Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis
Abstract
1. Introduction
2. Results
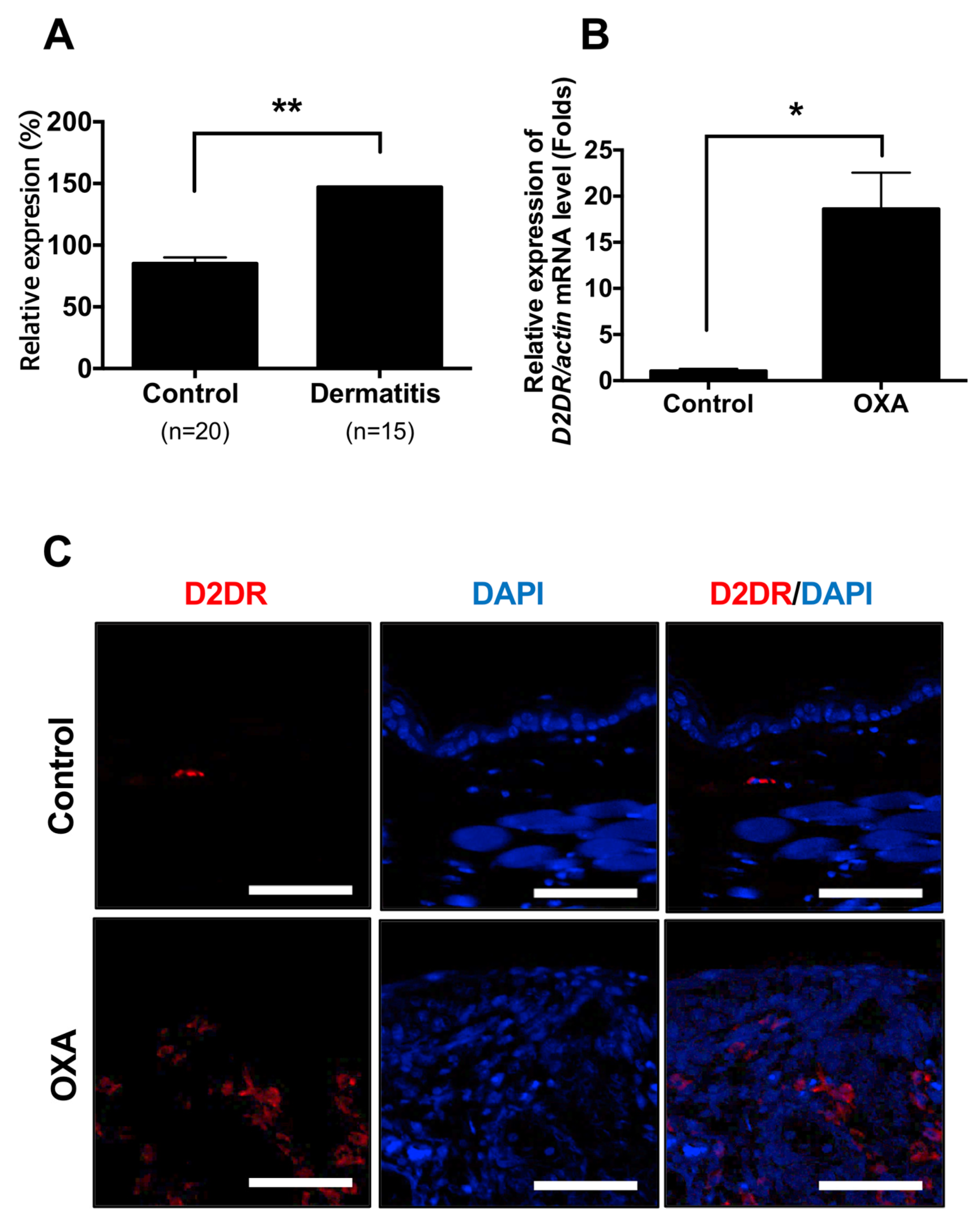
2.1. Dopamine Receptor D2 is Highly Expressed in Dermatitis Patients and Animal Model of Dermatitis
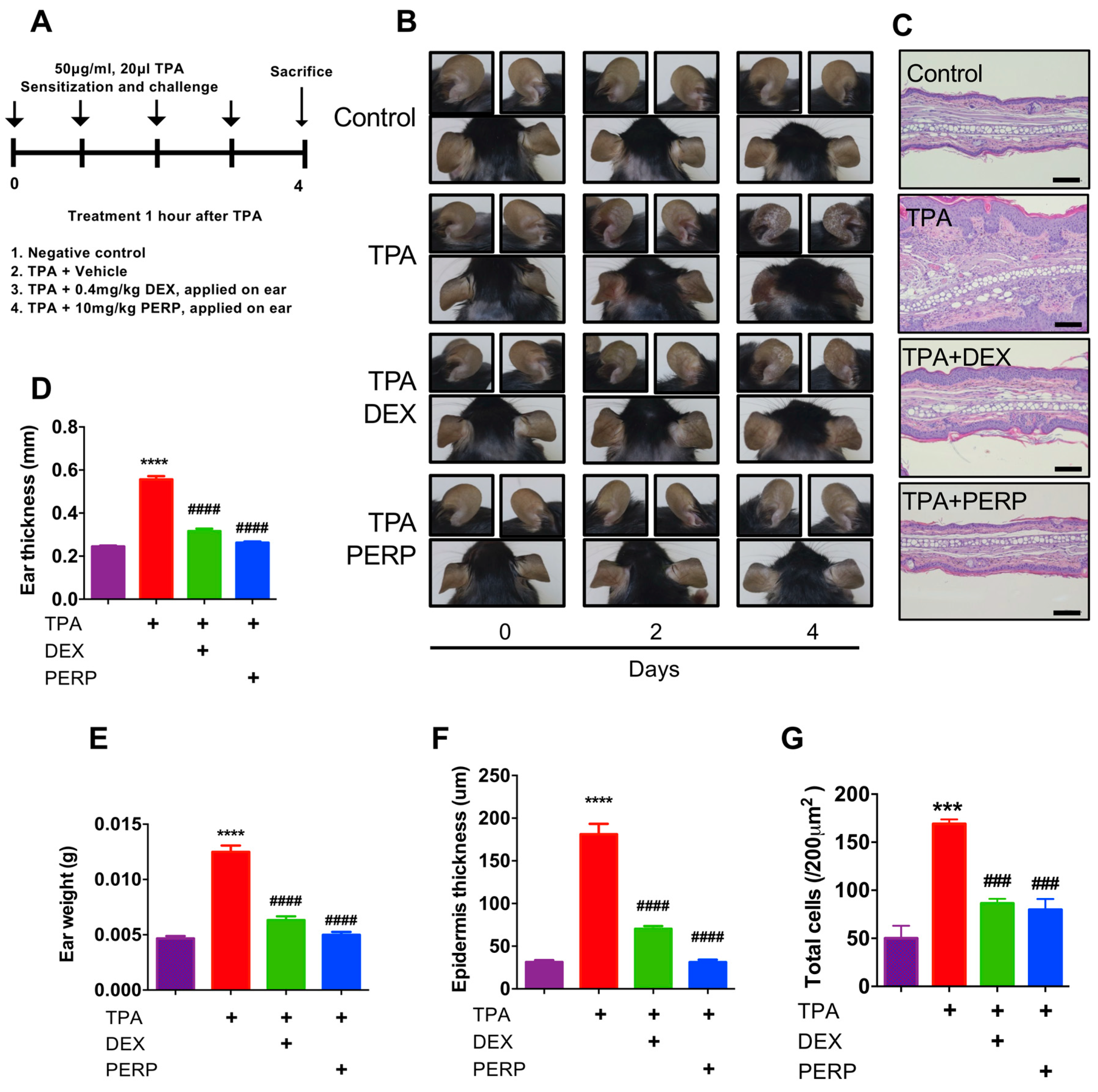
2.2. Perphenazine Ameliorates TPA-Induced Animal Model of Dermatitis
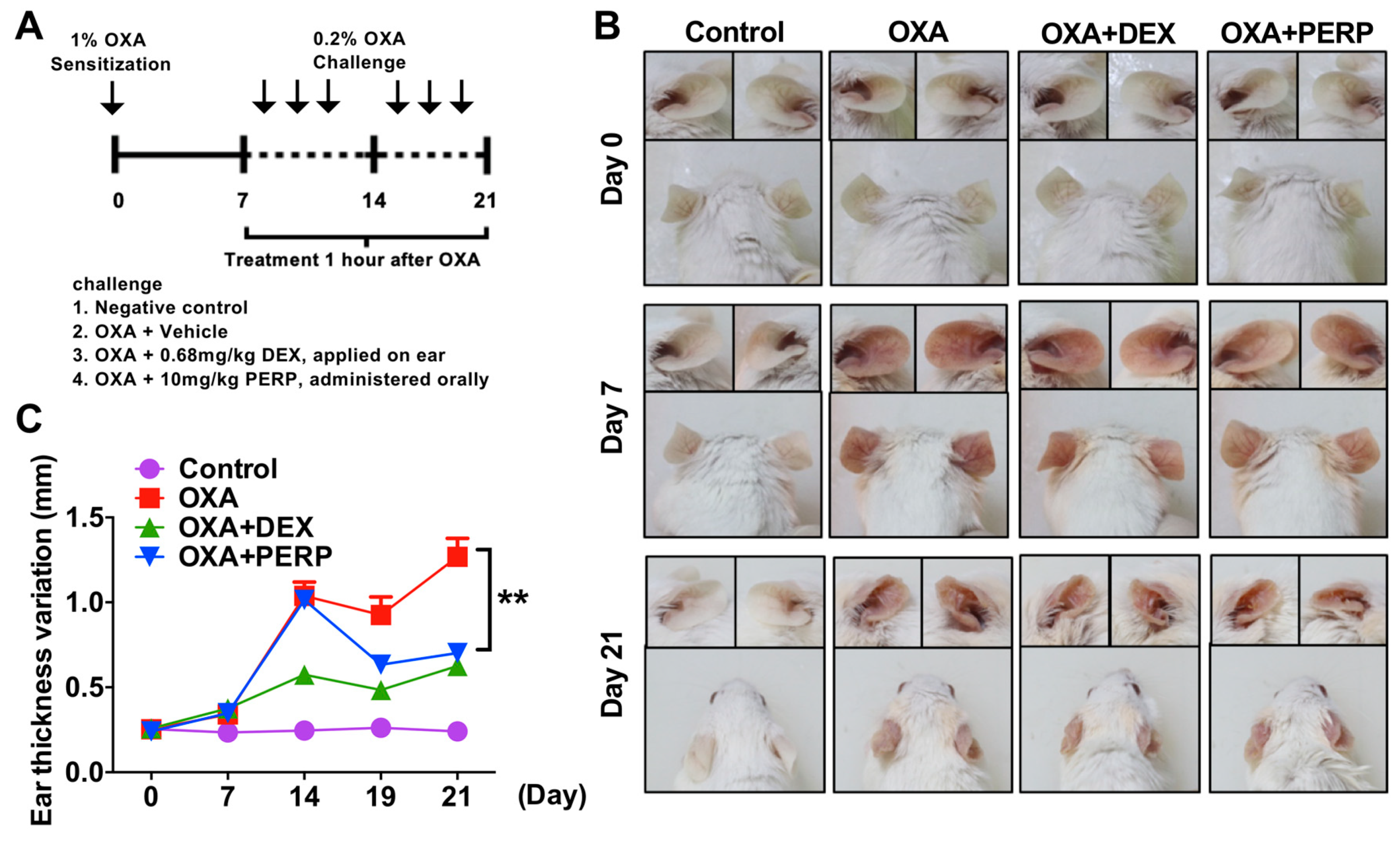
2.3. Perphenazine Ameliorates Morphological Phenotype of Oxazolone-Treated Animal Model of Dermatitis
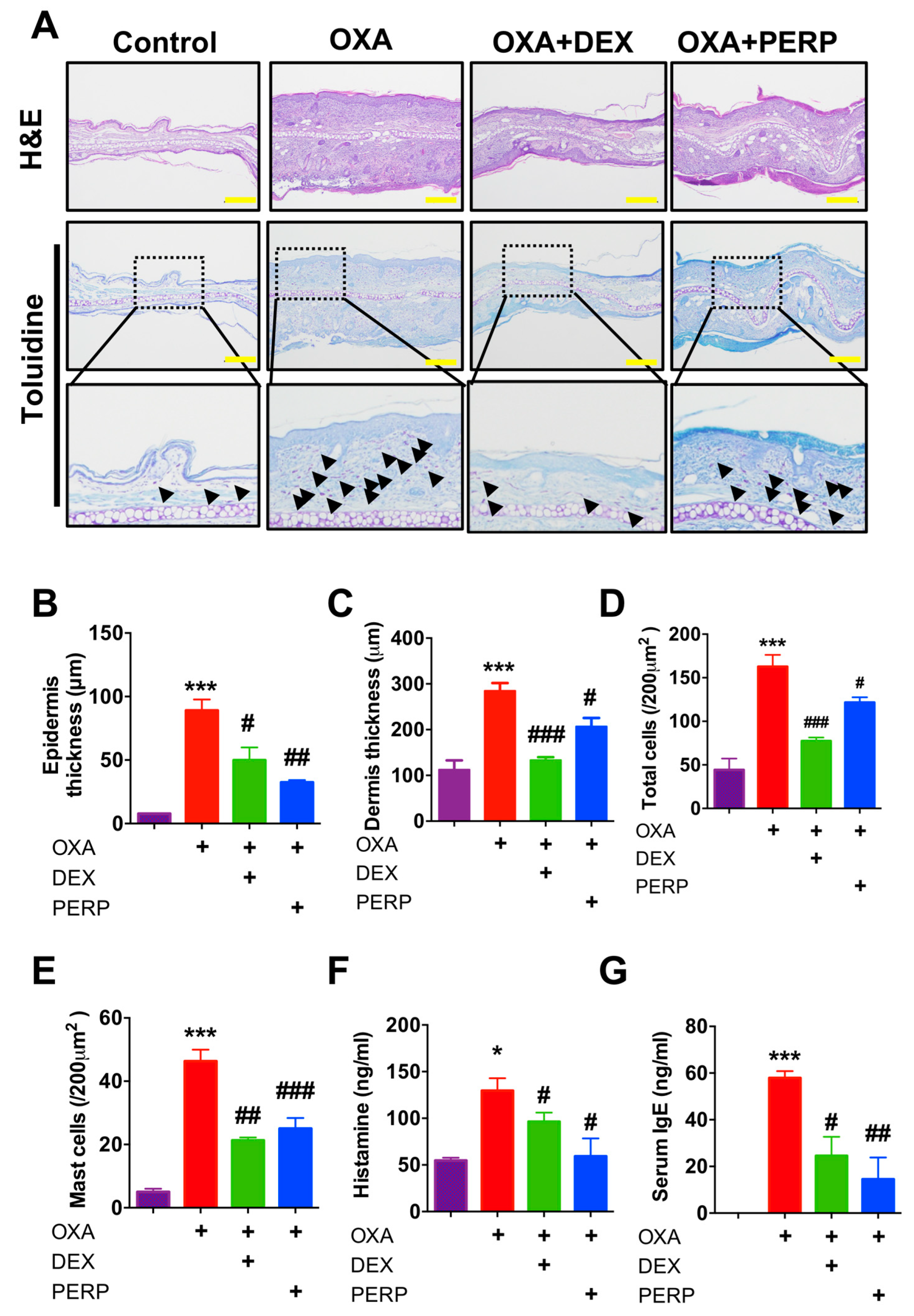
2.4. Perphenazine Ameliorates Histological Phenotype of Oxazolone-Treated Mice
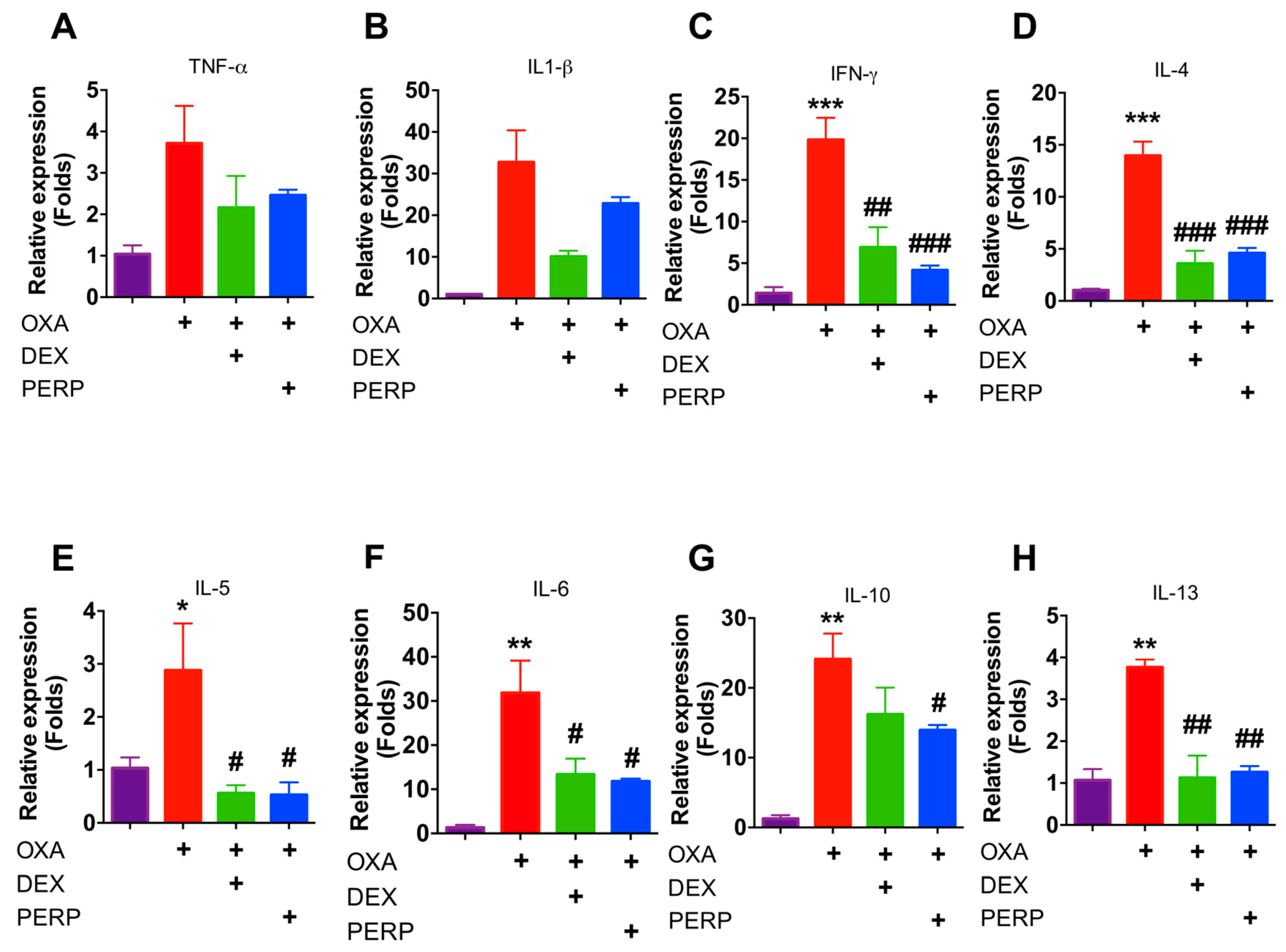
2.5. Perphenazine Attenuates Cytokine Expression in Oxazolone-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. TPA-Induced Acute Dermatitis in Mice
4.3. OXA-Induced Murine Model of Dermatitis
4.4. Histology
4.5. RNA Isolation and RT-PCR
4.6. Serum IgE ELISA
4.7. Histamine Release Assay
4.8. Cells and Reagents
4.9. Cell Viability Assay
4.10. Luciferase Assay
4.11. Immunofluorescence
4.12. Web-Based Meta-Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iams, J. Clinical Practice. Obstet. Anesthesia Dig. 2015, 35, 34. [Google Scholar] [CrossRef]
