Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures
Abstract
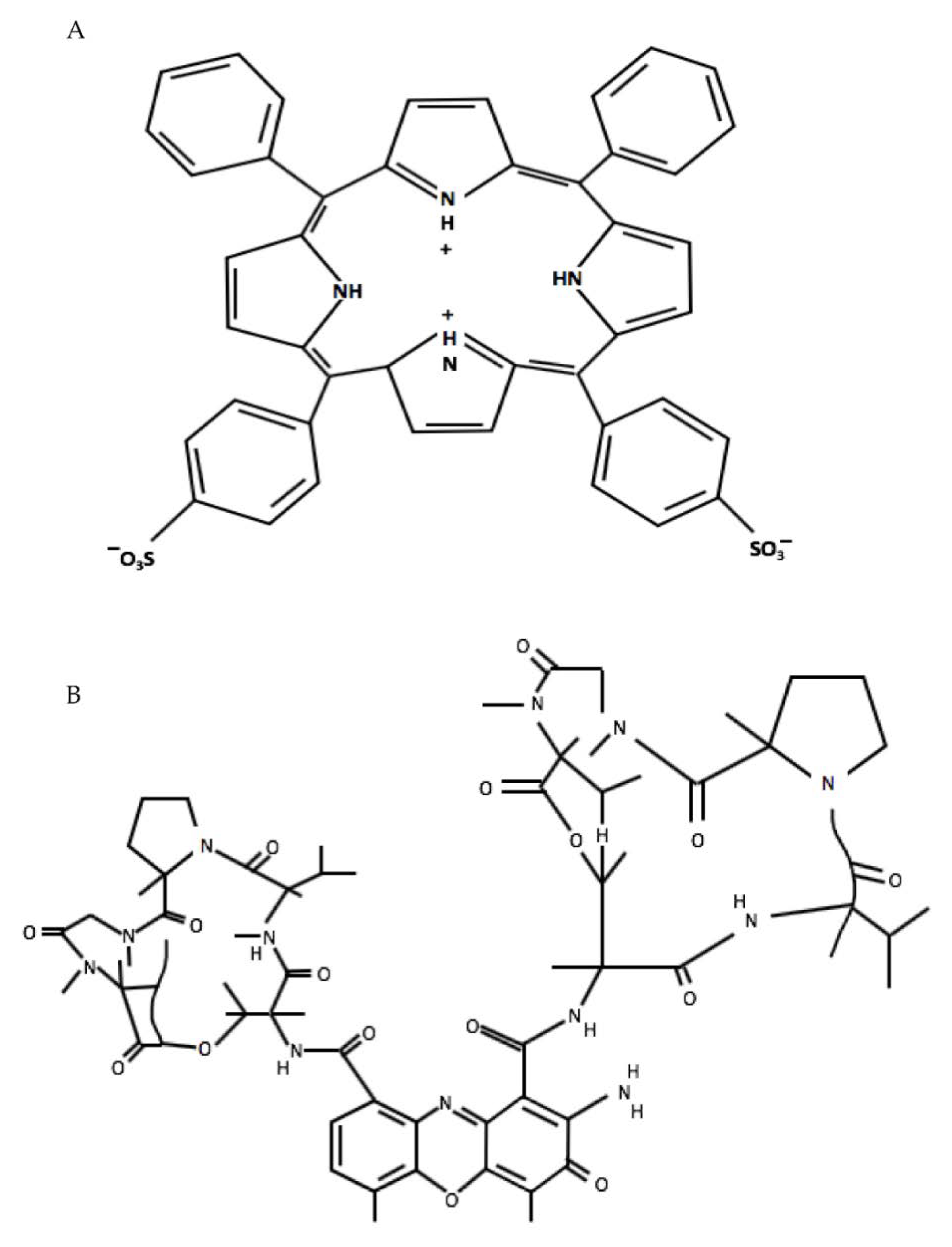
1. Introduction
2. Results
2.1. Combination Treatment and Phototoxicity Studies in 2D Cultures and 3D Constructs
2.2. Live-Dead Viability Assay of 3D Constructs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Manufacture of 3D Cancer Constructs
4.3. In Vitro PDT/Combination Treatment Phototoxicity Studies in 2D Cultures and 3D Constructs
4.4. Cell Viability Assay
4.5. Live/Dead Staining for Fluorescence Imaging
4.6. Evaluation of Synergistic Effects
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Mallidi, S.; Liu, J.; Chiang, C.T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Muhammad, S.; Khurshid, A.; Ikram, M.; Maqsood, M.; Fisher, C.; Cathcart, J.; Lilge, L. Effective Phthalocyanines mediated Photodynamic Therapy with Doxorubicin or Methotrexate combination therapy at sub-micromolar concentrations in vitro. Photodiagn. Photodyn. Ther. 2018, 22, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, S.; Moret, F.; Conte, C.; Fraix, A.; Tirino, P.; Ungaro, F.; Sortino, S.; Reddi, E.; Quaglia, F. Hyaluronan-decorated polymer nanoparticles targeting the CD44 receptor for the combined photo/ chemo-therapy of cancer. Nanoscale 2015, 7, 5643–5653. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.J.; Lai, P.S.; Shieh, M.J.; Macrobert, A.J.; Berg, K.; Bown, S.G. Reversal of doxorubicin resistance in breast cancer cells by photochemical internalization. Int. J. Cancer 2006, 119, 2692–2698. [Google Scholar] [CrossRef]
- Selbo, P.K.; Weyergang, A.; Bonsted, A.; Bown, S.G.; Berg, K. Photochemical internalization of therapeutic macromolecular agents: A novel strategy to kill multidrug-resistant cancer cells. J. Pharmacol. Exp. Ther. 2006, 319, 604–612. [Google Scholar] [CrossRef]
- Shi, L.; Buchner, A.; Pohla, H.; Pongratz, T.; Ruhm, A.; Zimmermann, W.; Gederaas, O.A.; Zhang, L.; Wang, X.; Stepp, H.; et al. Methadone enhances the effectiveness of 5-aminolevulinic acid-based photodynamic therapy for squamous cell carcinoma and glioblastoma in vitro. J. Biophotonics 2019, 12, e201800468. [Google Scholar] [CrossRef]
- Xia, Y.; Gupta, G.K.; Castano, A.P.; Mroz, P.; Avci, P.; Hamblin, M.R. CpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancer. J. Biophotonics 2013, 7, 897–905. [Google Scholar] [CrossRef]
- Huang, H.C.; Rizvi, I.; Liu, J.; Anbil, S.; Kalra, A.; Lee, H.; Baglo, Y.; Paz, N.; Hayden, D.; Pereira, S.; et al. Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res. 2018, 78, 558–571. [Google Scholar] [CrossRef]
- Berg, K.; Weyergang, A.; Prasmickaite, L.; Bonsted, A.; Hogset, A.; Strand, M.T.; Wagner, E.; Selbo, P.K. Photochemical internalization (PCI): A technology for drug delivery. Methods Mol. Biol. 2010, 635, 133–145. [Google Scholar]
