Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models
Abstract
1. Introduction
2. Results
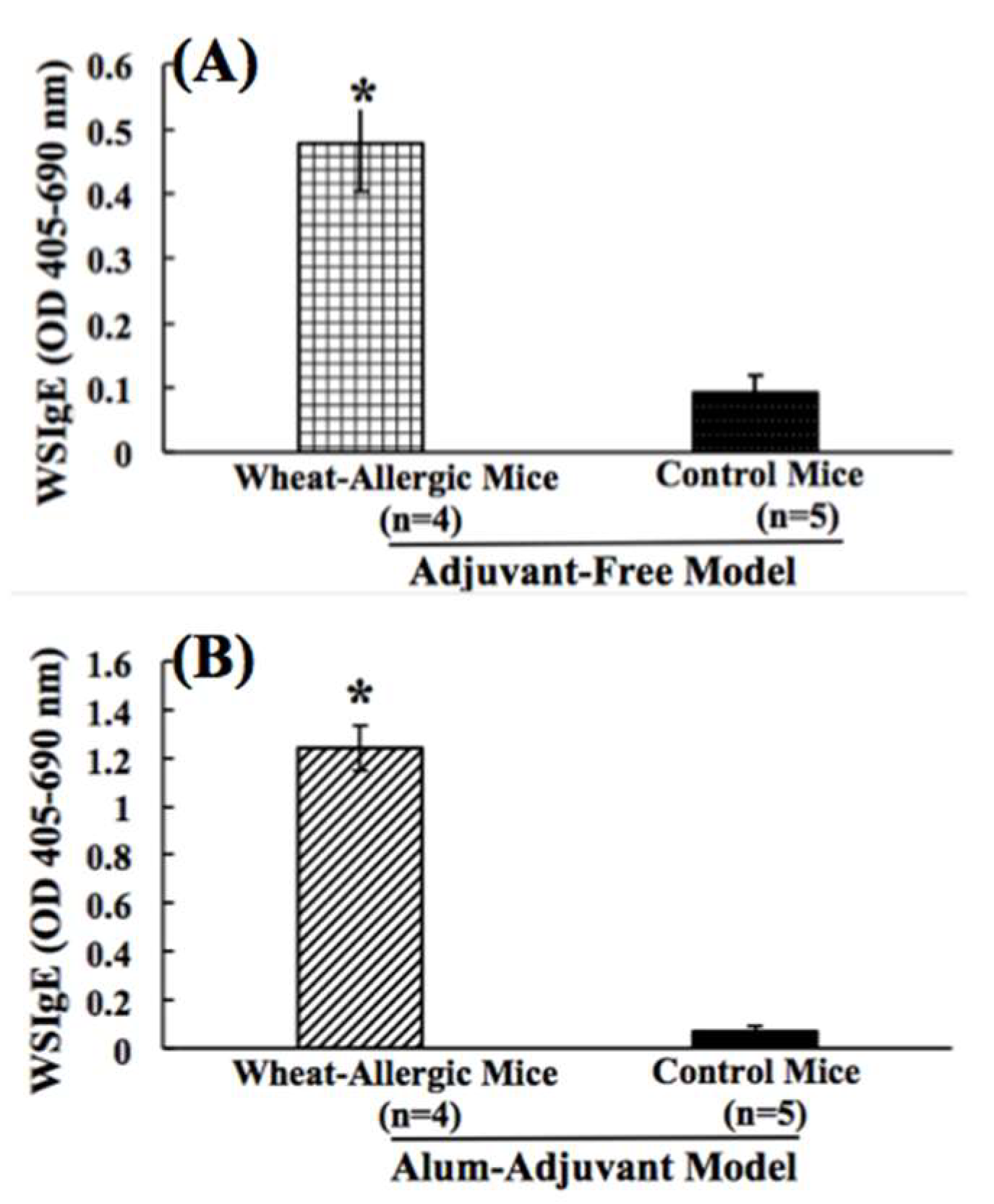
2.1. Comparison of Wheat Protein-Specfic IgE Antibody Responses in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
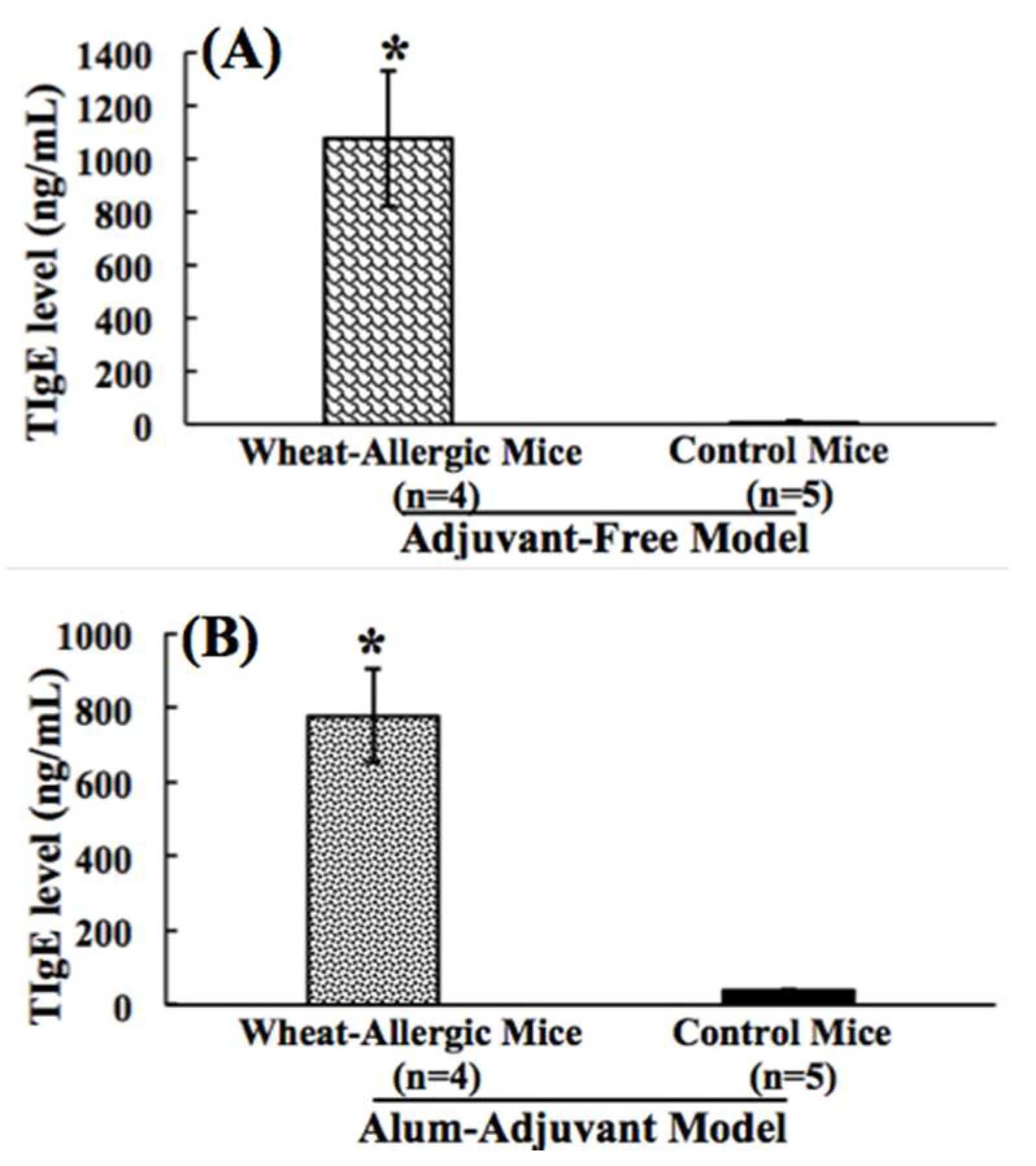
2.2. Comparison of Wheat Protein-Induced Elevation of Total Plasma IgE Antibody Levels in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.3. Comparison of Wheat Protein-Specfic IgG1 Antibody Responses in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.4. Comparison of Wheat Protein-Specfic IgG2a Antibody Responses in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.5. Comparison of Murine Mucosal Mast Cell Protease-1 Responses in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.6. Analysis of in Vivo Levels of Cytokines in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.7. Analysis of in Vivo Levels of Chemokines in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.8. Analysis of in Vivo Levels of Adhesion Molecules in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
2.9. Analysis of in Vivo Levels of Other Allergenicity Relevant Immune Markers in Adjuvant-Free vs. Alum-Adjuvant Mouse Models
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice
4.3. Preparation of Salt-Soluble Wheat Protein Extract from Durum Wheat Flour
4.4. Measurement of SSWP-Specific IgE Antibody Levels and Total Plasma IgE Concentration
4.5. Elicitation of Allergic Reaction and Quantitation of Plasma Level of Mucosal Mast Cell Protease-1
4.6. Spleen Tissue Collection, Protein Extraction and Analysis of Immune Markers
