Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine
Abstract
1. Introduction
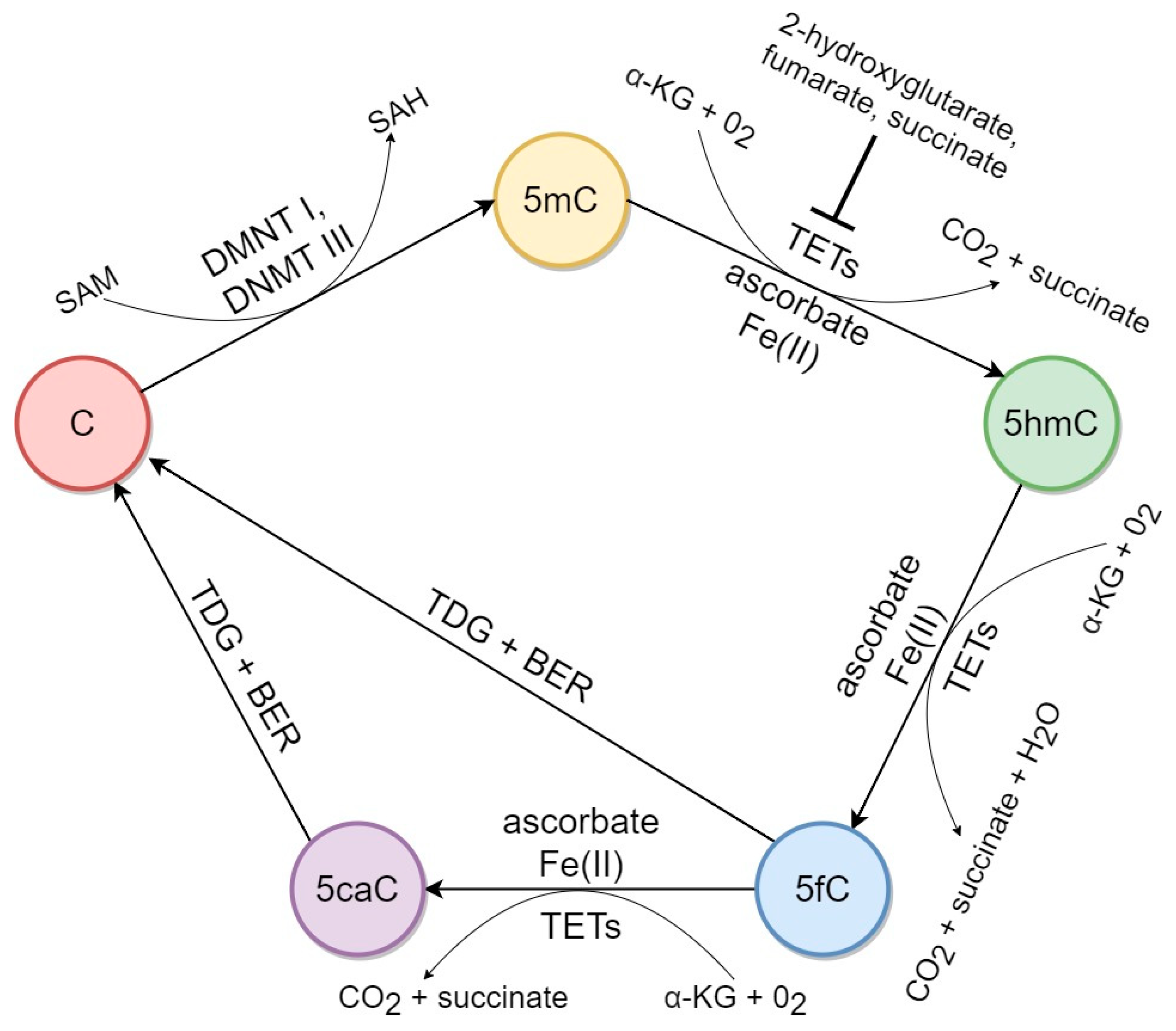
2. Factors Associated with 5hmC Biochemical Pathways in Mammalian DNA
3. Impact of External Factors on Genomic Hydroxymethylation
3.1. Hypnotics and Medications
3.1.1. Phenobarbital
3.1.2. Diethylstilbestrol
3.1.3. Cocaine
3.1.4. Methamphetamine
3.1.5. Ethanol
3.1.6. Dimethyl Sulfoxide
3.2. Anthropogenic Pollutants
3.2.1. Heavy Metals
3.2.2. Particulate Air Pollution
3.2.3. Bisphenol A
3.2.4. Hydroquinone
3.2.5. Pentachlorophenol metabolites
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5caC | 5-carboxylcytosine |
| 5fC | 5-formylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| 5mC | 5-methylcytosine |
| A549 | human Caucasian lung carcinoma cell line |
| ACE | angiotensin converting enzyme |
| ACE2 | angiotensin converting enzyme 2 |
| AMPAR | alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor |
| Avp | arginine vasopressin |
| BPA | bisphenol A |
| CAR | constitutive androstane receptor |
| COVID-19 | coronavirus disease 2019 |
| Crh/Crf | corticotropin-releasing hormone |
| DMSO | dimethyl sulfoxide |
| DNMTs | DNA methyltransferases |
| HEK293T | human embryonic kidney cell line |
| hESCs | human embryonic stem cells |
| HepG2 | human Caucasian hepatocyte carcinoma cell line |
| HIF | hypoxia-inducible factor |
| IDHs | isocitrate dehydrogenases human |
| MC3T3-E1 | mouse osteoblastic cell line |
| MCF-7 | human breast cancer cell line |
| mESCs | mouse embryonic stem cells |
| MRC5 | human embryonic lung fibroblasts cell culture (medical research council cell strain 5) |
| PCP | pentachlorophenol |
| SAM | S-adenosylmethionine |
| SARS-CoV | severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SH-SY5Y | human neuroblastoma cell line |
| TETs | Ten-Eleven Translocation enzymes |
References
- Penn, N.W.; Suwalski, R.; O’riley, C.; Bojanowski, K.; Yura, R. The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid. Biochem. J. 1972, 126, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Dean, W. Epigenetic reprogramming during early development in mammals. Reproduction 2004, 127, 643–651. [Google Scholar] [CrossRef]
- Pendina, A.A.; Efimova, O.A.; Fedorova, I.D.; Leont’eva, O.A.; Shilnikova, E.M.; Lezhnina, J.G.; Kuznetzova, T.V.; Baranov, V.S. DNA methylation patterns of metaphase chromosomes in human preimplantation embryos. Cytogenet. Genome Res. 2011, 132, 1–7. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Vasilyev, S.A.; Tolmacheva, E.N.; Lebedev, I.N. Epigenetic regulation and role of LINE-1 retrotransposon in embryogenesis. Russ. J. Genet. 2016, 52, 1219–1226. [Google Scholar] [CrossRef]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef]
- Iurlaro, M.; Ficz, G.; Oxley, D.; Raiber, E.A.; Bachman, M.; Booth, M.J.; Andrews, S.; Balasubramanian, S.; Reik, W. A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biol. 2013, 14, R119. [Google Scholar] [CrossRef]
- Spruijt, C.G.; Gnerlich, F.; Smits, A.H.; Pfaffeneder, T.; Jansen, P.W.; Bauer, C.; Eberl, H.C. Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives. Cell 2013, 152, 1146–1159. [Google Scholar] [CrossRef]
- Zhou, T.; Xiong, J.; Wang, M.; Yang, N.; Wong, J.; Zhu, B.; Xu, R.M. Structural basis for hydroxymethylcytosine recognition by the SRA domain of UHRF2. Mol. Cell 2014, 54, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Feng, S.; Kinney, S.M.; Pradhan, S.; Jacobsen, S.E. 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011, 12, R54. [Google Scholar] [CrossRef]
- Hon, G.C.; Song, C.X.; Du, T.; Jin, F.; Selvaraj, S.; Lee, A.Y.; Kuan, S. 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol. Cell 2014, 56, 286–297. [Google Scholar] [CrossRef]
- Lu, F.; Liu, Y.; Jiang, L.; Yamaguchi, S.; Zhang, Y. Role of Tet proteins in enhancer activity and telomere elongation. Genes Dev. 2014, 28, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Christensen, J.; Helin, K. DNA methylation: TET proteins—Guardians of CpG islands? EMBO Rep. 2012, 13, 28–35. [Google Scholar] [CrossRef]
- Song, J.; Pfeifer, G.P. Are there specific readers of oxidized 5-methylcytosine bases? Bioessays 2016, 38, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.R.; Ficz, G.; Reik, W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat. Rev. Genet. 2011, 13, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Kuznetzova, T.V.; Baranov, V.S. Oxidized form of 5-methylcytosine—5-hydroxymethylcytosine: A new insight into the biological significance in the mammalian genome. Russ. J. Genet. Appl. Res. 2015, 5, 75–81. [Google Scholar] [CrossRef]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Baranov, V.S. The evolution of ideas on the biological role of 5-methylcytosine oxidative derivatives in the mammalian genome. Russ. J. Genet. Appl. Res. 2018, 8, 11–21. [Google Scholar] [CrossRef]