- Martinez de Pinillos Bayona, A.; Moore, C.M.; Loizidou, M.; MacRobert, A.J.; Woodhams, J.H. Enhancing the efficacy of cytotoxic agents for cancer therapy using photochemical internalisation. Int. J. Cancer 2015, 138, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Adigbli, D.K.; Pye, H.; Seebaluck, J.; Loizidou, M.; MacRobert, A.J. The intracellular redox environment modulates the cytotoxic efficacy of single and combination chemotherapy in breast cancer cells using photochemical internalisation. RSC Adv. 2019, 9, 25861–25874. [Google Scholar] [CrossRef]
- Tsubone, T.M.; Martins, W.K.; Pavani, C.; Junqueira, H.C.; Itri, R.; Baptista, M.S. Enhanced efficiency of cell death by lysosome-specific photodamage. Sci. Rep. 2017, 7, 6734. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.O.; Angel-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218, 133–147. [Google Scholar] [CrossRef]
- Lilletvedt, M.; Tonnesen, H.H.; Hogset, A.; Sande, S.A.; Kristensen, S. Evaluation of physicochemical properties and aggregation of the photosensitizers TPCS2a and TPPS2a in aqueous media. Pharmazie 2011, 66, 325–333. [Google Scholar] [PubMed]
- Lilletvedt, M.; Tonnesen, H.H.; Høgset, A.; Nardo, L.; Kristensen, S. Physicochemical characterization of the photosensitizersTPCS2a andTPPS2a 1. Spectroscopic evaluation of drug–solvent interactions. Pharmazie 2010, 65, 588–595. [Google Scholar]
- Bull-Hansen, B.; Berstad, M.B.; Berg, K.; Cao, Y.; Skarpen, E.; Fremstedal, A.S.; Rosenblum, M.G.; Peng, Q.; Weyergang, A. Photochemical activation of MH3-B1/rGel: A HER2-targeted treatment approach for ovarian cancer. Oncotarget 2015, 6, 12436–12451. [Google Scholar] [CrossRef]
- Weyergang, A.; Berstad, M.E.; Bull-Hansen, B.; Olsen, C.E.; Selbo, P.K.; Berg, K. Photochemical activation of drugs for the treatment of therapy-resistant cancers. Photochem. Photobiol. Sci. 2015, 14, 1465–1475. [Google Scholar] [CrossRef]
- Fu, A.; Tang, R.; Hardie, J.; Farkas, M.E.; Rotello, V.M. Promises and pitfalls of intracellular delivery of proteins. Bioconjug. Chem. 2014, 25, 1602–1608. [Google Scholar] [CrossRef]
- Yaghini, E.; Dondi, R.; Edler, K.J.; Loizidou, M.; MacRobert, A.J.; Eggleston, I.M. Codelivery of a cytotoxin and photosensitiser via a liposomal nanocarrier: A novel strategy for light-triggered cytosolic release. Nanoscale 2018, 10, 20366–20376. [Google Scholar] [CrossRef]
- Dondi, R.; Yaghini, E.; Tewari, K.M.; Wang, L.; Giuntini, F.; Loizidou, M.; MacRobert, A.J.; Eggleston, I.M. Flexible synthesis of cationic peptide-porphyrin derivatives for light-triggered drug delivery and photodynamic therapy. Org. Biomol. Chem. 2016, 14, 11488–11501. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiller, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
- O’Rourke, C.; Hopper, C.; MacRobert, A.J.; Phillips, J.B.; Woodhams, J.H. Could clinical photochemical internalisation be optimised to avoid neuronal toxicity? Int. J. Pharm. 2017, 528, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; Jerjes, W.; Berg, K.; Hogset, A.; Mosse, C.A.; Hamoudi, R.; Hamdoon, Z.; Simeon, C.; Carnell, D.; Forster, M.; et al. Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: A phase 1, dose-escalation, first-in-man trial. Lancet Oncol. 2016, 17, 1217–1229. [Google Scholar] [CrossRef]
