Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors
Abstract
:1. Introduction
2. Legume Responses to Pathogen Attack
3. Phytohormones and PIs in Legumes
4. PIs Present in Legumes
5. Legume PIs as a Biopesticide against Insects and Nematodes
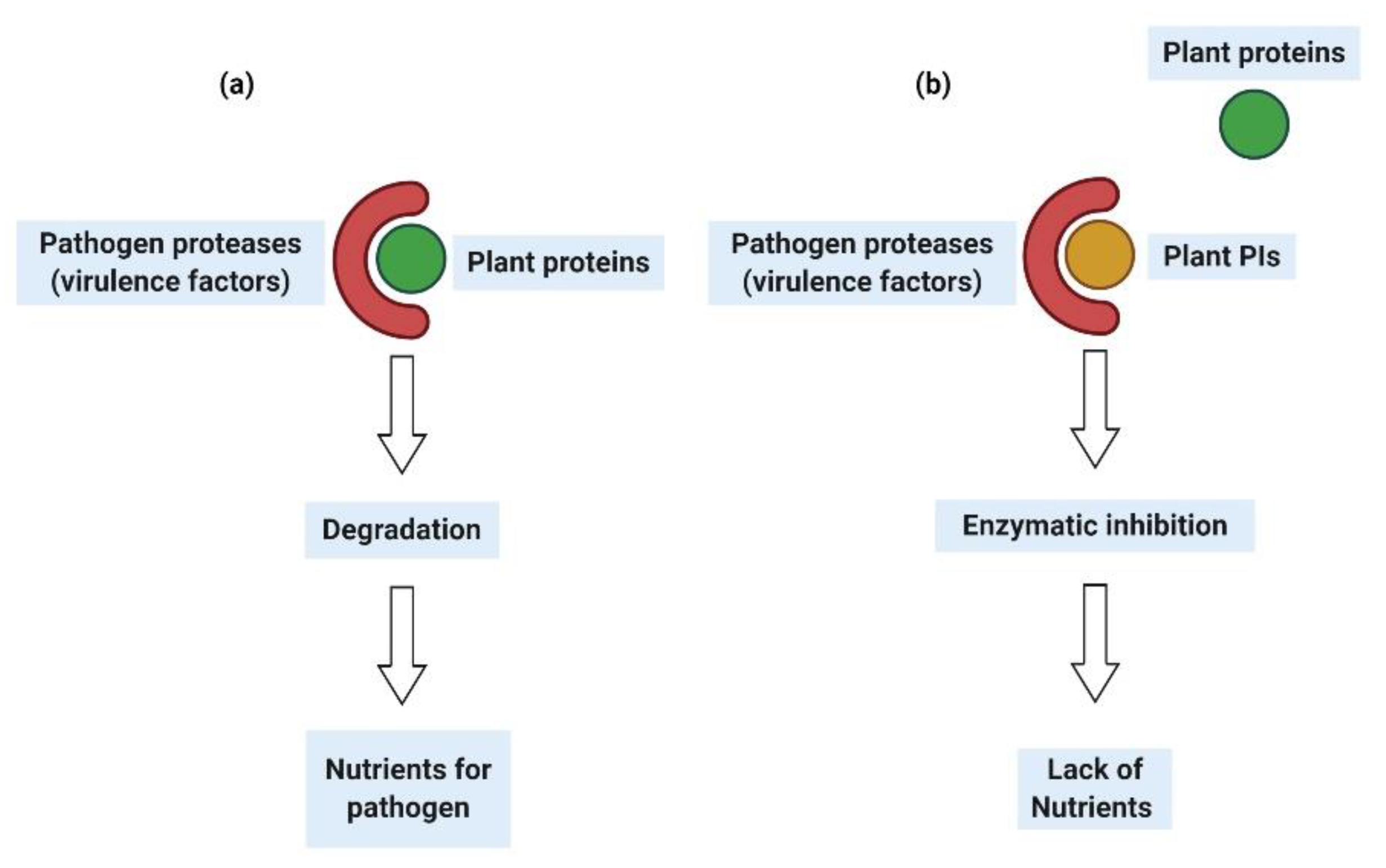
6. Legume PIs as a Biopesticide against Phytopathogenic Fungi and Bacteria
7. Recombinant PIs for Biotechnological Application
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ANFs | Anti-nutritional factors |
| ET | Ethylene |
| ETI | Effector-triggered immunity |
| ETS | Effector-triggered susceptibility |
| HGPs | H. armigera gut trypsin-like protease |
| HR | Hypersensitive cell death |
| JA | Jasmonic acid |
| NB-LRR | Nucleotide-binding and leucine-rich repeat domains |
| PA | Phosphatidic acid |
| PAMPs | Pathogen-associated molecular patterns |
| PIs | Protease inhibitors |
| PR | Pathogenesis-related |
| PRRs | Pathogen recognition receptor |
| PTI | PAMP-triggered immunity |
| SA | Salicylic acid |
References
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. CRC Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, R.; Nanda, V. Proximate composition, nutritional profile and health benefits of legumes—A review. Legum. Res. 2018, 41, 325–332. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. CRC Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.C.; van den Brand, G.J.; Vanlauwe, B.; Giller, K.E. Sustainable intensification through rotations with grain legumes in Sub-Saharan Africa: A review. Agric. Ecosyst. Environ. 2018, 261, 172–185. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. CRC Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef] [Green Version]
- Dhandaydham, M.; Charles, L.; Zhu, H.; Starr, J.L.; Huguet, T.; Cook, D.R.; Prosperi, J.M.; Opperman, C. Characterization of root-knot nematode resistance in Medicago truncatula. J. Nematol. 2008, 40, 46–54. [Google Scholar]
- Cruz, L.P.; de Sá, L.F.R.; Santos, L.A.; Gravina, G.A.; Carvalho, A.O.; Fernandes, K.V.S.; Freire Filho, F.R.; Gomes, V.M.; Oliveira, A.E.A. Evaluation of resistance in different cowpea cultivars to Callosobruchus maculatus infestation. J. Pest Sci. (2004) 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Mainali, B.P.; Kim, H.J.; Park, C.G.; Heon Kim, J.; Yoon, Y.N.; Oh, I.S.; Bae, S. Do Oviposition preference and development of azuki bean weevil, Callosobruchus chinensis, on five different leguminous seeds. J. Stored Prod. Res. 2015, 61, 97–101. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Martínez, L.X.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Effect of cooking and germination on bioactive compounds in pulses and their health benefits. J. Funct. Foods 2017, 38, 624–634. [Google Scholar] [CrossRef]
- Srinivasan, A.; Giri, A.P.; Harsulkar, A.M.; Gatehouse, J.A.; Gupta, V.S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae. Plant Mol. Biol. 2005, 57, 359–374. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Duxbury, Z.; Ma, Y.; Furzer, O.J.; Huh, S.U.; Cevik, V.; Jones, J.D.G.; Sarris, P.F. Pathogen perception by NLRs in plants and animals: Parallel worlds. BioEssays 2016, 38, 769–781. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Katagiri, F.; Tsuda, K. Understanding the plant immune system. Mol. Plant-Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryavtseva, N.N.; Sofyin, A.V.; Revina, T.A.; Gvozdeva, E.L.; Ievleva, E.V.; Valueva, T.A. Secretion of proteolytic enzymes by three phytopathogenic microorganisms. Appl. Biochem. Microbiol. 2013, 49, 514–520. [Google Scholar] [CrossRef]