- Kantidze, O.L.; Razin, S.V. 5-hydroxymethylcytosine in DNA repair: A new player or a red herring? Cell Cycle 2017, 16, 1499–1501. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Cantone, I.; Fisher, A.G. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Biol. 2013, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.A.; Pendina, A.A.; Krapivin, M.I.; Kopat, V.V.; Tikhonov, A.V.; Petrovskaia-Kaminskaia, A.V.; Navodnikova, P.M.; Talantova, O.E.; Glotov, O.S.; Baranov, V.S. Inter-Cell and Inter-Chromosome Variability of 5-Hydroxymethylcytosine Patterns in Noncultured Human Embryonic and Extraembryonic Cells. Cytogenet. Genome Res. 2018, 156, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.A.; Pendina, A.A.; Lezhnina, Y.G.; Tikhonov, A.V.; Chiryaeva, O.G.; Petrova, L.I.; Talantova, O.E.; Dudkina, V.S.; Koltsova, A.S.; Krapivin, M.I.; et al. Study of acetylated histone H3K9—An active chromatin mark—In chromosomes from adult and fetal human lymphocytes. Ecol. Genet. 2019, 17, 111–117. [Google Scholar] [CrossRef]
- Patkin, E.L.; Sofronov, G.A. Population epigenetics, ecotoxicology, and human diseases. Russ. J. Genet. Appl. Res. 2013, 3, 338–351. [Google Scholar] [CrossRef]
- Salemi, R.; Marconi, A.; Di Salvatore, V.; Franco, S.; Rapisarda, V.; Libra, M. Epigenetic alterations and occupational exposure to benzene, fibers, and heavy metals associated with tumor development. Mol. Med. Rep. 2017, 15, 3366–3371. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, N.A.; Vasilyev, S.A.; Lebedev, I.N. Epigenetic silencing of genomic structural variations. Russ. J. Genet. 2017, 53, 1072–1079. [Google Scholar] [CrossRef]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu. Rev. Public Health 2018, 39, 309–333. [Google Scholar] [CrossRef]
- Koltsova, A.S.; Pendina, A.A.; Efimova, O.A.; Chiryaeva, O.G.; Kuznetzova, T.V.; Baranov, V.S. On the Complexity of Mechanisms and Consequences of Chromothripsis: An Update. Front. Genet. 2019, 10, 393. [Google Scholar] [CrossRef]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Brocato, J.; Costa, M. Basic mechanics of DNA methylation and the unique landscape of the DNA methylome in metal-induced carcinogenesis. Crit. Rev. Toxicol. 2013, 43, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Washburn, K.A.; Moore, R.; Uno, T.; Teng, C.; Newbold, R.R.; McLachlan, J.A.; Negishi, M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997, 57, 4356–4359. [Google Scholar] [PubMed]
- Alworth, L.C.; Howdeshell, K.L.; Ruhlen, R.L.; Day, J.K.; Lubahn, D.B.; Huang, T.H.; Besch-Williford, C.L.; Vom Saal, F.S. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: Effects of low versus high doses. Toxicol. Appl. Pharmacol. 2002, 183, 10–22. [Google Scholar] [CrossRef]
- Sato, K.; Fukata, H.; Kogo, Y.; Ohgane, J.; Shiota, K.; Mori, C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr. J. 2008, 56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.M.; Blossom, S.J.; Erickson, S.W.; Reisfeld, B.; Zurlinden, T.J.; Broadfoot, B.; West, K.; Bai, S.; Cooney, C.A. Chronic exposure to water pollutant trichloroethylene increased epigenetic drift in CD4(+) T cells. Epigenomics 2016, 8, 633–649. [Google Scholar] [CrossRef]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef]
- Faulk, C.; Kim, J.H.; Anderson, O.S.; Nahar, M.S.; Jones, T.R.; Sartor, M.A.; Dolinoy, D.C. Detection of differential DNA methylation in repetitive DNA of mice and humans perinatally exposed to bisphenol A. Epigenetics 2016, 11, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Patkin, E.L.; Grudinina, N.A.; Sasina, L.K.; Noniashvili, E.M.; Pavlinova, L.I.; Suchkova, I.O.; Kustova, M.E.; Kolmakov, N.N.; Van Truong, T.; Sofronov, G.A. Asymmetric DNA methylation between sister chromatids of metaphase chromosomes in mouse embryos upon bisphenol A action. Reprod. Toxicol. 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Noniashvili, E.M.; Grudinina, N.A.; Kustova, M.E.; Suchkova, I.O.; Pavlinova, L.I.; Sasina, L.K.; Patkin, E.L. DNA methylation in early mice embryogenesis under the influence of bisphenol A. Ecol. Genet. 2017, 15, 42–53. [Google Scholar] [CrossRef]
- Suchkova, I.O.; Sasina, L.K.; Dergacheva, N.I.; Sofronov, G.A.; Patkin, E.L. The influence of low dose Bisphenol A on whole genome DNA methylation and chromatin compaction in different human cell lines. Toxicol. Vitr. 2019, 58, 26–34. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C. Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis. Semin. Cancer Biol. 2019, 57, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A.; Naumov, V.; Shtratnikova, V.; Logacheva, M.; Shershebnev, A.; Wu, H.; Gerasimov, E.; Zheludkevich, A.; Pilsner, J.R.; Sergeyev, O. Rat liver epigenome programing by perinatal exposure to 2,2’,4’4’-tetrabromodiphenyl ether. Epigenomics 2020, 12, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Cheng, R.Y.; Revelo, M.P.; Mitzner, W.; Tang, W. Hydroxymethylation as a Novel Environmental Biosensor. Curr. Environ. Health Rep. 2014, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kochmanski, J.; Bernstein, A.I. The Impact of Environmental Factors on 5-Hydroxymethylcytosine in the Brain. Curr. Environ. Health Rep. 2020, 1–12. [Google Scholar] [CrossRef]