- Rizk, P.; Rodenas, M.; De Benedetto, A. Allergen Immunotherapy and Atopic Dermatitis: the Good, the Bad, and the Unknown. Curr. Allergy Asthma Rep. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A. Adverse effects of topical corticosteroid use. West. J. Med. 1995, 162, 123–126. [Google Scholar] [PubMed]
- Ng, C.; Hon, K.E.; Robson, W.L.M. Atopic Dermatitis. Adv. Pediatr. 2007, 54, 241–273. [Google Scholar] [CrossRef]
- Cotter, D.G.; Schairer, D.; Eichenfield, L.F. Emerging therapies for atopic dermatitis: JAK inhibitors. J. Am. Acad. Dermatol. 2018, 78, S53–S62. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Werfel, T.; Allam, J.-P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.F.; Simon, H.-U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef]
- Klonowska, J.; Gleń, J.; Nowicki, R.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- Bitton, A.; Avlas, S.; Reichman, H.; Itan, M.; Karo-Atar, D.; Azouz, N.P.; Rozenberg, P.; Diesendruck, Y.; Nahary, L.; Rothenberg, M.E.; et al. A key role for IL-13 signaling via the type 2 IL-4 receptor in experimental atopic dermatitis. Sci. Immunol. 2020, 5, eaaw2938. [Google Scholar] [CrossRef]
- Andersson, K. Neurotransmitters: central and peripheral mechanisms. Int. J. Impot. Res. 2000, 12, S26–S33. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Basu, B.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. The immunoregulatory role of dopamine: An update. Brain, Behav. Immun. 