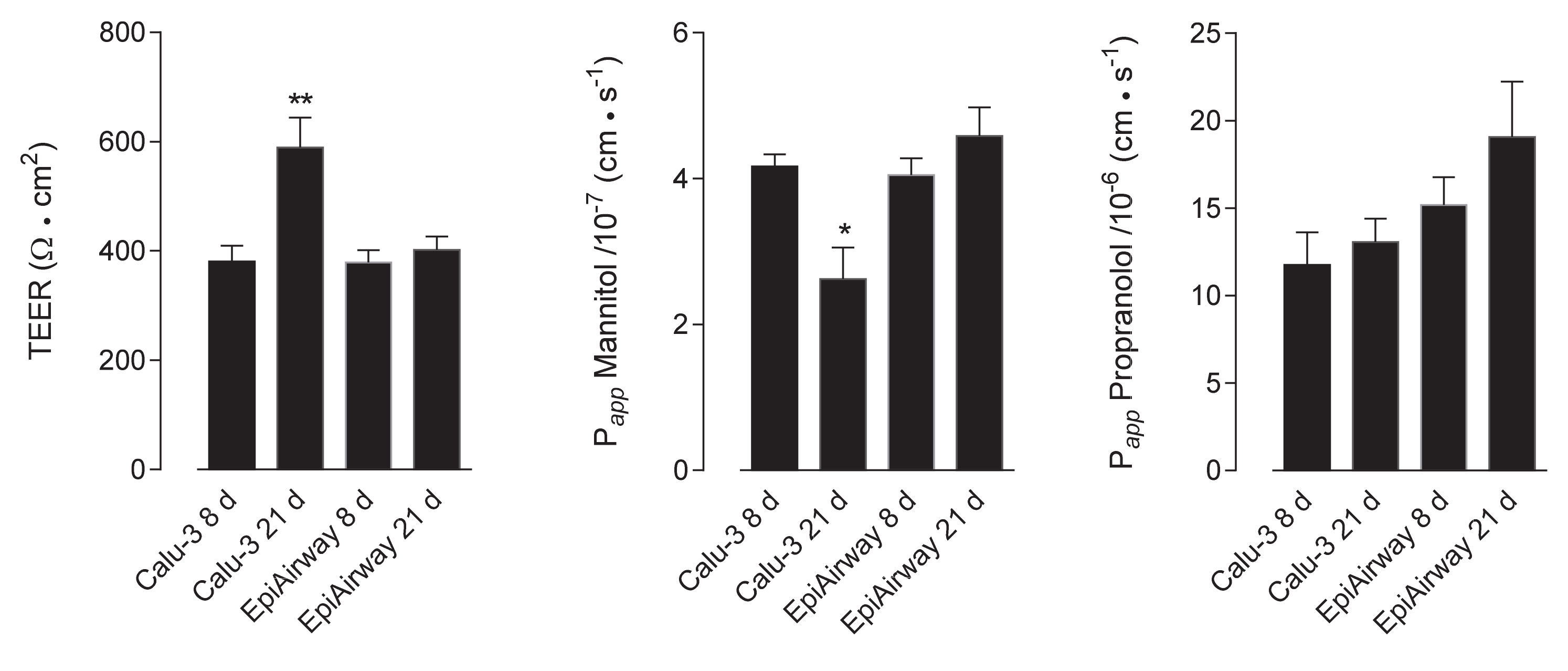
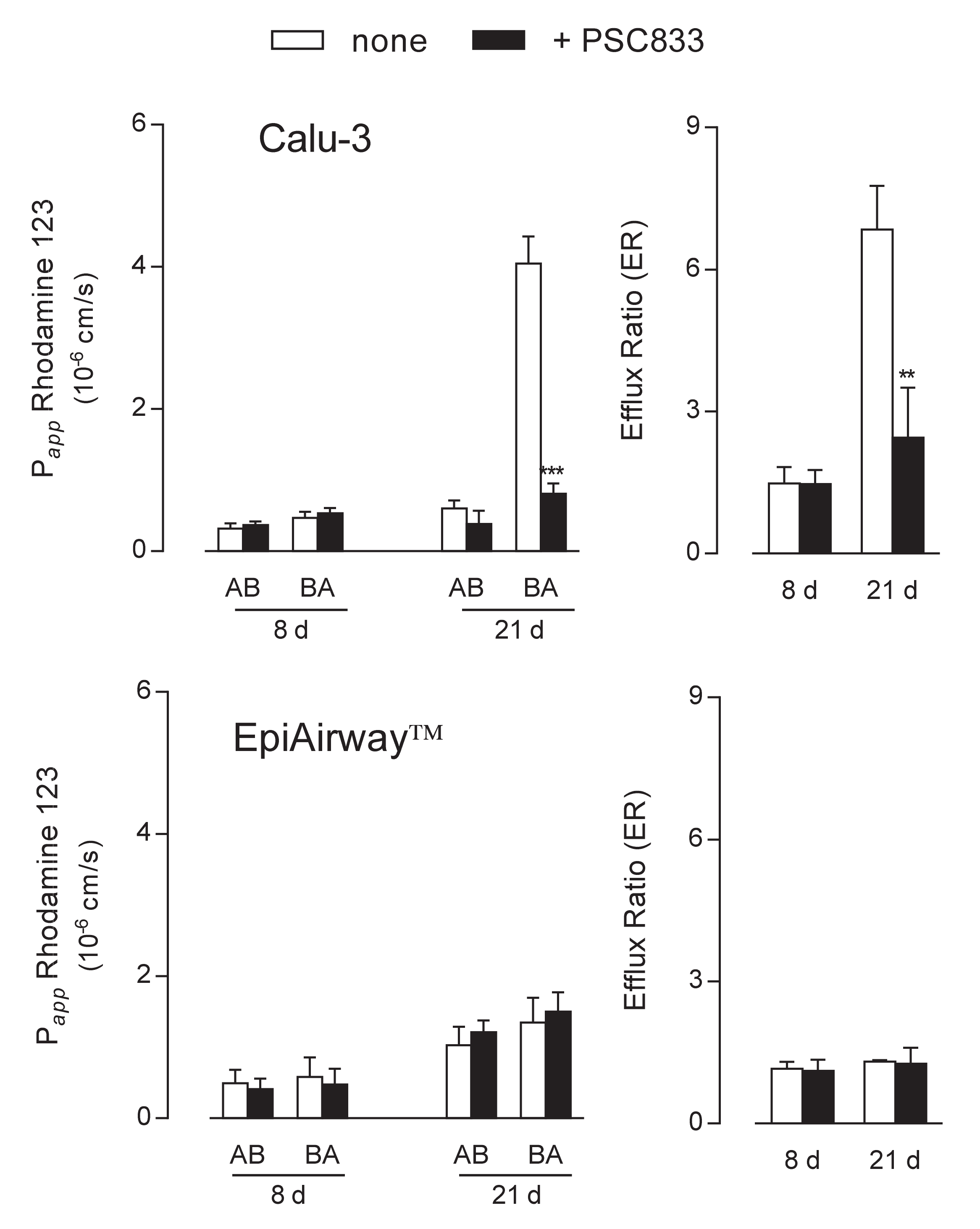
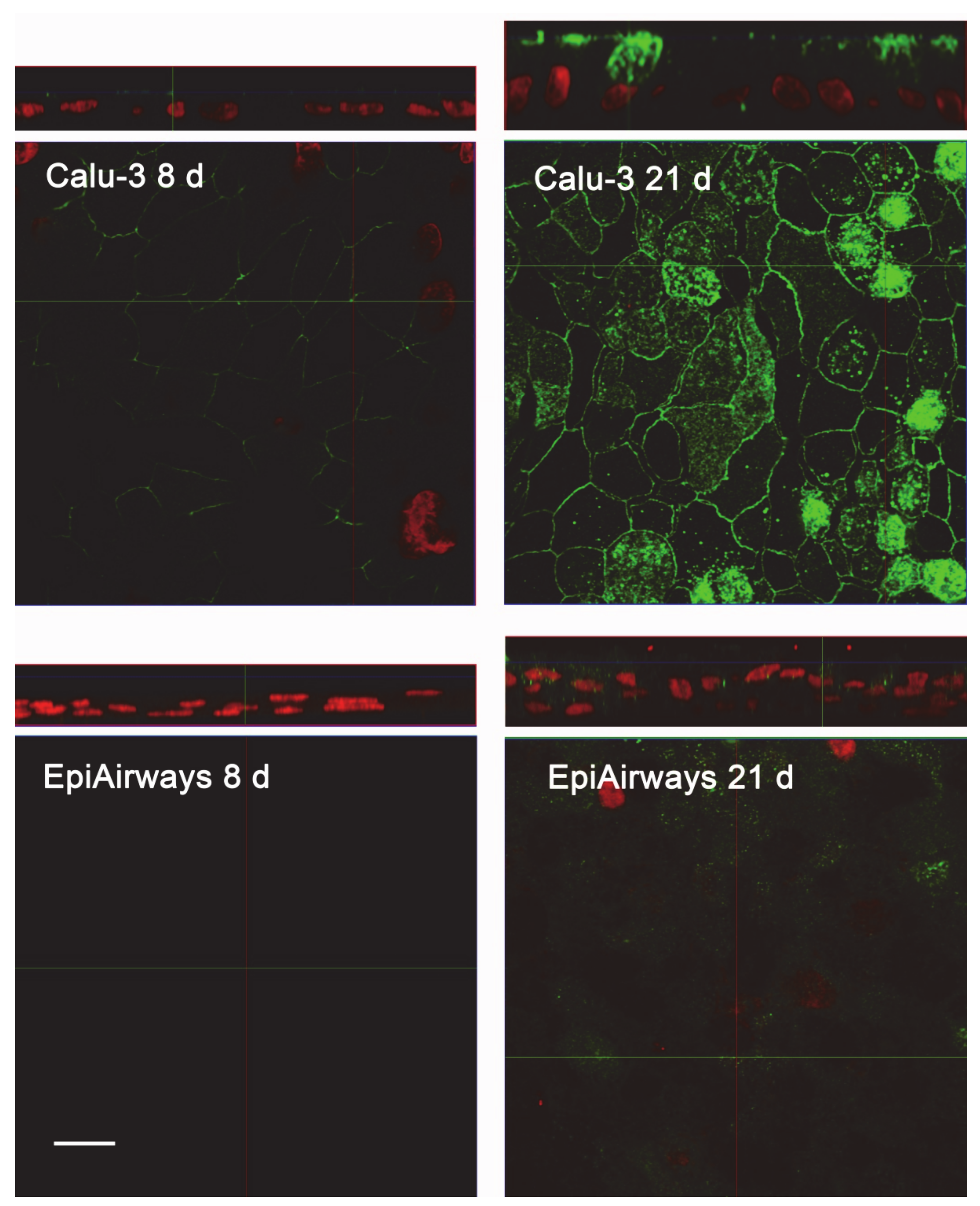
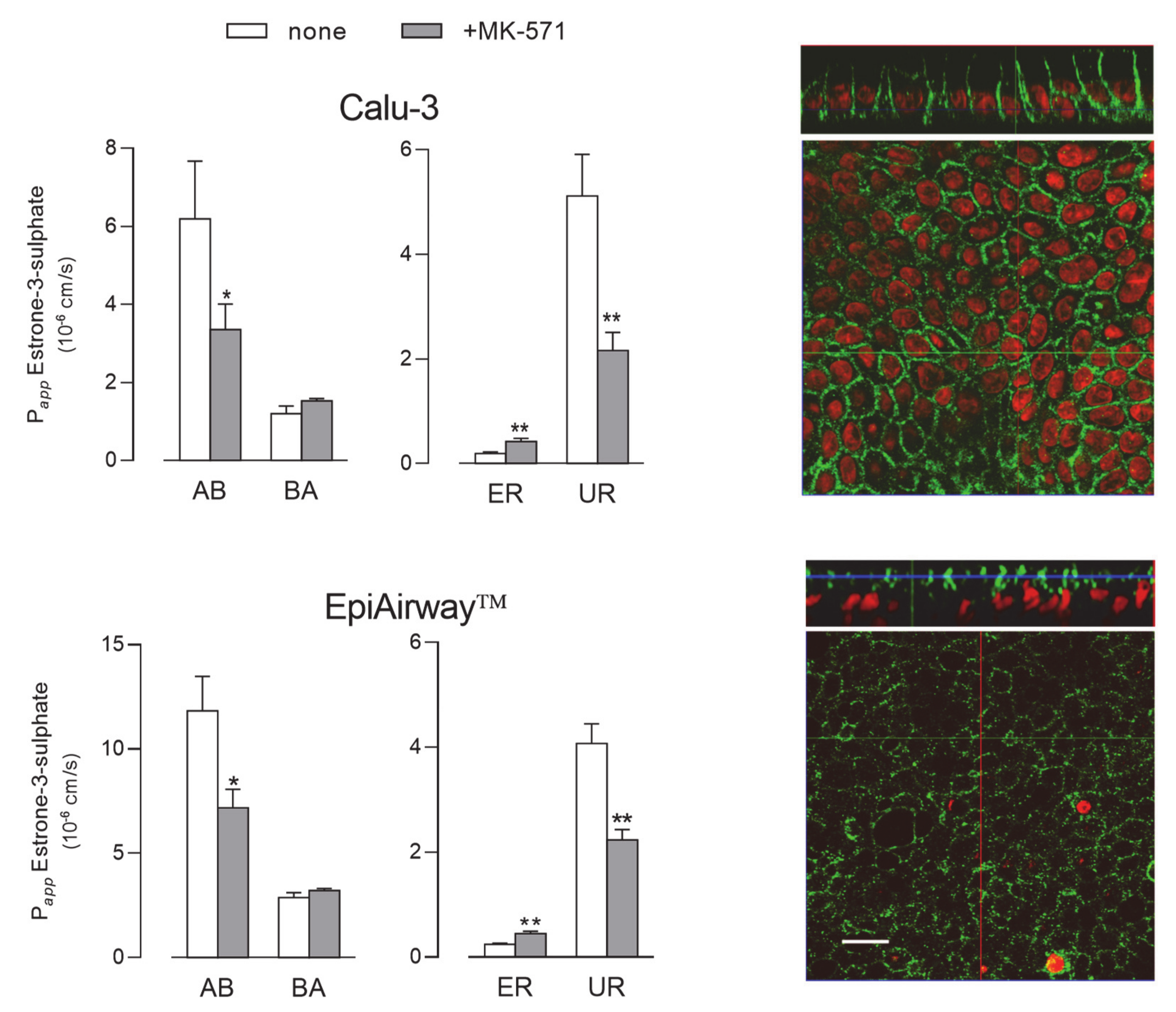
2. Results
3. Discussion
4. Material and Methods
4.1. Cell Cultures
4.2. Trans-epithelial Electrical Resistance (TEER) and Cellular Permeability
4.3. Bidirectional Transport Studies
4.4. Calculation of Papp
4.5. RT-qPCR Analysis
4.6. Western Blot Analysis
4.7. Immunocytochemistry and Immunohistochemistry
4.8. Statistical Analysis
4.9. Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCRP | Breast Cancer Resistance Protein; |
| HBSS | Hank’s Balanced Salt Solution; |
| MDR1 | Multidrug Resistance 1; |
| MRP1 | Multidrug Resistance-Associated Protein-1; |
| Papp | apparent permeability coefficient; |
| TEER | Transepithelial Electrical Resistance. |
References
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome. Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Van der Deen, M.; de Vries, E.G.; Timens, W.; Scheper, R.J.; Timmer-Bosscha, H.; Postma, D.S. ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir. Res. 2005, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Bosquillon, C. Drug transporters in the lung—Do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010, 99, 2240–2255. [Google Scholar] [CrossRef] [PubMed]
- Nickel, S.; Clerkin, C.G.; Selo, M.A.; Ehrhardt, C. Transport mechanisms at the pulmonary mucosa: Implications for drug delivery. Expert. Opin. Drug Deliv. 2016, 13, 667–690. [Google Scholar] [CrossRef]