- Pulczinski, J.; Yeung, B.H.; Wu, Q.; Cheng, R.Y.; Tang, W.Y. DNA Hydroxymethylation: Implications for Toxicology and Epigenetic Epidemiology. In Toxicoepigenetics: Core Principles and Applications, 1st ed.; McCullough, S.D., Dolinoy, D.C., Eds.; Elsevier: Amsterdam, Netherlands, 2018; pp. 191–214. ISBN 978-0-12-812433-8. [Google Scholar]
- Castro, G.D.; Díaz Gómez, M.I.; Castro, J.A. 5-Methylcytosine attack by hydroxyl free radicals and during carbon tetrachloride promoted liver microsomal lipid peroxidation: Structure of reaction products. Chem. Biol. Interact. 1996, 99, 289–299. [Google Scholar] [CrossRef]
- Madugundu, G.S.; Cadet, J.; Wagner, J.R. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Res. 2014, 42, 7450–7460. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. Radiation-induced damage to cellular DNA: Chemical nature and mechanisms of lesion formation. Radiat. Phys. Chem. 2016, 128, 54–59. [Google Scholar] [CrossRef]
- Cheng, X.; Kumar, S.; Posfai, J.; Pflugrath, J.W.; Roberts, R.J. Crystal structure of the Hhal DNA methyltransferase complexed with S-adenosyl-L-methionine. Cell 1993, 74, 299–307. [Google Scholar] [CrossRef]
- Okano, M.; Xie, S.; Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998, 19, 219–220. [Google Scholar] [CrossRef]
- Krebs, H.A.; Johnson, W.A. The role of citric acid in intermediate metabolism in animal tissues. Enzymologia 1937, 4, 148–156. [Google Scholar]
- Chou, N.H.; Tsai, C.Y.; Tu, Y.T.; Wang, K.C.; Kang, C.H.; Chang, P.M.; Li, G.C.; Lam, H.C.; Liu, S.I.; Tsai, K.W. Isocitrate Dehydrogenase 2 Dysfunction Contributes to 5-hydroxymethylcytosine Depletion in Gastric Cancer Cells. Anticancer Res. 2016, 36, 3983–3990. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Zhao, S. Inhibition of alpha--KG--dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- Mason, E.F.; Hornick, J.L. Succinate dehydrogenase deficiency is associated with decreased 5-hydroxymethylcytosine production in gastrointestinal stromal tumors: Implications for mechanisms of tumorigenesis. Mod. Pathol. 2013, 26, 1492–1497. [Google Scholar] [CrossRef]
- Yang, H.; Lin, H.; Xu, H.; Zhang, L.; Cheng, L.; Wen, B.; Shou, J.; Guan, K.; Xiong, Y.; Ye, D. TET-catalyzed 5-methylcytosine hydroxylation is dynamically regulated by metabolites. Cell Res. 2014, 24, 1017–1020. [Google Scholar] [CrossRef]
- Burr, S.; Caldwell, A.; Chong, M.; Beretta, M.; Metcalf, S.; Hancock, M.; Arno, M.; Balu, S.; Kropf, V.L.; Mistry, R.K.; et al. Oxygen gradients can determine epigenetic asymmetry and cellular differentiation via differential regulation of Tet activity in embryonic stem cells. Nucleic Acids Res. 2018, 46, 1210–1226. [Google Scholar] [CrossRef]
- Thienpont, B.; Steinbacher, J.; Zhao, H.; D’Anna, F.; Kuchnio, A.; Ploumakis, A.; Hermans, E. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 2016, 537, 63–68. [Google Scholar] [CrossRef]
- Koutsouraki, E.; Pells, S.; De Sousa, P.A. Sufficiency of hypoxia-inducible 2-oxoglutarate dioxygenases to block chemical oxidative stress-induced differentiation of human embryonic stem cells. Stem Cell Res. 2019, 34, 101358. [Google Scholar] [CrossRef]
- Mariani, C.J.; Vasanthakumar, A.; Madzo, J.; Yesilkanal, A.; Bhagat, T.; Yu, Y.; Verma, A. TET1--mediated hydroxymethylation facilitates hypoxic gene induction in neuroblastoma. Cell Rep. 2014, 7, 1343–1352. [Google Scholar] [CrossRef]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [PubMed]
- Dickson, K.M.; Gustafson, C.B.; Young, J.I.; Züchner, S.; Wang, G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem. Biophys. Res. Commun. 2013, 439, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic Acid Enhances Tet-Mediated 5-Methylcytosine Oxidation and Promotes DNA Demethylation in Mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M. Distribution of 5-hydroxymethylcytosine in different human tissues. J. Nucleic. Acids 2011, 2011, 870726. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenetics 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, Y.; Wang, X.; Chong, Z.; Yin, R.; Song, S.H.; Zhao, C.; Li, C.; Huang, H.; Sun, B.F.; et al. Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic Acids Res. 2014, 42, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.M.; Diwan, B.A.; Hu, H.; Ward, J.M.; Nims, R.W.; Lubet, R.A. Enhancement of hepatocarcinogenesis and induction of specific cytochrome P450-dependent monooxygenase activities by the barbiturates allobarbital, aprobarbital, pentobarbital, secobarbital and 5-phenyl-and 5-ethylbarbituric acids. Carcinogenesis 1994, 15, 395–402. [Google Scholar] [CrossRef]