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.R.; Chiaramonte, L.T. Public perception of food allergy. J. Allergy Clin. Immunol. 1996, 97, 1247–1251. [Google Scholar] [CrossRef]
- Biagini, R.E.; MacKenzie, B.A.; Sammons, D.L.; Smith, J.P.; Striley, C.A.; Robertson, S.K.; Snawder, J.E. Evaluation of the prevalence of antiwheat-, anti-flour dust, and anti-alpha-amylase specific IgE antibodies in US blood donors. Ann. Allergy Asthma Immunol. 2004, 92, 649–653. [Google Scholar] [CrossRef]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef]
- Osterballe, M.; Hansen, T.K.; Mortz, C.G.; Host, A.; Bindslev-Jensen, C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr. Allergy Immunol. 2005, 16, 567–573. [Google Scholar] [CrossRef]
- Bjornsson, E.; Janson, C.; Plaschke, P.; Norrman, E.; Sjoberg, O. Prevalence of sensitization to food allergens in adult Swedes. Ann. Allergy Asthma Immunol. 1996, 77, 327–332. [Google Scholar] [CrossRef]
- Gislason, D.; Bjornsson, E.; Gislason, T.; Janson, C.; Sjoberg, O.; Elfman, L.; Boman, G. Sensitization to airborne and food allergens in Reykjavik (Iceland) and Uppsala (Sweden)—A comparative study. Allergy 1999, 54, 1160–1167. [Google Scholar] [CrossRef]
- Woods, R.K.; Thien, F.; Raven, J.; Walters, E.H.; Abramson, M. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann. Allergy Asthma Immunol. 2002, 88, 183–189. [Google Scholar] [CrossRef]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef]
- Gupta, R.; Holdford, D.; Bilaver, L.; Dyer, A.; Holl, J.L.; Meltzer, D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013, 167, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Beaudouin, E.; Renaudin, J.M.; Morisset, M.; Codreanu, F.; Kanny, G.; Moneret-Vautrin, D.A. Food-dependent exercise-induced anaphylaxis—Update and current data. Eur. Ann. Allergy Clin. Immunol. 