- Akhtar, U.; Scott, J.; Chu, A.; GJ, E. In vivo and In vitro Assessment of Particulate Matter Toxicology. In Urban Airborne Particulate Matter. Environmental Science and Engineering (Environmental Engineering); Zereini, F.W.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 427–449. [Google Scholar]
- Haghi, M.; Ong, H.X.; Traini, D.; Young, P. Across the pulmonary epithelial barrier: Integration of physicochemical properties and human cell models to study pulmonary drug formulations. Pharmacol. Ther. 2014, 144, 235–252. [Google Scholar] [CrossRef]
- Hutter, V.; Chau, D.Y.; Hilgendorf, C.; Brown, A.; Cooper, A.; Zann, V.; Pritchard, D.I.; Bosquillon, C. Digoxin net secretory transport in bronchial epithelial cell layers is not exclusively mediated by P-glycoprotein/MDR1. Eur. J. Pharm. Biopharm. 2014, 86, 74–82. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Cho, H.J.; Bian, S.; Roh, H.J.; Lee, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J. Pharm. Sci. 2007, 96, 341–350. [Google Scholar] [CrossRef]
- Madlova, M.; Bosquillon, C.; Asker, D.; Dolezal, P.; Forbes, B. In-vitro respiratory drug absorption models possess nominal functional P-glycoprotein activity. J. Pharm. Pharmacol. 2009, 61, 293–301. [Google Scholar] [CrossRef]
- Zavala, J.; O’Brien, B.; Lichtveld, K.; Sexton, K.G.; Rusyn, I.; Jaspers, I.; Vizuete, W. Assessment of biological responses of EpiAirway 3-D cell constructs versus A549 cells for determining toxicity of ambient air pollution. Inhal. Toxicol. 2016, 28, 251–259. [Google Scholar] [CrossRef]