- Thomson, J.P.; Lempiainen, H.; Tefferi, A.; Nestor, C.E.; Muller, A.; Bolognani, F.; Oakeley, E.J.; Schubeler, D.; Terranova, R.; Reinhardt, D.; et al. Non-genotoxic carcinogen exposure induces defined changes in the 5-hydroxymethylome. Genome Biol. 2012, 13, R93. [Google Scholar] [CrossRef]
- Thomson, J.P.; Hunter, J.M.; Lempiainen, H.; Muller, A.; Terranova, R.; Moggs, J.G.; Meehan, R.R. Dynamic changes in 5-hydroxymethylation signatures underpin early and late events in drug exposed liver. Nucleic Acids Res. 2013, 41, 5639–5654. [Google Scholar] [CrossRef]
- Ohara, A.; Takahashi, Y.; Kondo, M.; Okuda, Y.; Takeda, S.; Kushida, M.; Kobayashi, K.; Sumida, K.; Yamada, T. Candidate genes responsible for early key events of phenobarbital-promoted mouse hepatocellular tumorigenesis based on differentiation of regulating genes between wild type mice and humanized chimeric mice. Toxicol. Res. 2017, 6, 795–813. [Google Scholar] [CrossRef]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Chevalier, D.M.; Phelps, J.Y.; Cantor, A.M.; Padilla-Banks, E.; Newbold, R.R.; Archer, T.K.; Kinyamu, H.K.; Williams, C.J. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol. Endocrinol. 2013, 27, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Mahalingaiah, S.; Hart, J.E.; Wise, L.A.; Terry, K.L.; Boynton-Jarrett, R.; Missmer, S.A. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses’ Health Study II. Am. J. Epidemiol. 2014, 179, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Yin, P.; Ono, M.; Monsivais, D.; Moravek, M.B.; Coon, J.S., 5th; Dyson, M.T.; Wei, J.J.; Bulun, S.E. 5-Hydroxymethylcytosine promotes proliferation of human uterine leiomyoma: A biological link to a new epigenetic modification in benign tumors. J. Clin. Endocrinol. Metab. 2014, 99, E2437-45. [Google Scholar] [CrossRef]
- Kol’tsova, A.S.; Pendina, A.A.; Efimova, O.A.; Kaminskaya, A.N.; Tikhonov, A.V.; Osinovskaya, N.S.; Sultanov, I.Y.; Shved, N.Y.; Kakhiani, M.I.; Baranov, V.S. Differential DNA Hydroxymethylation in Human Uterine Leiomyoma Cells Depending on the Phase of Menstrual Cycle and Presence of MED12 Gene Mutations. Bull. Exp. Biol. Med. 2017, 163, 646–649. [Google Scholar] [CrossRef]
- Chao, M.R.; Fragou, D.; Zanos, P.; Hu, C.W.; Bailey, A.; Kouidou, S.; Kovatsi, L. Epigenetically modified nucleotides in chronic heroin and cocaine treated mice. Toxicol. Lett. 2014, 229, 451–457. [Google Scholar] [CrossRef][Green Version]
- Feng, J.; Shao, N.; Szulwach, K.E.; Vialou, V.; Huynh, J.; Zhong, C.; Le, T.; Ferguson, D.; Cahill, M.E.; Li, Y.; et al. Role of Tet1 and 5 hydroxymethylcytosine in cocaine action. Nat. Neurosci. 2015, 18, 536–544. [Google Scholar] [CrossRef]
- Ploense, K.L.; Li, X.; Baker-Andresen, D.; Carr, A.E.; Woodward, N.; Bagley, J.; Szumlinski, K.K.; Bredy, T.W.; Kippin, T.E. Prolonged-access to cocaine induces distinct Homer2 DNA methylation, hydroxymethylation, and transcriptional profiles in the dorsomedial prefrontal cortex of Male Sprague-Dawley rats. Neuropharmacology 2018, 143, 299–305. [Google Scholar] [CrossRef]
- Saad, L.; Sartori, M.; Pol Bodetto, S.; Romieu, P.; Kalsbeek, A.; Zwiller, J.; Anglard, P. Regulation of Brain DNA Methylation Factors and of the Orexinergic System by Cocaine and Food Self-Administration. Mol. Neurobiol. 2019, 56, 5315–5331. [Google Scholar] [CrossRef]
- González, B.; Pantoja, C.R.G.; Sosa, M.H.; Vitullo, A.D.; Bisagno, V.; González, C.R. Cocaine alters the mouse testicular epigenome with direct impact on histone acetylation and DNA methylation marks. Reprod. Biomed. Online 2018, 37, 269–278. [Google Scholar] [CrossRef]
- Jayanthi, S.; Gonzalez, B.; McCoy, M.T.; Ladenheim, B.; Bisagno, V.; Cadet, J.L. Methamphetamine Induces TET1- and TET3-Dependent DNA Hydroxymethylation of Crh and Avp Genes in the Rat Nucleus Accumbens. Mol. Neurobiol. 2018, 55, 5154–5166. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; McCoy, M.T.; Chen, B.; Britt, J.P.; Kourrich, S.; Yau, H.J.; Ladenheim, B.; Krasnova, I.N.; Bonci, A.; Cadet, J.L. Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol. Psychiatry 2014, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Brannock, C.; Krasnova, I.N.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Walther, D.; Godino, A.; Pirooznia, M.; Lee, R.S. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol. Psychiatry 2017, 22, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ozturk, N.C.; Zhou, F.C. DNA methylation program in developing hippocampus and its alteration by alcohol. PLoS ONE 2013, 8, e60503. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.C.; Resendiz, M.; Öztürk, H.; Zhou, F.C. DNA Methylation program in normal and alcohol-induced thinning cortex. Alcohol 2017, 60, 135–147. [Google Scholar] [CrossRef]