- Yarullina, L.G.; Akhatova, A.R.; Kasimova, R.I. Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant–pathogen interactions. Russ. J. Plant Physiol. 2016, 63, 193–203. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Koiwa, H.; Bressan, R.A.; Hasegawa, P.M. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997, 2, 379–384. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Giacometti, R.; Barneto, J.; Barriga, L.G.; Sardoy, P.M.; Balestrasse, K.; Andrade, A.M.; Pagano, E.A.; Alemano, S.G.; Zavala, J.A. Early perception of stink bug damage in developing seeds of field-grown soybean induces chemical defences and reduces bug attack. Pest Manag. Sci. 2016, 72, 1585–1594. [Google Scholar] [CrossRef]
- Lee, S.; Hirt, H.; Lee, Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001, 26, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Paudel, J.R.; Bede, J.C. Ethylene signaling modulates herbivore-induced defense responses in the model legume Medicago truncatula. Mol. Plant-Microbe Interact. 2015, 28, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, H.; Ma, Y.; Yang, W.; Yang, Q.; Yu, D. Identification of soybean herbivory-regulated genes and a transgenic investigation of their potential in insect resistance. Plant Cell. Tissue Organ Cult. 2015, 123, 321–340. [Google Scholar] [CrossRef]
- Gao, L.L.; Anderson, J.P.; Klingler, J.P.; Nair, R.M.; Edwards, O.R.; Singh, K.B. Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Mol. Plant-Microbe Interact. 2007, 20, 82–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphuis, L.G.; Guo, S.M.; Gao, L.L.; Singh, K.B. Genetic mapping of a major resistance gene to pea aphid (Acyrthosipon pisum) in the model legume Medicago truncatula. Int. J. Mol. Sci. 2016, 17, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamchi, A.; Ben, C.; Rossignol, M.; Zareie, S.R.; Mirlohi, A.; Sayed-Tabatabaei, B.E.; Pichereaux, C.; Sarrafi, A.; Rickauer, M.; Gentzbittel, L. Proteomics analysis of Medicago truncatula response to infection by the phytopathogenic bacterium Ralstonia solanacearum points to jasmonate and salicylate defence pathways. Cell. Microbiol. 2018, 20, e12796. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakitani, M.; Iwamatsu, A.; Yoshikawa, M.; Yamaoka, N.; Ishida, I. The structure and function of a soybean β-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. USA 1997, 94, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef]
- Sharma, K. Protease inhibitors in crop protection from insects. Int. J. Curr. Res. Acad. Rev. 2015, 3, 55–70. [Google Scholar]
- Martinez, M.; Santamaria, M.E.; Diaz-Mendoza, M.; Arnaiz, A.; Carrillo, L.; Ortego, F. Phytocystatins: Defense proteins against phytophagous insects and acari. Int. J. Mol. Sci. 2016, 17, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.; dos Reis, S.; de Souza, C. Phytocystatins and their potential to control plant diseases caused by fungi. Protein Pept. Lett. 2015, 22, 104–111. [Google Scholar] [CrossRef] [PubMed]