2006, 38, 45–51. [Google Scholar] [PubMed]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2016, 46, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Fox, S.R.; Smith, B.M.; James, C.; Palmisano, E.L.; Mohammed, A.; Zahid, Z.; Assaad, A.H.; Tobin, M.C.; Gupta, R.S. Racial Differences in Food Allergy Phenotype and Health Care Utilization among US Children. J. Allergy Clin. Immunol. Pract. 2017, 5, 352–357. [Google Scholar] [CrossRef]
- Cianferoni, A.; Khullar, K.; Saltzman, R.; Fiedler, J.; Garrett, J.P.; Naimi, D.R.; Spergel, J.M. Oral food challenge to wheat: A near-fatal anaphylaxis and review of 93 food challenges in children. World Allergy Organ. J. 2013, 6, 14. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Kushimoto, H.; Aoki, T. Masked type I wheat allergy. Relation to exercise-induced anaphylaxis. Arch. Dermatol. 1985, 121, 355–360. [Google Scholar] [CrossRef]
- Du Toit, G. Food-dependent exercise-induced anaphylaxis in childhood. Pediatr. Allergy Immunol. 2007, 18, 455–463. [Google Scholar] [CrossRef]
- Yokooji, T.; Kurihara, S.; Murakami, T.; Chinuki, Y.; Takahashi, H.; Morita, E.; Harada, S.; Ishii, K.; Hiragun, M.; Hide, M.; et al. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol. Int. 2013, 62, 435–445. [Google Scholar] [CrossRef]
- United States Food and Drug Administration Food Allergies. What You Need to Know about Food Allergies. Available online: https://www.fda.gov/food/resourcesforyou/consumers/ucm079311.htm (accessed on 16 August 2019).
- United States Department of Agriculture. Wheat’s Role in the US Diet. Available online: https://www.ers.usda.gov/topics/crops/wheat/wheats-role-in-the-us-diet/ (accessed on 20 August 2019).