- Liyanage, V.R.; Zachariah, R.M.; Davie, J.R.; Rastegar, M. Ethanol deregulates Mecp2/MeCP2 in differentiating neural stem cells via interplay between 5-methylcytosine and 5-hydroxymethylcytosine at the Mecp2 regulatory elements. Exp. Neurol. 2015, 265, 102–117. [Google Scholar] [CrossRef]
- Koller, G.; Zill, P.; Soyka, M.; Adorjan, K.; Weiss, C.; Kern, A.; Nguyen-Thien, M.L.; Kamp, F.; Proebstl, L.; Krause, D.; et al. Short-term changes in global methylation and hydroxymethylation during alcohol detoxification. Eur. Neuropsychopharmacol. 2019, 29, 897–903. [Google Scholar] [CrossRef]
- Tammen, S.A.; Park, J.E.; Shin, P.K.; Friso, S.; Chung, J.; Choi, S.W. Iron Supplementation Reverses the Reduction of Hydroxymethylcytosine in Hepatic DNA Associated with Chronic Alcohol Consumption in Rats. J. Cancer. Prev. 2016, 21, 264–270. [Google Scholar] [CrossRef]
- Ji, C.; Nagaoka, K.; Zou, J.; Casulli, S.; Lu, S.; Cao, K.Y.; Zhang, H.; Iwagami, Y.; Carlson, R.I.; Brooks, K.; et al. Chronic ethanol mediated hepatocyte apoptosis links to decreased TET1 and 5-hydroxymethylcytosine formation. FASEB J. 2019, 33, 1824–1835. [Google Scholar] [CrossRef]
- Thaler, R.; Spitzer, S.; Karlic, H.; Klaushofer, K.; Varga, F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics 2012, 7, 635–651. [Google Scholar] [CrossRef]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Song, K.H.; Chung, J.Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA methylation in cancer: Too much, but also too little. Oncogene 2002, 21, 5400–5413. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, X.; Xu, W.; Martin, F.L.; Alamdar, A.; Liu, L.; Tian, M.; Huang, Q.; Shen, H. Exposure to arsenic via drinking water induces 5-hydroxymethylcytosine alteration in rat. Sci. Total Environ. 2014, 497–498, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Tian, M.; Wang, X.; Zhang, J.; Huang, Q.; Liu, L.; Shen, H. Cortex and hippocampus DNA epigenetic response to a long-term arsenic exposure via drinking water. Environ. Pollut. 2018, 234, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Liu, X.; Cheng, Q.Y.; Xiao, S.; Xia, L.X.; Yuan, B.F.; Feng, Y.Q. Heavy Metals Induce Decline of Derivatives of 5-Methycytosine in Both DNA and RNA of Stem Cells. ACS Chem. Biol. 2017, 12, 1636–1643. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Li, L.; Amato, N.J.; Wang, Z.; Wang, Y. Arsenite Targets the Zinc Finger Domains of Tet Proteins and Inhibits Tet-Mediated Oxidation of 5-Methylcytosine. Environ. Sci. Technol. 2015, 49, 11923–11931. [Google Scholar] [CrossRef]
- Müller, S.M.; Finke, H.; Ebert, F.; Kopp, J.F.; Schumacher, F.; Kleuser, B.; Francesconi, K.A.; Raber, G.; Schwerdtle, T. Arsenic-containing hydrocarbons: Effects on gene expression, epigenetics, and biotransformation in HepG2 cells. Arch. Toxicol. 2018, 92, 1751–1765. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Tang, W.Y.; Shang, Y.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Ledesma, M.; Leon, M.; Laclaustra, M.; Pollak, J.; et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ. Health Perspect. 2014, 122, 946–954. [Google Scholar] [CrossRef]
- Niedzwiecki, M.M.; Liu, X.; Hall, M.N.; Thomas, T.; Slavkovich, V.; Ilievski, V.; Levy, D.; Alam, S.; Siddique, A.B.; Parvez, F.; et al. Sex-specific associations of arsenic exposure with global DNA methylation and hydroxymethylation in leukocytes: Results from two studies in Bangladesh. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1748–1757. [Google Scholar] [CrossRef]
- Xu, P.; Chen, Z.; Chen, Y.; Feng, L.; Wu, L.; Xu, D.; Wang, X.; Lou, X.; Lou, J. Body burdens of heavy metals associated with epigenetic damage in children living in the vicinity of a municipal waste incinerator. Chemosphere 2019, 229, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Rifas-Shiman, S.L.; Godderis, L.; Duca, R.C.; Navas-Acien, A.; Litonjua, A.A.; DeMeo, D.L.; Brennan, K.J.; Amarasiriwardena, C.J.; Hivert, M.F.; et al. Prenatal Exposure to Mercury: Associations with Global DNA Methylation and Hydroxymethylation in Cord Blood and in Childhood. Environ. Health Perspect. 2017, 125, 087022. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Mo, J.; Dai, J.; Wang, H. Nickel(ii) inhibits the oxidation of DNA 5-methylcytosine in mammalian somatic cells and embryonic stem cells. Metallomics 2018, 10, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Cingolani, P.; Senut, M.C.; Land, S.; Mercado-Garcia, A.; Tellez-Rojo, M.M.; Baccarelli, A.A.; Wright, R.O.; Ruden, D.M. Lead exposure induces changes in 5-hydroxymethylcytosine clusters in CpG islands in human embryonic stem cells and umbilical cord blood. Epigenetics 2015, 10, 607–621. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Leray, E.; Heydarpour, P.; Torres-Jardón, R.; Reis, J. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Rev. Neurol. 2016, 172, 69–80. [Google Scholar] [CrossRef]