- Chemuturi, N.V.; Hayden, P.; Klausner, M.; Donovan, M.D. Comparison of human tracheal/bronchial epithelial cell culture and bovine nasal respiratory explants for nasal drug transport studies. J. Pharm. Sci. 2005, 94, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal. Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Reus, A.A.; Maas, W.J.; Jansen, H.T.; Constant, S.; Staal, Y.C.; van Triel, J.J.; Kuper, C.F. Feasibility of a 3D human airway epithelial model to study respiratory absorption. Toxicol. In Vitro 2014, 28, 258–264. [Google Scholar] [CrossRef]
- Min, K.A.; Talattof, A.; Tsume, Y.; Stringer, K.A.; Yu, J.Y.; Lim, D.H.; Rosania, G.R. The extracellular microenvironment explains variations in passive drug transport across different airway epithelial cell types. Pharm. Res. 2013, 30, 2118–2132. [Google Scholar] [CrossRef]
- Haghi, M.; Young, P.M.; Traini, D.; Jaiswal, R.; Gong, J.; Bebawy, M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev. Ind. Pharm. 2010, 36, 1207–1214. [Google Scholar] [CrossRef]
- Hamilton, K.O.; Topp, E.; Makagiansar, I.; Siahaan, T.; Yazdanian, M.; Audus, K.L. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 2001, 298, 1199–1205. [Google Scholar]
- Archinal-Mattheis, A.; Rzepka, R.W.; Watanabe, T.; Kokubu, N.; Itoh, Y.; Combates, N.J.; Bair, K.W.; Cohen, D. Analysis of the interactions of SDZ PSC 833 ([3’-keto-Bmt1]-Val2]-Cyclosporine), a multidrug resistance modulator, with P-glycoprotein. Oncol. Res. 1995, 7, 603–610. [Google Scholar]
- Marsousi, N.; Doffey-Lazeyras, F.; Rudaz, S.; Desmeules, J.A.; Daali, Y. Intestinal permeability and P-glycoprotein-mediated efflux transport of ticagrelor in Caco-2 monolayer cells. Fundam. Clin. Pharmacol. 2016, 30, 577–584. [Google Scholar] [CrossRef]