- El-Latif, A.O.A. Protease purification and characterization of a serine protease inhibitor from Egyptian varieties of soybean seeds and its efficacy against Spodoptera littoralis. J. Plant Prot. Res. 2015, 55, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Swathi, M.; Mohanraj, S.S.; Swaroop, V.; Gujjarlapudi, M.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Proteinase inhibitors from Cajanus platycarpus accessions active against pod borer Helicoverpa armigera. Acta Physiol. Plant. 2015, 37, 242. [Google Scholar] [CrossRef]
- Vasudev, A.; Sohal, S.K. Partially purified Glycine max proteinase inhibitors: Potential bioactive compounds against tobacco cutworm, Spodoptera litura (Fabricius, 1775) (Lepidoptera: Noctuidae). Turkish J. Zool. 2016, 40, 379–387. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, A.; Kaur, A.P.; Rup, P.J.; Sohal, S.K. Assessment of soybean inhibitor as a biopesticide against melon fruit fly, Bactrocera cucurbitae (Coquillett). J. Plant Dis. Prot. 2017, 124, 445–451. [Google Scholar] [CrossRef]
- Golla, S.K.; Rajasekhar, P.; Akbar, S.M.D.; Sharma, H.C. Proteolytic activity in the midgut of Helicoverpa armigera (Noctuidae: Lepidoptera) larvae fed on wild relatives of chickpea, Cicer arietinum. J. Econ. Entomol. 2018, 111, 2409–2415. [Google Scholar] [CrossRef]
- Negi, P.; Chand, S.; Thakur, N.; Nath, A.K. Biological Activity of Serine Protease Inhibitor Isolated from the Seeds of Phaseolus vulgaris. Agric. Res. 2018, 7, 265–270. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Conti, E. Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. (2004) 2018, 91, 671–679. [Google Scholar] [CrossRef]
- Ramalho, S.R.; Bezerra, C.D.S.; Lourenço De Oliveira, D.G.; Souza Lima, L.; Maria Neto, S.; Ramalho De Oliveira, C.F.; Valério Verbisck, N.; Rodrigues Macedo, M.L. Novel peptidase Kunitz inhibitor from Platypodium elegans seeds is active against Spodoptera frugiperda larvae. J. Agric. Food Chem. 2018, 66, 1349–1358. [Google Scholar] [CrossRef]
- Rocha, R.O.; Morais, J.K.S.; Oliveira, J.T.A.; Oliveira, H.D.; Sousa, D.O.B.; Souza, C.E.A.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Antonino De Souza, J.D.; Grossi De Sá, M.F.; et al. Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 5335–5343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, L.B.D.S.; Oliveira, A.S.; Ribeiro, J.K.C.; Kiyota, S.; Vasconcelos, I.M.; De Oliveira, J.T.A.; De Sales, M.P. Effects of a novel pathogenesis-related class 10 (PR-10) protein from crotalaria pallida roots with papain inhibitory activity against root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2010, 58, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Dawei, L.; Shaoxu, Y.; Jingsheng, C.; Lijie, C.; Yuxi, D. Effects on trypsin inhibitor in roots of resistant soybeans after Heterodera glycines invasion. Int. J. Agric. Biol. 2016, 18, 965–968. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Wang, X.; Fang, W.; Bidochka, M.J.; She, R.; Xiao, Y.; Pei, Y. Psc-AFP, an antifungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides 2006, 27, 1726–1731. [Google Scholar] [CrossRef]
- Lopes, J.L.S.; Valadares, N.F.; Moraes, D.I.; Rosa, J.C.; Araújo, H.S.S.; Beltramini, L.M. Physico-chemical and antifungal properties of protease inhibitors from Acacia plumosa. Phytochemistry 2009, 70, 871–879. [Google Scholar] [CrossRef]