- Wei, H.; Feng, Y.; Liang, F.; Cheng, W.; Wu, X.; Zhou, R.; Wang, Y. Role of oxidative stress and DNA hydroxymethylation in the neurotoxicity of fine particulate matter. Toxicology 2017, 380, 94–103. [Google Scholar] [CrossRef]
- De Oliveira, A.A.F.; De Oliveira, T.F.; Dias, M.F.; Medeiros, M.H.G.; Di Mascio, P.; Veras, M.; Lemos, M.; Marcourakis, T.; Saldiva, P.H.N.; Loureiro, A.P.M. Genotoxic and epigenotoxic effects in mice exposed to concentrated ambient fine particulate matter (PM(2.5)) from São Paulo city, Brazil. Part. Fibre. Toxicol. 2018, 15, 40. [Google Scholar] [CrossRef]
- Wang, C.; Chen, R.; Cai, J.; Shi, J.; Yang, C.; Tse, L.A.; Li, H.; Lin, Z.; Meng, X.; Liu, C.; et al. Personal exposure to fine particulate matter and blood pressure: A role of angiotensin converting enzyme and its DNA methylation. Environ Int. 2016, 94, 661–666. [Google Scholar] [CrossRef]
- Lin, C.I.; Tsai, C.H.; Sun, Y.L.; Hsieh, W.Y.; Lin, Y.C.; Chen, C.Y.; Lin, C.S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018, 14, 253–265. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Corley, M.J.; Ndhlovu, L.C. DNA Methylation Analysis of the COVID-19 Host Cell Receptor, Angiotensin I Converting Enzyme 2 Gene (ACE2) in the Respiratory System Reveal Age and Gender Differences. Preprints 2020. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Zhao, M.; Coit, P.; Lu, Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020, 215, 108410. [Google Scholar] [CrossRef]
- Faraji, M.; Pourpak, Z.; Naddafi, K.; Nodehi, R.N.; Nicknam, M.H.; Shamsipour, M.; Rezaei, S.; Ghozikali, M.G.; Ghanbarian, M.; Mesdaghinia, A. Effects of airborne particulate matter (PM10) from dust storm and thermal inversion on global DNA methylation in human peripheral blood mononuclear cells (PBMCs) in vitro. Atmos. Environ. 2018, 195, 170–178. [Google Scholar] [CrossRef]
- Sanchez-Guerra, M.; Zheng, Y.; Osorio-Yanez, C.; Zhong, J.; Chervona, Y.; Wang, S.; Chang, D.; McCracken, J.P.; Díaz, A.; Bertazzi, P.A.; et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: Results from the Beijing truck driver air pollution study. Epigenetics 2015, 10, 633–642. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Nawrot, T.; Hoet, P.; Van Meerbeek, B.; Van Landuyt, K.L.; Godderis, L. Temporal variability of global DNA methylation and hydroxymethylation in buccal cells of healthy adults: Association with air pollution. Environ. Int. 2018, 111, 301–308. [Google Scholar] [CrossRef]
- Laws, S.C.; Carey, S.A.; Ferrell, J.M.; Bodman, G.J.; Cooper, R.L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000, 54, 154–167. [Google Scholar] [CrossRef]
- Gioiosa, L.; Fissore, E.; Ghirardelli, G.; Parmigiani, S.; Palanza, P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm. Behav. 2007, 52, 307–316. [Google Scholar] [CrossRef]
- Koike, E.; Yanagisawa, R.; Win-Shwe, T.T.; Takano, H. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 2018, 32, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, L.J.; Feng, Y.N.; Chen, B.; Feng, Y.M.; Liang, G.J.; Shen, W. Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol. Biol. Rep. 2012, 39, 8621–8628. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Navarro, C.; Robles, V.; Herráez, M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure. Environ. Pollut. 2015, 206, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.I.; Makino, T.; Suzumori, K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005, 20, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, X.; Cheng, B.; Sun, Y.; Li, C.; Li, T.; Huang, K. Bisphenol A accelerates toxic amyloid formation of human islet amyloid polypeptide: A possible link between bisphenol A exposure and type 2 diabetes. PLoS ONE 2013, 8, e54198. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, X.; Li, D.K.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome wide alteration in DNA hydroxymethylation in the sperm from bisphenol A exposed men. PLoS ONE. 2017, 12, e178535. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Miao, M.; Li, D.K.; Wang, Z.; Li, R.; Liang, H.; Yuan, W. Association of Bisphenol A Exposure with LINE-1 Hydroxymethylation in Human Semen. Int. J. Environ. Res. Public Health 2018, 15, 1770. [Google Scholar] [CrossRef]
- Song, X.; Miao, M.; Zhou, X.; Li, D.; Tian, Y.; Liang, H.; Li, R.; Yuan, W. Bisphenol A Exposure and Sperm ACHE Hydroxymethylation in Men. Int. J. Environ. Res. Public Health 2019, 16, 152. [Google Scholar] [CrossRef]