- Food and Agriculture Organization of the United Nations. Wheat. Available online: http://www.fao.org/land-water/databases-and-software/crop-information/wheat/en/ (accessed on 20 August 2019).
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef]
- Lauriere, M.; Pecquet, C.; Bouchez-Mahiout, I.; Snegaroff, J.; Bayrou, O.; Raison-Peyron, N.; Vigan, M. Hydrolysed wheat proteins present in cosmetics can induce immediate hypersensitivities. Contact Dermat. 2006, 54, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, Y.; Itagaki, Y.; Taniguchi, M.; Saito, A.; Yasueda, H.; Nakazawa, T.; Hasegawa, M.; Nakamura, H.; Akiyama, K. Rhinoconjunctival sensitization to hydrolyzed wheat protein in facial soap can induce wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Chinuki, Y.; Morita, E. Wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat protein in soap. Allergol. Int. 2012, 61, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, A.S.; Makinen-Kiljunen, S.; Hilvo, S.; Siltanen, M.; Makela, M.J. Severe allergic reaction to gluten hydrolysate without reaction to wheat. Ann. Allergy Asthma Immunol. 2011, 106, 343–344. [Google Scholar] [CrossRef]
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a hypoallergenic wheat line lacking omega-5 gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef]
- Mishra, A.; Arora, N. Allergenicity Assessment of Transgenic Wheat Lines In Silico. Methods Mol. Biol. 2017, 1679, 97–111. [Google Scholar]
- Rey, M.D.; Calderon, M.C.; Rodrigo, M.J.; Zacarias, L.; Alos, E.; Prieto, P. Novel Bread Wheat Lines Enriched in Carotenoids Carrying Hordeum chilense Chromosome Arms in the ph1b Background. PLoS ONE 2015, 10, e0134598. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and validation of a mouse-based primary screening method for testing relative allergenicity of proteins from different wheat genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Adamidi, C.; Lozano, R.M.; Yee, B.C.; Momma, M.; Kobrehel, K.; Ermel, R.; Frick, O.L. Thioredoxin-linked mitigation of allergic responses to wheat. Proc. Natl. Acad. Sci. USA 1997, 94, 5372–5377. [Google Scholar] [CrossRef]
- Kroghsbo, S.; Rigby, N.M.; Johnson, P.E.; Adel-Patient, K.; Bogh, K.L.; Salt, L.J.; Mills, E.N.; Madsen, C.B. Assessment of the sensitizing potential of processed peanut proteins in Brown Norway rats: Roasting does not enhance allergenicity. PLoS ONE 2014, 9, e96475. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, A.R.; Madsen, C.B.; Bogh, K.L. An Animal Model for Wheat Allergy Skin Sensitisation: A Comparative Study in Naive versus Tolerant Brown Norway Rats. Int. Arch. Allergy Immunol. 2019, 178, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Kozai, H.; Yano, H.; Matsuda, T.; Kato, Y. Wheat-dependent exercise-induced anaphylaxis in mice is caused by gliadin and glutenin treatments. Immunol. Lett. 2006, 102, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nagano, T.; Yano, H.; Matsuda, T.; Ikeda, T.M.; Haruma, K.; Kato, Y. Impact of omega-5 gliadin on wheat-dependent exercise-induced anaphylaxis in mice. Biosci. Biotechnol. Biochem. 