- Nair, M.; Sandhu, S.S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts an antimicrobial effect on Fusarium oxysporum f.sp. ciceris. Agric. Sci. 2013, 4, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Scarafoni, A.; Ronchi, A.; Prinsi, B.; Espen, L.; Assante, G.; Venturini, G.; Duranti, M. The proteome of exudates from germinating Lupinus albus seeds is secreted through a selective dual-step process and contains proteins involved in plant defence. FEBS J. 2013, 280, 1443–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, V.; Bonacci, G.; Batthyany, C.; Amé, M.V.; Carrari, F.; Gieco, J.; Asis, R. Peanut seed cultivars with contrasting resistance to Aspergillus parasiticus colonization display differential temporal response of protease inhibitors. Phytopathology 2017, 107, 474–482. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Lu, W.; Hong, J.; Rao, P. Isolation of a trypsin-chymotrypsin inhibitor and its functional properties. Prep. Biochem. Biotechnol. 2014, 44, 545–557. [Google Scholar] [CrossRef]
- Wang, S.; Lin, J.; Ye, M.; Ng, T.B.; Rao, P.; Ye, X. Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities. Peptides 2006, 27, 3129–3136. [Google Scholar] [CrossRef]
- Urwin, P.E.; Lilley, C.J.; McPherson, M.J.; Atkinson, H.J. Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J. 1997, 12, 455–461. [Google Scholar] [CrossRef]
- Alkharouf, N.W.; Klink, V.P.; Chouikha, I.B.; Beard, H.S.; MacDonald, M.H.; Meyer, S.; Knap, H.T.; Khan, R.; Matthews, B.F. The Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 2006, 224, 838–852. [Google Scholar] [CrossRef] [PubMed]
- De Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-De-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef] [PubMed]
- Migliolo, L.; de Oliveira, A.S.; Santos, E.A.; Franco, O.L.; de Sales, M.P. Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine- and cysteine-proteinases. J. Mol. Graph. Model. 2010, 29, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yuan, S.S.; Jiang, L.L.; Ye, X.J.; Ng, T.B.; Wu, Z.J. Plant antifungal proteins and their applications in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 4961–4981. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.C.; Kim, J.Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K.S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Da Silva Bezerra, C.; De Oliveira, C.F.R.; Machado, O.L.T.; De Mello, G.S.V.; Da Rocha Pitta, M.G.; De Melo Rêgo, M.J.B.; Napoleão, T.H.; Paiva, P.M.G.; De Fátima Ferreira Ribeiro, S.; Gomes, V.M.; et al. Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: A multifunctional Kunitz inhibitor. Process Biochem. 2016, 51, 792–803. [Google Scholar] [CrossRef]
- Brito, M.S.d.; Melo, M.B.; Alves, J.P.d.A.; Fontenelle, R.O.d.S.; Mata, M.F.; Andrade, L.B.d.S. Partial purification of trypsin/papain inhibitors from Hymenaea courbaril L. seeds and antibacterial effect of protein fractions. Hoehnea 2016, 43, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Haque, Q.M.R.; Fatma, T.; Manzoor, N.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 15, 939–950. [Google Scholar] [CrossRef]
- Dona, A.; Arvanitoyannis, I.S. Health risks of genetically modified foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 164–175. [Google Scholar] [CrossRef]