2009, 24, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Contreras, F.; Zouali, M. The Dopaminergic System in Autoimmune Diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Álvarez-Herrera, S.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutiérrez, E.O.; Garcés-Alvarez, M.E.; De La Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-H.; Liu, Y.-Q.; Deng, Q.-W.; Peng, Y.-P.; Qiu, Y.-H. Dopamine D2 Receptor Is Involved in Alleviation of Type II Collagen-Induced Arthritis in Mice. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Kawahara, R.; Hashimura, H.; Yamanaka, N.; Iimori, M.; Amagase, K.; Kato, S.; Takeuchi, K. Dopamine D2–Receptor Antagonists Ameliorate Indomethacin-Induced Small Intestinal Ulceration in Mice by Activating α7 Nicotinic Acetylcholine Receptors. J. Pharmacol. Sci. 2011, 116, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Takagi, R.; Kaneko, A.; Matsushita, S. Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J. Neuroimmunol. 2015, 289, 43–55. [Google Scholar] [CrossRef]
- Contreras, F.; Prado, C.; Gonzalez, H.; Franz, D.; Osorio-Barrios, F.; Osorio, F.; Ugalde, V.; Lopez, E.; Elgueta, D.; Figueroa, A.; et al. Dopamine Receptor D3 Signaling on CD4+ T Cells Favors Th1- and Th17-Mediated Immunity. J. Immunol. 2016, 196, 4143–4149. [Google Scholar] [CrossRef]