2011, 75, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Denery-Papini, S.; Bodinier, M.; Pineau, F.; Triballeau, S.; Tranquet, O.; Adel-Patient, K.; Moneret-Vautrin, D.A.; Bakan, B.; Marion, D.; Mothes, T.; et al. Immunoglobulin-E-binding epitopes of wheat allergens in patients with food allergy to wheat and in mice experimentally sensitized to wheat proteins. Clin. Exp. Allergy 2011, 41, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery-Papini, S.; Larre, C.; Gaudin, J.C.; Brossard, C.; Bodinier, M. Wheat gliadins modified by deamidation are more efficient than native gliadins in inducing a Th2 response in Balb/c mice experimentally sensitized to wheat allergens. Mol. Nutr. Food Res. 2012, 56, 336–344. [Google Scholar] [CrossRef]
- Adachi, R.; Nakamura, R.; Sakai, S.; Fukutomi, Y.; Teshima, R. Sensitization to acid-hydrolyzed wheat protein by transdermal administration to BALB/c mice, and comparison with gluten. Allergy 2012, 67, 1392–1399. [Google Scholar] [CrossRef]
- Abe, R.; Shimizu, S.; Yasuda, K.; Sugai, M.; Okada, Y.; Chiba, K.; Akao, M.; Kumagai, H.; Kumagai, H. Evaluation of reduced allergenicity of deamidated gliadin in a mouse model of wheat-gliadin allergy using an antibody prepared by a peptide containing three epitopes. J. Agric. Food Chem. 2014, 62, 2845–2852. [Google Scholar] [CrossRef]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse Model of Anaphylaxis and Atopic Dermatitis to Salt-Soluble Wheat Protein Extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef]
- Birmingham, N.P.; Parvataneni, S.; Hassan, H.M.; Harkema, J.; Samineni, S.; Navuluri, L.; Kelly, C.J.; Gangur, V. An adjuvant-free mouse model of tree nut allergy using hazelnut as a model tree nut. Int. Arch. Allergy Immunol. 2007, 144, 203–210. [Google Scholar] [CrossRef]
- Parvataneni, S.; Gonipeta, B.; Tempelman, R.J.; Gangur, V. Development of an adjuvant-free cashew nut allergy mouse model. Int. Arch. Allergy Immunol. 2009, 149, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Parvataneni, S.; Gonipeta, B.; Acharya, H.G.; Gangur, V. An Adjuvant-Free Mouse Model of Transdermal Sensitization and Oral Elicitation of Anaphylaxis to Shellfish. Int. Arch. Allergy Immunol. 2015, 168, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gonipeta, B.; Parvataneni, S.; Tempelman, R.J.; Gangur, V. An adjuvant-free mouse model to evaluate the allergenicity of milk whey protein. J. Dairy Sci. 2009, 92, 4738–4744. [Google Scholar] [CrossRef] [PubMed]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse models of food allergy: How well do they simulate the human disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Navuluri, L.; Parvataneni, S.; Hassan, H.; Birmingham, N.P.; Kelly, C.; Gangur, V. Allergic and anaphylactic response to sesame seeds in mice: Identification of Ses i 3 and basic subunit of 11s globulins as allergens. Int. Arch. Allergy Immunol. 2006, 140, 270–276. [Google Scholar] [CrossRef]
- Gonipeta, B.; Parvataneni, S.; Paruchuri, P.; Gangur, V. Long-term characteristics of hazelnut allergy in an adjuvant-free mouse model. Int. Arch. Allergy Immunol. 2010, 152, 219–225. [Google Scholar] [CrossRef]
- Khodoun, M.V.; Strait, R.; Armstrong, L.; Yanase, N.; Finkelman, F.D. Identification of markers that distinguish IgE- from IgG-mediated anaphylaxis. Proc. Natl. Acad. Sci. USA 2011, 108, 12413–12418. [Google Scholar] [CrossRef]