- Paparini, A.; Romano-Spica, V. Public health issues related with the consumption of food obtained from genetically modified organisms. Biotechnol. Annu. Rev. 2004, 10, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielkopolan, B.; Krawczyk, K.; Obrępalska-Stęplowska, A. Gene expression of serine and cysteine proteinase inhibitors during cereal leaf beetle larvae feeding on wheat: The role of insect-associated microorganisms. Arthropod. Plant. Interact. 2018, 12, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Wielkopolan, B.; Obrępalska-Stęplowska, A. Three-way interaction among plants, bacteria, and coleopteran insects. Planta 2016, 244, 313–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emani, C. Gossypium. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berling/Heidelberg, Germany, 2011; pp. 109–122. ISBN 978-3-642-20449-4. [Google Scholar]
- Mohanraj, S.S.; Tetali, S.D.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Biochemical properties of a bacterially expressed Bowman-Birk inhibitor from Rhynchosia sublobata (Schumach.) Meikle seeds and its activity against gut proteases of Achaea janata. Phytochemistry 2018, 151, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro Júnior, J.E.; Valadares, N.F.; Pereira, H.D.M.; Dyszy, F.H.; da Costa Filho, A.J.; Uchôa, A.F.; de Oliveira, A.S.; da Silveira Carvalho, C.P.; Grangeiro, T.B. Expression in Escherichia coli of cysteine protease inhibitors from cowpea (Vigna unguiculata): The crystal structure of a single-domain cystatin gives insights on its thermal and pH stability. Int. J. Biol. Macromol. 2017, 102, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.D.; Ahn, J.E.; Salzman, R.A.; Moon, J.; Chi, Y.H.; Yun, D.J.; Lee, S.Y.; Koiwa, H.; Zhu-Salzman, K. Functional expression of an insect cathepsin B-like counter-defence protein. Insect Mol. Biol. 2008, 17, 235–245. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Koiwa, H.; Salzman, R.A.; Shade, R.E.; Ahn, J.-E. Cowpea bruchid Callosobruchus maculatus uses a three-component strategy to overcome a plant defensive cysteine protease inhibitor. Insect Mol. Biol. 2003, 12, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.M.; Xie, C.J.; Wang, D.; Wei, Y.M.; Cai, J.; Cheng, S.S.; Yang, X.Y.; Sui, A.P. Psc-AFP from Psoralea corylifolia L. overexpressed in Pichia pastoris increases antimicrobial activity and enhances disease resistance of transgenic tobacco. Appl. Microbiol. Biotechnol. 2017, 101, 1073–1084. [Google Scholar] [CrossRef]

| Legume Seed | Protein | Fat | Minerals | Crude Fiber | Carbohydrates |
|---|---|---|---|---|---|
| Chickpea (Cicer arietinum) | 17.1 | 5.3 | 3.0 | 3.9 | 60.9 |
| Soybean (Glycine max) | 43.2 | 19.5 | 19.5 | 3.7 | 20.9 |
| Lentil (Lens esculenta) | 25.1 | 0.7 | 2.1 | 0.7 | 59.0 |
| Cowpea (Vigna catjang) | 24.1 | 1.0 | 3.2 | 3.8 | 54.5 |
| Peas dry (Pisum sativum) | 19.7 | 1.1 | 2.2 | 4.5 | 56.5 |
| Pigeon pea (Cajanus cajan) | 22.3 | 1.7 | 3.5 | 1.5 | 57.6 |
| Kidney bean (Phaseolus vulgaris) | 22.9 | 1.3 | 3.2 | 4.8 | 60.6 |
| Seed Legumes | Protease Target | Insect Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
|---|---|---|---|---|---|
| Soybean (Glycine max) | Trypsin and chymotrypsin | Spodoptera littoralis | 17.9 | ND | [43] |
| Pigeonpea (Cajanus cajan, Cajanus platycarpus) | Trypsin and chymotrypsin | Helicoverpa armigera | ND | ND | [44] |