- Kochmanski, J.J.; Marchlewicz, E.H.; Cavalcante, R.G.; Perera, B.P.U.; Sartor, M.A.; Dolinoy, D.C. Longitudinal Effects of Developmental Bisphenol A Exposure on Epigenome Wide DNA Hydroxymethylation at Imprinted Loci in Mouse Blood. Environ. Health Perspect. 2018, 126, 077006. [Google Scholar] [CrossRef]
- Malloy, M.A.; Kochmanski, J.J.; Jones, T.R.; Colacino, J.A.; Goodrich, J.M.; Dolinoy, D.C.; Svoboda, L.K. Perinatal Bisphenol A Exposure and Reprogramming of Imprinted Gene Expression in the Adult Mouse Brain. Front. Genet. 2019, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lyu, C.; Ren, Y.; Wang, H. Role of TET Dioxygenases and DNA Hydroxymethylation in Bisphenols-Stimulated Proliferation of Breast Cancer Cells. Environ. Health Perspect. 2020, 128, 27008. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.B.; O’Driscoll, C.M.; Bressler, J.P. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. J. Biol. Chem. 2013, 288, 28792–28800. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, I.E.; Van Tonder, J.J.; Steenkamp, V. Comparative toxicity of pentachlorophenol with its metabolites tetrachloro-1, 2-hydroquinone and tetrachloro-1, 4-benzoquinone in HepG2 cells. Open Toxicol. J. 2012, 5, 11–20. [Google Scholar]
- Li, C.; Wang, F.; Wang, H. Tetrachloro-1,4-benzoquinone induces apoptosis of mouse embryonic stem cells. J. Environ. Sci. 2017, 51, 5–12. [Google Scholar] [CrossRef]
- Amouroux, R.; Nashun, B.; Shirane, K.; Nakagawa, S.; Hill, P.W.; D’Souza, Z.; Encheva, V. De novo DNA methylation drives 5hmC accumulation in mouse zygotes. Nat. Cell. Biol. 2016, 18, 225–233. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hong, K.; Liu, R.; Inoue, A.; Shen, L.; Zhang, K. Dynamics of 5-methylcytosine and 5-hydroxymethylcytosine during germ cell reprogramming. Cell Res. 2013, 23, 329–339. [Google Scholar] [CrossRef]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Fedorova, I.D.; Krapivin, M.I.; Chiryaeva, O.G.; Shilnikova, E.M.; Bogdanova, M.A.; Kogan, I.Y.; Kuznetzova, T.V.; et al. Chromosome hydroxymethylation patterns in human zygotes and cleavage-stage embryos. Reproduction 2015, 149, 223–233. [Google Scholar] [CrossRef]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Parfenyev, S.E.; Mekina, I.D.; Komarova, E.M.; Mazilina, M.A.; Daev, E.V.; Chiryaeva, O.G.; Galembo, I.A.; et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget 2017, 8, 88294–88307. [Google Scholar] [CrossRef]
- Pendina, A.A.; Efimova, O.A.; Krapivin, M.I.; Mekina, I.D.; Tikhonov, A.V.; Koltsova, A.S.; Petrovskaia-Kaminskaia, A.V.; Chiryaeva, O.G.; Kogan, I.Y.; Gzgzyan, A.M.; et al. Genomic distribution of 5-formylcytosine and 5-carboxylcytosine in human preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 893–895. [Google Scholar] [CrossRef]
- Tikhodeyev, O.N. Heredity determined by the environment: Lamarckian ideas in modern molecular biology. Sci. Total. Environ. 2020, 710, 135521. [Google Scholar] [CrossRef] [PubMed]
| External Factor | Species | Condition | Organ/Tissue/Cell Line | Genomic Region | 5hmC Alteration | Ref. | |
|---|---|---|---|---|---|---|---|
| Hypnotics and Medications | |||||||
| Phenobarbital | Mouse | In vivo | Liver | Upstream, promoter, and gene body regions of multiple genes from Cyp2b and 2c families | Increase | [69] | |
| Multiple genes | Differential DNA hydroxymethylation | [70] | |||||
| Phenobarbital-induced hepatocellular adenoma | Multiple genes | Differential DNA hydroxymethylation | [71] | ||||
| Diethylstilbestrol | Mouse | In vivo | Uterus | Genomic DNA | Decrease | [73] | |
| Cocaine | Mouse | In vivo | Liver | Genomic DNA | Decrease | [77] | |
| Brain | Genomic DNA | No change | [77] | ||||
| Brain (nucleus accumbens) | Genomic DNA | No change | [78] | ||||
| Multiple genes | Differential DNA hydroxymethylation | [78] | |||||
| Rat | In vivo | Brain (prefrontal cortex) | Promoter of Homer2 gene | Decrease | [79] | ||
| Methamphetamine | Rat | In vivo | Brain (striatum) | Promoters of GluA1 and GluA2 genes | Decrease | [83] | |
| Brain (nucleus accumbens) | Transcription start site of Crh gene; intragenic sites of Avp gene | Increase | [82] | ||||
| Multiple genes | Differential DNA hydroxymethylation | [84] | |||||
| Ethanol | Human | In vivo | Blood | Genomic DNA | Decrease during consumption; Increase after detoxification | [88] | |
| Liver | Genomic DNA | Decrease | [90] | ||||
| Rat | In vivo | Liver | Genomic DNA | Decrease | [90] | ||
| Genomic DNA | Decrease | [89] | |||||
| Mouse | In vivo | Brain (hippocampus) | Genomic DNA | Decrease | [85] | ||
| Brain (cortex: cortical plate) | Genomic DNA | Increase | [86] | ||||
| Brain (cortex: subplate) | Genomic DNA | Decrease | [86] | ||||
| Brain (cortex: subventricular zone/ventricular zone) | Genomic DNA | Decrease | [86] | ||||
| In vitro | Forebrains neural stem cells | Promoters R1, R2, R3, R5 of MeCP2 gene | Increase | [87] | |||
| Genomic DNA | No change during exposure, Decrease after withdrawal | [87] | |||||