- Ortiz, T.; Para, R.; Gonipeta, B.; Reitmeyer, M.; He, Y.; Srkalovic, I.; Ng, P.K.; Gangur, V. Effect of extrusion processing on immune activation properties of hazelnut protein in a mouse model. Int. J. Food Sci. Nutr. 2016, 67, 660–669. [Google Scholar] [CrossRef]
- Li, X.M.; Serebrisky, D.; Lee, S.Y.; Huang, C.K.; Bardina, L.; Schofield, B.H.; Stanley, J.S.; Burks, A.W.; Bannon, G.A.; Sampson, H.A. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J. Allergy Clin. Immunol. 2000, 106, 150–158. [Google Scholar] [CrossRef]
- Li, X.M.; Kleiner, G.; Huang, C.K.; Lee, S.Y.; Schofield, B.; Soter, N.A.; Sampson, H.A. Murine model of atopic dermatitis associated with food hypersensitivity. J. Allergy Clin. Immunol. 2001, 107, 693–702. [Google Scholar] [CrossRef]
- Kow, A.S.F.; Chik, A.; Soo, K.M.; Khoo, L.W.; Abas, F.; Tham, C.L. Identification of Soluble Mediators in IgG-Mediated Anaphylaxis via Fcgamma Receptor: A Meta-Analysis. Front. Immunol. 2019, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 2017, 278, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Bush, A. Cytokines and Chemokines as Biomarkers of Future Asthma. Front. Pediatr. 2019, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Gangur, V.; Birmingham, N.P.; Thanesvorakul, S.; Joseph, S. CCR3 and CXCR3 as drug targets for allergy: Principles and potential. Curr. Drug Targets Inflamm. Allergy 2003, 2, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, C. IL-25 in allergic inflammation. Immunol. Rev. 2017, 278, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Wagner, S.R.; Wu, Q.; Shilling, R.A. Th17 cells are not required for maintenance of IL-17A-producing gammadelta T cells in vivo. Immunol. Cell Biol. 2017, 95, 280–286. [Google Scholar] [CrossRef]
- Gangur, V.; Oppenheim, J.J. Are chemokines essential or secondary participants in allergic responses? Ann. Allergy Asthma Immunol. 2000, 84, 569–579. [Google Scholar] [CrossRef]
- Sinz, H.; Renz, H.; Skevaki, C. Cellular and noncellular bloodborne biomarkers in asthma. Ann. Allergy Asthma Immunol. 2017, 118, 672–679. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Bukiej, A.; Marcinkiewicz, C.; Musial, J. Integrins in pulmonary inflammatory diseases. Curr. Pharm. Des. 2005, 11, 893–901. [Google Scholar] [CrossRef]
- Teoh, C.M.; Tan, S.S.; Tran, T. Integrins as Therapeutic Targets for Respiratory Diseases. Curr. Mol. Med. 2015, 15, 714–734. [Google Scholar] [CrossRef]
- Dahl, M. Genetic and biochemical markers of obstructive lung disease in the general population. Clin. Respir. J. 2009, 3, 121–122. [Google Scholar] [PubMed]
- Agassandian, M.; Shurin, G.V.; Ma, Y.; Shurin, M.R. C-reactive protein and lung diseases. Int. J. Biochem. Cell Biol. 2014, 53, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Kordowski, A.; Reinicke, A.T.; Wu, D.; Orinska, Z.; Hagemann, P.; Huber-Lang, M.; Lee, J.B.; Wang, Y.H.; Hogan, S.P.; Kohl, J. C5a receptor 1(-/-) mice are protected from the development of IgE-mediated experimental food allergy. Allergy 2019, 74, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.U.; Lim, J.Y.; Kim, N.G.; Shin, J.H.; Ro, J.Y. IgE and IgA produced by OX40-OX40L or CD40-CD40L interaction in B cells-mast cells re-activate FcepsilonRI or FcalphaRI on mast cells in mouse allergic asthma. Eur. J. Pharmacol. 2015, 754, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Verma, A.K.; Das, M.; Dwivedi, P.D. A molecular insight of CTLA-4 in food allergy. Immunol. Lett. 2013, 149, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Frick, O.L. The dog as a model for food allergy. Ann. N. Y. Acad. Sci. 2002, 964, 173–183. [Google Scholar] [CrossRef]