- Elgueta, D.; Contreras, F.; Prado, C.; Montoya, A.; Ugalde, V.; Chovar, O.; Villagra, R.; Henríquez, C.; Abellanas, M.A.; Aymerich, M.S.; et al. Dopamine Receptor D3 Expression Is Altered in CD4+ T-Cells From Parkinson’s Disease Patients and Its Pharmacologic Inhibition Attenuates the Motor Impairment in a Mouse Model. Front. Immunol. 2019, 10, 981. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
- Boyd, K.N.; Mailman, R.B. Dopamine receptor signaling and current and future antipsychotic drugs. Handb. Exp. Pharmacol. 2012, 212, 53–86. [Google Scholar] [CrossRef]
- Ascher-Svanum, H.; Zhu, B.; Faries, D.E.; Landbloom, R.; Swartz, M.S.; Swanson, J.W. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olsson, M.; Broberg, A.; Jernas, M.; Carlsson, L.; Rudemo, M.; Suurküla, M.; Svensson, P.-A.; Benson, M. Increased expression of aquaporin 3 in atopic eczema. Allergy 2006, 61, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Mobini, R.; Andersson, B.; Erjefält, J.; Hahn-Zoric, M.; Langston, M.A.; Perkins, A.; Cardell, L.O.; Benson, M. A module-based analytical strategy to identify novel disease-associated genes shows an inhibitory role for interleukin 7 Receptor in allergic inflammation. BMC Syst. Boil. 2009, 3, 19. [Google Scholar] [CrossRef]
- Esaki, H.; Ewald, D.A.; Ungar, B.; Rozenblit, M.; Zheng, X.; Xu, H.; Estrada, Y.D.; Peng, X.; Mitsui, H.; Litman, T.; et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J. Allergy Clin. Immunol. 2015, 135, 153–163. [Google Scholar] [CrossRef]