- Mouly, S.; Paine, M.F. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm. Res. 2003, 20, 1595–1599. [Google Scholar] [CrossRef]
- Blokzijl, H.; Vander Borght, S.; Bok, L.I.; Libbrecht, L.; Geuken, M.; van den Heuvel, F.A.; Dijkstra, G.; Roskams, T.A.; Moshage, H.; Jansen, P.L.; et al. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm. Bowel. Dis. 2007, 13, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.; Kauffmann, H.M.; Ito, K.; Leslie, E.M.; Deeley, R.G.; Schrenk, D.; Cole, S.P. A naturally occurring mutation in MRP1 results in a selective decrease in organic anion transport and in increased doxorubicin resistance. Pharmacogenetics 2002, 12, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Gekeler, V.; Ise, W.; Sanders, K.H.; Ulrich, W.R.; Beck, J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995, 208, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef]
- Toyoda, Y.; Takada, T.; Suzuki, H. Inhibitors of Human ABCG2: From Technical Background to Recent Updates With Clinical Implications. Front. Pharmacol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Bosquillon, C.; Madlova, M.; Patel, N.; Clear, N.; Forbes, B. A Comparison of Drug Transport in Pulmonary Absorption Models: Isolated Perfused rat Lungs, Respiratory Epithelial Cell Lines and Primary Cell Culture. Pharm. Res. 2017, 34, 2532–2540. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasic, E.; Hevir-Kene, N.; Rizner, T.L.; Peternel, L.; Kristan, K. The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function. Eur. J. Pharm. Sci. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Mathia, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability characteristics of calu-3 human bronchial epithelial cells: In vitro-in vivo correlation to predict lung absorption in rats. J. Drug Target. 2002, 10, 31–40. [Google Scholar] [CrossRef]
- Mukherjee, M.; Pritchard, D.I.; Bosquillon, C. Evaluation of air-interfaced Calu-3 cell layers for investigation of inhaled drug interactions with organic cation transporters in vitro. Int. J. Pharm. 2012, 426, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Suzuki, S.; Uchida, Y.; Tachikawa, M.; Terasaki, T. Drug Transporter Protein Quantification of Immortalized Human Lung Cell Lines Derived from Tracheobronchial Epithelial Cells (Calu-3 and BEAS2-B), Bronchiolar-Alveolar Cells (NCI-H292 and NCI-H441), and Alveolar Type II-like Cells (A549) by Liquid Chromatography-Tandem Mass Spectrometry. J. Pharm. Sci. 2015, 104, 3029–3038. [Google Scholar] [PubMed]
- Berg, T.; Hegelund Myrback, T.; Olsson, M.; Seidegard, J.; Werkstrom, V.; Zhou, X.H.; Grunewald, J.; Gustavsson, L.; Nord, M. Gene expression analysis of membrane transporters and drug-metabolizing enzymes in the lung of healthy and COPD subjects. Pharmacol. Res. Perspect. 2014, 2, e00054. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, P.A.; Abati, A.; Litman, T.; Morisaki, K.; Honjo, Y.; Mittal, K.; Bates, S.E. Localization of the ABCG2 mitoxantrone resistance-associated protein in normal tissues. Cancer Lett. 2006, 235, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, G.L.; Pijnenborg, A.C.; Smit, E.F.; Muller, M.; Postma, D.S.; Timens, W.; van der Valk, P.; de Vries, E.G.; Scheper, R.J. Multidrug resistance related molecules in human and murine lung. J. Clin. Pathol. 