- Bodinier, M.; Leroy, M.; Ah-Leung, S.; Blanc, F.; Tranquet, O.; Denery-Papini, S.; Wal, J.M.; Adel-Patient, K. Sensitization and elicitation of an allergic reaction to wheat gliadins in mice. J. Agric. Food Chem. 2009, 57, 1219–1225. [Google Scholar] [CrossRef]
- Lee, J.H.; Noh, G. Polydesensitisation with reducing elevated serum total IgE by IFN-gamma therapy in atopic dermatitis: IFN-gamma and polydesensitisation (PDS). Cytokine 2013, 64, 395–403. [Google Scholar] [CrossRef]
- Eigenmann, P.A. T lymphocytes in food allergy: Overview of an intricate network of circulating and organ-resident cells. Pediatr. Allergy Immunol. 2002, 13, 162–171. [Google Scholar] [CrossRef]
- Laouini, D.; Alenius, H.; Bryce, P.; Oettgen, H.; Tsitsikov, E.; Geha, R.S. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J. Clin. Invest. 2003, 112, 1058–1066. [Google Scholar] [CrossRef]
- Van Wijk, F.; Hoeks, S.; Nierkens, S.; Koppelman, S.J.; van Kooten, P.; Boon, L.; Knippels, L.M.; Pieters, R. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J. Immunol. 2005, 174, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, N.; Payankaulam, S.; Thanesvorakul, S.; Stefura, B.; HayGlass, K.; Gangur, V. An ELISA-based method for measurement of food-specific IgE antibody in mouse serum: An alternative to the passive cutaneous anaphylaxis assay. J. Immunol. Methods 2003, 275, 89–98. [Google Scholar] [CrossRef]






| Immune Markers * | Control (n = 5) | SSWP ** (n = 4) | Student’s t-Test, p < |
|---|---|---|---|
| Cytokines | |||
| IL-4 (Th2) | 0.4 ± 0.1 | 1.1 ± 0.1 | <0.01 |
| IL-5 (Th2) | 1.3 ± 0.7 | 3.8 ± 0.2 | <0.05 |
| IL-7 | 0 ± 0 | 83.9 ± 27.6 | <0.05 |
| IL-10 (T-Regulatory) | 89.9 ± 13.6 | 141.8 ± 12.2 | <0.05 |
| IL-12p70 | 1.3 ± 1.3 | 11.6 ± 2.1 | <0.01 |
| IL-20 | 0.7 ± 0.7 | 54.0 ± 13.6 | <0.01 |
| IL-23 | 3408.0 ± 1028.6 | 10,505.3 ± 2652.8 | <0.01 |
| IL-17B (Th17) | 105.5 ± 4.5 | 219.5 ± 27.1 | <0.01 |
| IL-17E (IL-25; Th2, Th17) | 54.8 ± 28.4 | 198.2 ± 18.4 | <0.01 |
| IL-17F (Th17) | 0.3 ± 0.3 | 41.9 ± 9.6 | <0.01 |
| Chemokines | |||
| CXCL4 (PF-4) | 757.8 ± 25.9 | 946.8 ± 21.0 | <0.05 |
| CXCL11 (I-TAC) | 13.7 ± 12.8 | 66.6 ± 9.8 | <0.05 |
| CCL4 (MIP1b) | 10.6 ± 0.3 | 20.3 ± 0.9 | <0.001 |
| CCL5 (RANTES) | 234.1 ± 30.4 | 624.1 ± 19.5 | <0.0001 |
| CCL9 (MIP-1g) | 192.1 ± 19.7 | 289.0 ± 7.5 | <0.05 |
| CCL11 (Eotaxin) | 7.2 ± 0.2 | 12.2 ± 0.9 | <0.01 |
| CCL19 (MIP-3b) | 4.2 ± 0.4 | 8.4 ± 0.7 | <0.01 |
| CCL22 (MDC) | 13.5 ± 0.5 | 19.1 ± 1.2 | <0.01 |
| Adhesion Molecules | |||
| E-selectin | 31.2 ± 6.9 | 74.7 ± 2.6 | <0.01 |
| VCAM-1 | 5559.8 ± 88.8 | 7608.9 ± 264.0 | <0.001 |
| MadCAM-1 | 68.7 ± 14.8 | 148.8 ± 25.3 | <0.05 |
| P-Cadherin | 126.8 ± 36.9 | 378.7 ± 4.6 | <0.01 |
| E-Cadherin | 465.6 ± 30.3 | 863.3 ± 47.1 | <0.001 |
| Other Immune Markers | |||
| C5a | 11.2 ± 0.7 | 16.3 ± 1.8 | <0.05 |
| CRP | 61.6 ± 2.9 | 126.4 ± 14.3 | <0.01 |