- WCR Fund. Cancer Trends, Ovarian Cancer Statistics; World Cancer Research Fund; American Cancer Institute: New York, NY, USA, 2018. [Google Scholar]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. PNAS 2016, 113, 10666–10671. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, H.N.; Moganty, R.R. Structural aspects of the interaction of anticancer drug Actinomycin-D to the GC rich region of hmgb1 gene. Int. J. Biol. Macromol. 2016, 87, 433–442. [Google Scholar] [CrossRef]
- Bensaude, O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription 2011, 2, 103–108. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Salanti, G.; Pavlidis, N.; Paraskevaidis, E.; Ioannidis, J.P. Survival benefits with diverse chemotherapy regimens for ovarian cancer: Meta-analysis of multiple treatments. J. Natl. Cancer Inst. 2006, 98, 1655–1663. [Google Scholar] [CrossRef]
- Sumi, T.; Ishiko, O.; Yoshida, H.; Ogita, S. Bleomycin, actinomycin-D, and cisplatin treatment of ovarian germ-cell malignancies contributes to reducing adverse drug reactions. Oncol. Rep. 2000, 7, 1235–1238. [Google Scholar] [CrossRef][Green Version]
- Chinsky, L.; Turpin, P.Y. Fluorescence of actinomycin D and its DNA complex. Biochim. Biophys. Acta 1977, 475, 54–63. [Google Scholar] [CrossRef]
- Hutt, D.M.; Herman, D.; Rodrigues, A.P.; Noel, S.; Pilewski, J.M.; Matteson, J.; Hoch, B.; Kelly, J.W.; Schmidt, A.; Thomas, P.J.; et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat. Chem. Biol. 2010, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Tannock, I.F. Inhibition of endosomal sequestration of basic anticancer drugs: Influence on cytotoxicity and tissue penetration. Br. J. Cancer 2006, 94, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Simon, S.M. Subcellular localization and activity of multidrug resistance proteins. Mol. Biol. Cell 2003, 14, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Hadi, L.; MacRobert, A.J.; Loizidou, M.; Yaghini, E. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale 2018, 10, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, Y.; Chen, D.; Yang, C.; Kai, C.; Wang, X.; Shi, F.; Dou, J. Observation of ovarian cancer stem cell behavior and investigation of potential mechanisms of drug resistance in three-dimensional cell culture. J. Biosci. Bioeng. 2014, 118, 214–222. [Google Scholar] [CrossRef]
- Yamada, T.; Hattori, K.; Satomi, H.; Okazaki, T.; Mori, H.; Hirose, Y. Establishment and characterization of a cell line (HCH-1) originating from a human clear cell carcinoma of the ovary. J. Ovarian Res. 2016, 9, 32. [Google Scholar] [CrossRef]
- Hill, C.R.; Jamieson, D.; Thomas, H.D.; Brown, C.D.; Boddy, A.V.; Veal, G.J. Characterisation of the roles of ABCB1, ABCC1, ABCC2 and ABCG2 in the transport and pharmacokinetics of actinomycin D in vitro and in vivo. Biochem. Pharmacol. 2013, 85, 29–37. [Google Scholar] [CrossRef]