2002, 55, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Terasaki, T. Quantitative expression of human drug transporter proteins in lung tissues: Analysis of regional, gender, and interindividual differences by liquid chromatography-tandem mass spectrometry. J. Pharm. Sci. 2013, 102, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Paturi, D.K.; Kwatra, D.; Ananthula, H.K.; Pal, D.; Mitra, A.K. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3). Int. J. Pharm. 2010, 384, 32–38. [Google Scholar] [CrossRef]
- Endter, S.; Francombe, D.; Ehrhardt, C.; Gumbleton, M. RT-PCR analysis of ABC, SLC and SLCO drug transporters in human lung epithelial cell models. J. Pharm. Pharmacol. 2009, 61, 583–591. [Google Scholar] [CrossRef]
- Berg, T.; Hegelund-Myrback, T.; Ockinger, J.; Zhou, X.H.; Brannstrom, M.; Hagemann-Jensen, M.; Werkstrom, V.; Seidegard, J.; Grunewald, J.; Nord, M.; et al. Expression of MATE1, P-gp, OCTN1 and OCTN2, in epithelial and immune cells in the lung of COPD and healthy individuals. Respir. Res. 2018, 19, 68. [Google Scholar] [CrossRef]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim. Biophys. Acta 2015, 1848, 1563–1572. [Google Scholar] [CrossRef][Green Version]
- Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Dall’Asta, V. y+LAT1 and y+LAT2 contribution to arginine uptake in different human cell models: Implications in the pathophysiology of Lysinuric Protein Intolerance. J. Cell Mol. Med. 2020, 24, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Milioli, M.; Di Lascia, M.; Bernuzzi, G.; Dall’Asta, V. Human macrophage differentiation induces OCTN2-mediated L-carnitine transport through stimulation of mTOR-STAT3 axis. J. Leukoc. Biol. 2017, 101, 665–674. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotoli, B.M.; Barilli, A.; Visigalli, R.; Ferrari, F.; Frati, C.; Lagrasta, C.A.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. Int. J. Mol. Sci. 2020, 21, 3190. https://doi.org/10.3390/ijms21093190
Rotoli BM, Barilli A, Visigalli R, Ferrari F, Frati C, Lagrasta CA, Di Lascia M, Riccardi B, Puccini P, Dall’Asta V. Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. International Journal of Molecular Sciences. 2020; 21(9):3190. https://doi.org/10.3390/ijms21093190
Chicago/Turabian StyleRotoli, Bianca Maria, Amelia Barilli, Rossana Visigalli, Francesca Ferrari, Caterina Frati, Costanza Annamaria Lagrasta, Maria Di Lascia, Benedetta Riccardi, Paola Puccini, and Valeria Dall’Asta. 2020. "Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium" International Journal of Molecular Sciences 21, no. 9: 3190. https://doi.org/10.3390/ijms21093190
APA StyleRotoli, B. M., Barilli, A., Visigalli, R., Ferrari, F., Frati, C., Lagrasta, C. A., Di Lascia, M., Riccardi, B., Puccini, P., & Dall’Asta, V. (2020). Characterization of ABC Transporters in EpiAirway™, a Cellular Model of Normal Human Bronchial Epithelium. International Journal of Molecular Sciences, 21(9), 3190. https://doi.org/10.3390/ijms21093190