- Choi, S.Y.; Heo, M.J.; Lee, C.; Choi, Y.M.; An, I.S.; Bae, S.; An, S.; Jung, J.H. 2-deoxy-d-glucose Ameliorates Animal Models of Dermatitis. Biomed. 2020, 8, 20. [Google Scholar] [CrossRef]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse Skin Inflammation Induced by Multiple Topical Applications of 12-O-Tetradecanoylphorbol-13-Acetate. Ski. Pharmacol. Physiol. 1991, 4, 262–271. [Google Scholar] [CrossRef]
- Jin, H. Animal models of atopic dermatitis. J. Invest Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef]
- Talhada, D.; Rabenstein, M.; Ruscher, K. The role of dopaminergic immune cell signalling in poststroke inflammation. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774225. [Google Scholar] [CrossRef]
- Figueroa, C.; Gálvez-Cancino, F.; Oyarce, C.; Contreras, F.; Prado, C.; Valeria, C.; Cruz, S.; Lladser, A.; Pacheco, R.; Gomez, S.C. Inhibition of dopamine receptor D3 signaling in dendritic cells increases antigen cross-presentation to CD8+ T-cells favoring anti-tumor immunity. J. Neuroimmunol. 2017, 303, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Sarkar, C.; Chakroborty, D.; Ganguly, S.; Shome, S.; Dasgupta, P.S.; Basu, S. D1 and D2 Dopamine Receptor-mediated Inhibition of Activated Normal T Cell Proliferation Is Lost in Jurkat T Leukemic Cells. J. Boil. Chem. 2010, 285, 27026–27032. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.; Gowda, R.; A Noory, M.; Robertson, G.P. Modulating cancer cell survival by targeting intracellular cholesterol transport. Br. J. Cancer 2017, 117, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Seol, I.-W.; Kuo, N.Y.; Kim, K.M. Effects of dopaminergic drugs on the mast cell degranulation and nitric oxide generation in RAW 264.7 cells. Arch. Pharmacal Res. 2004, 27, 94–98. [Google Scholar] [CrossRef]
- Mori, T.; Kabashima, K.; Fukamachi, S.; Kuroda, E.; Sakabe, J.-I.; Kobayashi, M.; Nakajima, S.; Nakano, K.; Tanaka, Y.; Matsushita, S.; et al. D1-like dopamine receptors antagonist inhibits cutaneous immune reactions mediated by Th2 and mast cells. J. Dermatol. Sci. 2013, 71, 37–44. [Google Scholar] [CrossRef]
- Ozdemir, E.I.; Gursoy, S. Role of D(1)/D(2) dopamin receptors antagonist perphenazine in morphine analgesia and tolerance in rats. Bosn J. Basic Med. Sci. 2013, 13, 119–125. [Google Scholar] [CrossRef]
- Heo, M.-J.; Lee, C.; Choi, S.Y.; Choi, Y.M.; An, I.-S.; Bae, S.; An, S.; Jung, J.H. Nintedanib ameliorates animal model of dermatitis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Jung, J.H.; Wang, X.D.; Loeken, M.R. Mouse embryonic stem cells established in physiological-glucose media express the high KM Glut2 glucose transporter expressed by normal embryos. STEM CELLS Transl. Med. 2013, 2, 929–934. [Google Scholar] [CrossRef]