| Dimethyl sulfoxide | Mouse | In vitro | MC3T3-E1 | Genomic DNA | Short-term increase | [91] | |
| Promoters of Fas and Dlx5 genes | Short-term increase | [91] | |||||
| Anthropogenic pollutants | |||||||
| Heavy metals | Arsenic | Human | In vivo | Blood | Genomic DNA | Decrease | [100] |
| Blood | Genomic DNA | Increase (males); Decrease (females) | [101] | ||||
| In vitro | HepG2 | Genomic DNA | Increase | [99] | |||
| HEK293T | Genomic DNA | Decrease | [98] | ||||
| Rat | In vivo | Brain (cortex) | Genomic DNA | Decrease | [96] | ||
| Brain (Hippocampus) | Genomic DNA | Decrease | [96] | ||||
| Heart | Genomic DNA | Increase | [95] | ||||
| Spleen | Genomic DNA | Increase | [95] | ||||
| Lung | Genomic DNA | Increase | [95] | ||||
| Pancreas | Genomic DNA | Decrease | [95] | ||||
| Liver | Genomic DNA | No changes | [95] | ||||
| Kidney | Genomic DNA | No changes | [95] | ||||
| Mouse | In vitro | mESCs | Genomic DNA | Decrease | [98] | ||
| Genomic DNA | Decrease | [97] | |||||
| Mercury | Human | In vivo | Blood | Genomic DNA | Decrease | [103] | |
| Nickel | Human | In vitro | HEK293T | Genomic DNA | Decrease | [104] | |
| MRC5 | Genomic DNA | Decrease | [104] | ||||
| Mouse | In vitro | mESCs | Genomic DNA | Decrease | [104] | ||
| Cadmium | Human | In vivo | Blood | Genomic DNA | Increase (males); No changes (females) | [102] | |
| Mouse | In vitro | mESCs | Genomic DNA | Decrease | [97] | ||
| Chromium | Human | In vivo | Blood | Genomic DNA | No change | [102] | |
| Mouse | In vitro | mESCs | Genomic DNA | Decrease | [97] | ||
| Antimony | Mouse | In vitro | mESCs | Genomic DNA | Decrease | [97] | |
| Lead | Human | In vivo | Umbilical cord blood | Transcription start sites of GSTM1 and GSTM5 genes; Imprinted loci PEG10, SGCE | Decrease | [105] | |
| Blood | Genomic DNA | No change | [102] | ||||
| In vitro | hESCs | Transcription start sites of GSTM1 and GSTM5 genes; Imprinted loci PEG10, SGCE | Decrease | [105] | |||
| Particulate air pollution | PM2.5 | Human | In vivo | Buccal cells | Genomic DNA | Decrease | [118] |
| In vitro | SH-SY5Y | Genomic DNA | Increase | [108] | |||
| Promoters of MeCP2, GRIN1, GABRB3, NRXN1, NLGN3 genes | Increase | [108] | |||||
| Mouse | In vivo | Lung | Genomic DNA | Decrease | [109] | ||
| Liver | Genomic DNA | Decrease | [109] | ||||
| Kidney | Genomic DNA | No change | [109] | ||||
| PM10 | Human | In vivo | Blood | Genomic DNA | Increase | [117] | |
| Buccal cells | Genomic DNA | Decrease | [118] | ||||
| In vitro | Blood | Genomic DNA | Increase | [116] | |||
| Bisphenol A | Human | In vivo | Sperm | LINE1 | Increase | [129] | |
| Increase | [128] | ||||||
| Genomic DNA | Increase | [128] | |||||
| ACHE gene | Increase | [130] | |||||
| In vitro | MCF-7 | Genomic DNA | Decrease | [133] | |||
| Mouse | In vivo | Brain (cortex) | Kcnq1 locus | No change | [132] | ||
| Brain (midbrain) | Kcnq1 locus | No change | [132] | ||||
| Blood | Gnas, Grb10, Plagl1, Pde10a, Pde4d genes | Increase | [131] | ||||
| Klf14,Airn, Cmah, Snrpn, Ppp1r9a, Kcnq1, Phactr2 genes | Decrease | [131] | |||||
| Hydroquinone | Human | In vitro | HEK293 | Genomic DNA | Increase | [134] | |
| Open reading frame 2 of LINE1 | Decrease | [134] | |||||
| Promoters of GCLC and 14-3-3σ genes | Increase | [134] | |||||
| Pentachlorophenol metabolites | Tetrachloro-1,4-benzoquinone | Human | In vitro | A549 | Genomic DNA | Increase | [67] |
| HepG2 | Genomic DNA | Increase | [67] | ||||
| MRC5 | Genomic DNA | Increase | [67] | ||||
| Mouse | In vitro | mESCs | Genomic DNA | Increase | [136] | ||
| Tetrachloro-1,4-hydroquinone | Human | In vitro | A549 | Genomic DNA | Increase | [67] | |
| HepG2 | Genomic DNA | Increase | [67] | ||||
| MRC5 | Genomic DNA | Increase | [67] | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, O.A.; Koltsova, A.S.; Krapivin, M.I.; Tikhonov, A.V.; Pendina, A.A. Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine. Int. J. Mol. Sci. 2020, 21, 3223. https://doi.org/10.3390/ijms21093223
Efimova OA, Koltsova AS, Krapivin MI, Tikhonov AV, Pendina AA. Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine. International Journal of Molecular Sciences. 2020; 21(9):3223. https://doi.org/10.3390/ijms21093223
Chicago/Turabian StyleEfimova, Olga A., Alla S. Koltsova, Mikhail I. Krapivin, Andrei V. Tikhonov, and Anna A. Pendina. 2020. "Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine" International Journal of Molecular Sciences 21, no. 9: 3223. https://doi.org/10.3390/ijms21093223
APA StyleEfimova, O. A., Koltsova, A. S., Krapivin, M. I., Tikhonov, A. V., & Pendina, A. A. (2020). Environmental Epigenetics and Genome Flexibility: Focus on 5-Hydroxymethylcytosine. International Journal of Molecular Sciences, 21(9), 3223. https://doi.org/10.3390/ijms21093223