- Mohammad Hadi, L.; Yaghini, E.; Stamati, K.; Loizidou, M.; MacRobert, A.J. Therapeutic enhancement of a cytotoxic agent using photochemical internalisation in 3D compressed collagen constructs of ovarian cancer. Acta Biomater. 2018, 81, 80–92. [Google Scholar] [CrossRef]
- Cheema, U.; Rong, Z.; Kirresh, O.; Macrobert, A.J.; Vadgama, P.; Brown, R.A. Oxygen diffusion through collagen scaffolds at defined densities: Implications for cell survival in tissue models. J. Tissue Eng. Regen. Med. 2012, 6, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nyga, A.; Loizidou, M.; Emberton, M.; Cheema, U. A novel tissue engineered three-dimensional in vitro colorectal cancer model. Acta Biomater. 2013, 9, 7917–7926. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Chen, Y.D.; Huang, S.F.; Wang, H.M.; Wu, M.H. The Effect of Primary Cancer Cell Culture Models on the Results of Drug Chemosensitivity Assays: The Application of Perfusion Microbioreactor System as Cell Culture Vessel. BioMed Res. Int. 2015, 2015, 470283. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.L.; Jiang, Q.; Han, S.; Wu, Y.; Cui Tomshine, J.; Wang, D.; Gan, Y.; Zou, G.; Liang, X.J. Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Mol. Imaging 2012, 11, 487–498. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Zhang, M.; Boughton, P.; Rose, B.; Lee, C.S.; Hong, A.M. The Use of Porous Scaffold as a Tumor Model. Int. J. Biomater. 2013, 2013, 396056. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Sabanayagam, C.R.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials 2014, 35, 3319–3330. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef]
- Shin, C.S.; Kwak, B.; Han, B.; Park, K. Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Mol. Pharm. 2013, 10, 2167–2175. [Google Scholar] [CrossRef]
- Celli, J.P.; Rizvi, I.; Blanden, A.R.; Massodi, I.; Glidden, M.D.; Pogue, B.W.; Hasan, T. An imaging-based platform for high-content, quantitative evaluation of therapeutic response in 3D tumour models. Sci. Rep. 2014, 4, 3751. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Wong, S.; Lu, M.; Xiao, P.; Stenzel, M.H. Influence of nanoparticle shapes on cellular uptake of paclitaxel loaded nanoparticles in 2D and 3D cancer models. Polym. Chem. 2017, 2017, 3317–3326. [Google Scholar] [CrossRef]
- Magdeldin, T.; Lopez-Davila, V.; Pape, J.; Cameron, G.W.; Emberton, M.; Loizidou, M.; Cheema, U. Engineering a vascularised 3D in vitro model of cancer progression. Sci. Rep. 2017, 7, 44045. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Magdeldin, T.; Ali, M.; Walsh, C.; Lythgoe, M.; Emberton, M.; Cheema, U. Cancer invasion regulates vascular complexity in a three-dimensional biomimetic model. Eur. J. Cancer 2019, 119, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Berg, K.; Hogset, A.; Bown, S.G.; MacRobert, A.J. Photophysical and photobiological properties of a sulfonated chlorin photosensitiser TPCS(2a) for photochemical internalisation (PCI). Photochem. Photobiol. Sci. 2013, 12, 519–526. [Google Scholar] [CrossRef]