| Soybean (Glycine max) | Trypsin | Spodoptera litura | ND | ND | [45] |
| Soybean (Glycine max) | Trypsin | B. cucurbitae | ND | ND | [46] |
| Chickpea (Cicer arietinum, Cicer cuneatum, Cicer bijugum, Cicer chrossanicum, Cicer reticulatum) | Trypsin and chymotrypsin | Helicoverpa armigera | ND | ND | [47] |
| Common bean (Phaseolus vulgaris) | Trypsin | Spodoptera litura | ND | ND | [48] |
| Faba bean (Vicia faba) | Cysteine protease | Halyomorpha halys | ND | ND | [49] |
| Uruvalheira (Platypodium elegans) | Trypsin and chymotrypsin | Spodoptera frugiperda | 19.7 | FVVDTDGDPLRNGGSYYILPVFRGRGGGIEQAAIGTETCPLTVVQSPSEVSKGLPLR | [50] |
| Soybean (Glycine max) | Cysteine protease | Nezara viridula L. | ND | ND | [30] |
| Seed Legume | Protease Target | Nematode Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
| Soybean (Glycine max) | Trypsin and papain | Meloidogyne incognita | 4.53–22.546 | ND | [51] |
| Crotalaria pallida | Papain | Meloidogyne incognita | 15 | FAFEDENTSPVAPAKLFKALTKDADVIIPKVIEPDQ | [52] |
| Soybean (Glycine max) | Trypsin | Heterodera glycines | ND | ND | [53] |
| Seed Legume | Protease Target | Fungus Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
| Psoralea corylifolia | Trypsin | Alternaria brassicae, Aspergillus niger, Fusarium oxyxporum, Rhizoctonia cerealis | 18 | EWEPVQNGGSSYYMVPRIWA | [54] |
| Acacia plumosa | Trypsin and chymotrypsin | Aspergillus niger, Thielaviopsis paradoxa, Colletotrichum sp. | 20 | KELLVDNEGEI | [55] |
| Chickpea (Cicer arietinum) | Trypsin | Fusarium oxysporum | 20 | ND | [56] |
| Lupin (Lupinus albus) | Cysteine protease | Fusarium oxysporum, Botrytis cinerea, Alternaria solani,Aspergillus niger, Penicillium expansum | 10.7–11.8 | ND | [57] |
| Peanut (Arachis hypogaea) | Trypsin and chymotrypsin | Aspergillus parasiticus | 16.82 | ND | [58] |
| Black soybean (Glycine max L. merr) | Trypsin and chymotrypsin | Alternaria alternata, Fusarium oxysporum, Pythium aphanidermatum, Physalospora piricola, Botrytis cinereal, Fusarium solani | 20 | DEYSKPCCDLCMCTRRCPPQ | [59] |
| Mung bean (Phaseolus mungo) | Trypsin and chymotrypsin | Physalospora piricola, Mycosphaerella arachidicola, Botrytis cinerea, Pythium aphanidermatum, Sclerotium rolfsii, and Fusarium oxysporum | 10 | EMPGKPACLDTDDFCYKP | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sifuentes, L.; Marszalek, J.E.; Chuck-Hernández, C.; Serna-Saldívar, S.O. Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. Int. J. Mol. Sci. 2020, 21, 3322. https://doi.org/10.3390/ijms21093322
Rodríguez-Sifuentes L, Marszalek JE, Chuck-Hernández C, Serna-Saldívar SO. Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. International Journal of Molecular Sciences. 2020; 21(9):3322. https://doi.org/10.3390/ijms21093322
Chicago/Turabian StyleRodríguez-Sifuentes, Lucio, Jolanta Elzbieta Marszalek, Cristina Chuck-Hernández, and Sergio O. Serna-Saldívar. 2020. "Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors" International Journal of Molecular Sciences 21, no. 9: 3322. https://doi.org/10.3390/ijms21093322
APA StyleRodríguez-Sifuentes, L., Marszalek, J. E., Chuck-Hernández, C., & Serna-Saldívar, S. O. (2020). Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. International Journal of Molecular Sciences, 21(9), 3322. https://doi.org/10.3390/ijms21093322