| CD40 | 2088.4 ± 121.5 | 2800.4 ± 181.6 | <0.05 |
| CD40L | 124.1 ± 14.6 | 337.5 ± 15.3 | <0.0001 |
| CTLA4 | 7.1 ± 1.2 | 18.8 ± 2.7 | <0.001 |
| Immune Markers * | Control (n = 5) | Alum (n = 4) | Student’s t-Test, p < |
|---|---|---|---|
| Cytokines | |||
| IL-1ra | 72.5 ± 5.3 | 196.4 ± 4.1 | <0.00001 |
| IL-4 (Th2) | 0.4 ± 0.1 | 0.8 ± 0.05 | <0.01 |
| IL-7 | 0 | 42.4 ± 9.2 | <0.01 |
| IL-9 (Th9) | 100.2 ± 18.8 | 389.7 ± 47 | <0.01 |
| IL-12p70 | 1.3 ± 1.3 | 15.7 ± 5.5 | <0.05 |
| IL-20 | 0.8 ± 0.8 | 35.6 ± 14 | <0.05 |
| IL-28 | 21.7 ± 4.1 | 40.0 ± 5.5 | <0.05 |
| IL-33 (Th2) | 156.9 ± 12.8 | 230.2 ± 8.2 | <0.05 |
| Chemokines | |||
| CXCL4 (PF4) | 757.8 ± 25.9 | 1030.4 ± 50 | <0.01 |
| CXCL9 (MIG) | 242.9 ± 9.5 | 352.7 ± 9.0 | <0.001 |
| CCL1 (TCA-3) | 4.6 ± 1.5 | 11.0 ± 2.0 | <0.05 |
| CCL9 (MIP-1g) | 192.0 ± 19.7 | 291.4 ± 18.8 | <0.01 |
| CCL11 (Eotaxin) | 7.1± 0.1 | 16.8 ± 0.8 | <0.001 |
| CCL22 (MDC) | 13.5 ± 0.5 | 24.0 ± 1.6 | <0.001 |
| CCL19 (MIP-3b) | 4.2 ± 0.4 | 6.5 ± 0.5 | <0.01 |
| Adhesion Molecules | |||
| P-Cadherin | 233.9 ± 36.9 | 428.0 ± 24.7 | <0.01 |
| VCAM-1 | 5559.8 ± 88.8 | 7046.7 ± 196.8 | <0.001 |
| Other Immune Markers | |||
| C5a | 11.1 ± 0.7 | 19.6 ± 0.4 | <0.0001 |
| CRP | 61.5 ± 2.9 | 119.9 ± 11 | <0.01 |
| MBL-2 | 1485.8 ± 217.6 | 2117.3 ± 71.1 | <0.05 |
| Immune Markers * | Alum (n = 5) | Alum + SSWP ** (n = 4) | Student’s t-Test, p < |
|---|---|---|---|
| Cytokines | |||
| IFN-g (Th1) | 0.7 ± 0.5 | 42.1 ± 4.6 | <0.001 |
| IL-2 | 0.1 ± 0.1 | 27.7 ± 4.0 | <0.0001 |
| IL-5 (Th2) | 0.9 ± 0.6 | 9.5 ± 0.6 | <0.0001 |
| IL-13 (Th2) | 0.4 ± 0.4 | 25.9 ± 4.6 | <0.01 |
| IL-21 | 0.1 ± 0.1 | 15.8 ± 4.8 | <0.05 |
| Chemokines | |||
| CXCL12 (SDF-1a) | 1.7 ± 0.03 | 8.8 ± 0.6 | <0.0001 |
| CXCL13 (BLC) | 159.9 ± 20.4 | 304.2 ± 13.5 | <0.01 |
| CCL3 (MIP1a) | 3.6 ± 1.5 | 41.1 ± 4.4 | <0.001 |
| CCL12 (MCP-5) | 0.9 ± 0.4 | 4.7 ± 1.1 | <0.05 |
| CCL21 (6Ckine) | 11.1 ± 1.7 | 71.6 ± 16.3 | <0.05 |
| XCL1 (Lymphotactin) | 69.4 ± 16.7 | 433.5 ± 94 | <0.01 |
| Adhesion Molecules | |||
| P-selectin | 14,861.9 ± 344.5 | 23,402.1 ± 380.6 | <0.01 |
| MadCAM-1 | 12.2 ± 3.5 | 286.2 ± 14.6 | <0.00001 |
| Other Immune Markers | |||
| CD40 | 1829.92 ± 88.1 | 2274.11 ± 138.1 | <0.05 |
| CD40L | 57.9 ± 5.7 | 124.6 ± 13.6 | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Gao, H.; Jorgensen, R.; Salloum, J.; Jian, D.I.; Ng, P.K.W.; Gangur, V. Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. Int. J. Mol. Sci. 2020, 21, 3205. https://doi.org/10.3390/ijms21093205
Jin Y, Gao H, Jorgensen R, Salloum J, Jian DI, Ng PKW, Gangur V. Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. International Journal of Molecular Sciences. 2020; 21(9):3205. https://doi.org/10.3390/ijms21093205
Chicago/Turabian StyleJin, Yining, Haoran Gao, Rick Jorgensen, Jillian Salloum, Dan Ioan Jian, Perry K.W. Ng, and Venugopal Gangur. 2020. "Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models" International Journal of Molecular Sciences 21, no. 9: 3205. https://doi.org/10.3390/ijms21093205
APA StyleJin, Y., Gao, H., Jorgensen, R., Salloum, J., Jian, D. I., Ng, P. K. W., & Gangur, V. (2020). Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. International Journal of Molecular Sciences, 21(9), 3205. https://doi.org/10.3390/ijms21093205