- Dietze, A.; Bonsted, A.; Hogset, A.; Berg, K. Photochemical internalization enhances the cytotoxic effect of the protein toxin gelonin and transgene expression in sarcoma cells. Photochem. Photobiol. 2003, 78, 283–289. [Google Scholar] [CrossRef]
- Lu, D.F.; Wang, Y.S.; Li, C.; Wei, G.J.; Chen, R.; Dong, D.M.; Yao, M. Actinomycin D inhibits cell proliferations and promotes apoptosis in osteosarcoma cells. Int. J. Clin. Exp. Med. 2015, 8, 1904–1911. [Google Scholar]
- Kleeff, J.; Kornmann, M.; Sawhney, H.; Korc, M. Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. Int. J. Cancer 2000, 86, 399–407. [Google Scholar] [CrossRef]
- Shiraishi, N.; Akiyama, S.; Kobayashi, M.; Kuwano, M. Lysosomotropic agents reverse multiple drug resistance in human cancer cells. Cancer Lett. 1986, 30, 251–259. [Google Scholar] [CrossRef]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget 2015, 6, 1143–1156. [Google Scholar] [CrossRef]
- Bowen, D.; Goldman, I.D. The relationship among transport, intracellular binding, and inhibition of RNA synthesis by actinomycin D in Ehrlich ascites tumor cells in vitro. Cancer Res. 1975, 35, 3054–3060. [Google Scholar]
- Kessel, D.; Wodinsky, I. Uptake in vivo and in vitro of actinomycin D by mouse leukemias as factors in survival. Biochem. Pharmacol. 1968, 17, 161–164. [Google Scholar] [CrossRef]
- Gronewold, A.; Horn, M.; Randelovic, I.; Tovari, J.; Munoz Vazquez, S.; Schomacker, K.; Neundorf, I. Corrigendum: Characterization of a Cell-Penetrating Peptide with Potential Anticancer Activity. ChemMedChem 2017, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.; Shin, H.; Han, H.; Na, K.; Kim, H. Combination of chemotherapy and photodynamic therapy for cancer treatment with sonoporation effects. J. Control. Release 2018, 283, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bazylinska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart PLGA Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef]
- Tran, M.G.B.; Neves, J.; Stamati, K.; Redondo, P.; Cope, A.; Brew-Graves, C.; Williams, N.R.; Grierson, J.; Cheema, U.; Loizidou, M.; et al. Acceptability and feasibility study of patient-specific ’tumouroids’ as personalised treatment screening tools: Protocol for prospective tissue and data collection of participants with confirmed or suspected renal cell carcinoma. Int. J. Surg. Protoc. 2019, 14, 24–29. [Google Scholar] [CrossRef]
- Magdeldin, T.; Lopez-Davila, V.; Villemant, C.; Cameron, G.; Drake, R.; Cheema, U.; Loizidou, M. The efficacy of cetuximab in a tissue-engineered three-dimensional in vitro model of colorectal cancer. J. Tissue Eng. 2014, 5, 2041731414544183. [Google Scholar] [CrossRef]