- Bae, S.; Jung, J.H.; Kim, K.; An, I.-S.; Kim, S.-Y.; Lee, J.H.; Park, I.-C.; Jin, Y.-W.; Lee, S.-J.; An, S. TRIAD1 inhibits MDM2-mediated p53 ubiquitination and degradation. FEBS Lett. 2012, 586, 3057–3063. [Google Scholar] [CrossRef]
- Bouma, G.; Td, S.; Kj, L. Faculty Opinions recommendation of Analyzing real-time PCR data by the comparative C(T) method. Faculty Opinions – Post-Publication Peer Review of the Biomedical Literature 2010, 3, 1101–1108. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, S.-M.; Bae, S.; Lee, S.-J.; Park, I.-C.; Jin, Y.-W.; Lee, J.H.; An, S. Triad 1 induces apoptosis by p53 activation. FEBS Lett. 2010, 584, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Choi, Y.M.; Choi, S.-Y.; An, I.-S.; Bae, S.; An, S.; Jung, J.H. Glucose metabolism regulates expression of hair-inductive genes of dermal papilla spheres via histone acetylation. Sci. Rep. 2020, 10, 4887. [Google Scholar] [CrossRef]
- Jung, J.H.; Bae, S.; Lee, J.Y.; Woo, S.R.; Cha, H.J.; Yoon, Y.; Suh, K.-S.; Lee, S.-J.; Park, I.-C.; Jin, Y.-W.; et al. E3 ubiquitin ligase Hades negatively regulates the exonuclear function of p53. Cell Death Differ. 2011, 18, 1865–1875. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adam, K.; Oswald, I. The hypnotic effects of an antihistamine: promethazine. Br. J. Clin. Pharmacol. 1986, 22, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, R.J. Some Endocrine Effects of Two Phenothiazine Derivatives, Chlorpromazine and Perphenazine, in the Female Mouse. Br. J. Pharmacol. Chemother. 1963, 20, 497–506. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, M.-J.; Choi, S.Y.; Lee, C.; Choi, Y.M.; An, I.-s.; Bae, S.; An, S.; Jung, J.H. Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis. Int. J. Mol. Sci. 2020, 21, 3241. https://doi.org/10.3390/ijms21093241
Heo M-J, Choi SY, Lee C, Choi YM, An I-s, Bae S, An S, Jung JH. Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis. International Journal of Molecular Sciences. 2020; 21(9):3241. https://doi.org/10.3390/ijms21093241
Chicago/Turabian StyleHeo, Min-Jeong, Soo Young Choi, Chanmi Lee, Yeong Min Choi, In-sook An, Seunghee Bae, Sungkwan An, and Jin Hyuk Jung. 2020. "Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis" International Journal of Molecular Sciences 21, no. 9: 3241. https://doi.org/10.3390/ijms21093241
APA StyleHeo, M.-J., Choi, S. Y., Lee, C., Choi, Y. M., An, I.-s., Bae, S., An, S., & Jung, J. H. (2020). Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis. International Journal of Molecular Sciences, 21(9), 3241. https://doi.org/10.3390/ijms21093241