- Yaghini, E.; Dondi, R.; Tewari, K.M.; Loizidou, M.; Eggleston, I.M.; MacRobert, A.J. Endolysosomal targeting of a clinical chlorin photosensitiser for light-triggered delivery of nano-sized medicines. Sci. Rep. 2017, 7, 6059. [Google Scholar] [CrossRef]
- Martinez de Pinillos Bayona, A.; Woodhams, J.; Pye, H.; Hamoudi, R.A.; Moore, C.M.; MacRobert, A.J. Efficacy of photochemical internalisation using disulfonated chlorin and porphyrin photosensitisers: An in vitro study in 2D and 3D prostate cancer models. Cancer Lett. 2017, 393, 68–75. [Google Scholar] [CrossRef]
- Mathews, M.S.; Blickenstaff, J.W.; Shih, E.C.; Zamora, G.; Vo, V.; Sun, C.H.; Hirschberg, H.; Madsen, S.J. Photochemical internalization of bleomycin for glioma treatment. J. Biomed. Opt. 2012, 17, 058001. [Google Scholar] [CrossRef]








| 2D Culture | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | TPPS2a Concentration (µg/mL) | Light Exposure Period (Minute) | PDT only (% Mean Viability ±% SD) | Dactinomycin only (% Mean Viability ±% SD) | Combination Treatment (% Mean Viability ±% SD) | Combination Treatment Efficacy Ratio vs. PDT | Combination Treatment Efficacy Ratio vs. Dactinomycin only | Alpha Values |
| SKOV3 | 0.3 | 3 | 70.7 ± 2.9 | 1.9 | 2.5 | 1.8 | ||
| HEY | 0.4 | 3 | 2.5 | 3.2 | 2.2 | |||
| SKOV3 | 0.3 | 5 | 2.1 | 6.3 | 1.9 | |||
| HEY | 0.4 | 5 | 3.8 | 8.9 | 3.4 | |||
| SKOV3 | 0.3 | 7 | 2.7 | 14.8 | 2.4 | |||
| HEY | 0.4 | 7 | 7.7 | 38.2 | 8.3 | |||
| 3D Culture | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | TPPS2a Concentration (µg/mL) | Light Exposure Period (Minute) | PDT only (% Mean Viability ±%SD) | Dactinomycin only (% Mean Viability ± % SD) | Combination Treatment (% Mean Viability ± % SD) | Combination Treatment Efficacy Ratio vs. PDT | Combination Treatment Efficacy Ratio vs. Dactinomyin only | Alpha Values |
| SKOV3 | 0.3 | 3 | 1.9 | 2.3 | 1.8 | |||
| HEY | 0.4 | 3 | 2.2 | 2.8 | 2.0 | |||
| SKOV3 | 0.3 | 5 | 1.7 | 4.7 | 1.6 | |||
| HEY | 0.4 | 5 | 2.9 | 5.9 | 2.5 | |||
| SKOV3 | 0.3 | 7 | 2.2 | 10.1 | 2.0 | |||
| HEY | 0.4 | 7 | 5.5 | 22.3 | 4.9 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad Hadi, L.; Yaghini, E.; MacRobert, A.J.; Loizidou, M. Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures. Int. J. Mol. Sci. 2020, 21, 3203. https://doi.org/10.3390/ijms21093203
Mohammad Hadi L, Yaghini E, MacRobert AJ, Loizidou M. Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures. International Journal of Molecular Sciences. 2020; 21(9):3203. https://doi.org/10.3390/ijms21093203
Chicago/Turabian StyleMohammad Hadi, Layla, Elnaz Yaghini, Alexander J. MacRobert, and Marilena Loizidou. 2020. "Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures" International Journal of Molecular Sciences 21, no. 9: 3203. https://doi.org/10.3390/ijms21093203
APA StyleMohammad Hadi, L., Yaghini, E., MacRobert, A. J., & Loizidou, M. (2020). Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures. International Journal of Molecular Sciences, 21(9), 3203. https://doi.org/10.3390/ijms21093203






