Biochemical Convergence of Mitochondrial Hsp70 System Specialized in Iron–Sulfur Cluster Biogenesis
Abstract
:1. Introduction
2. Results
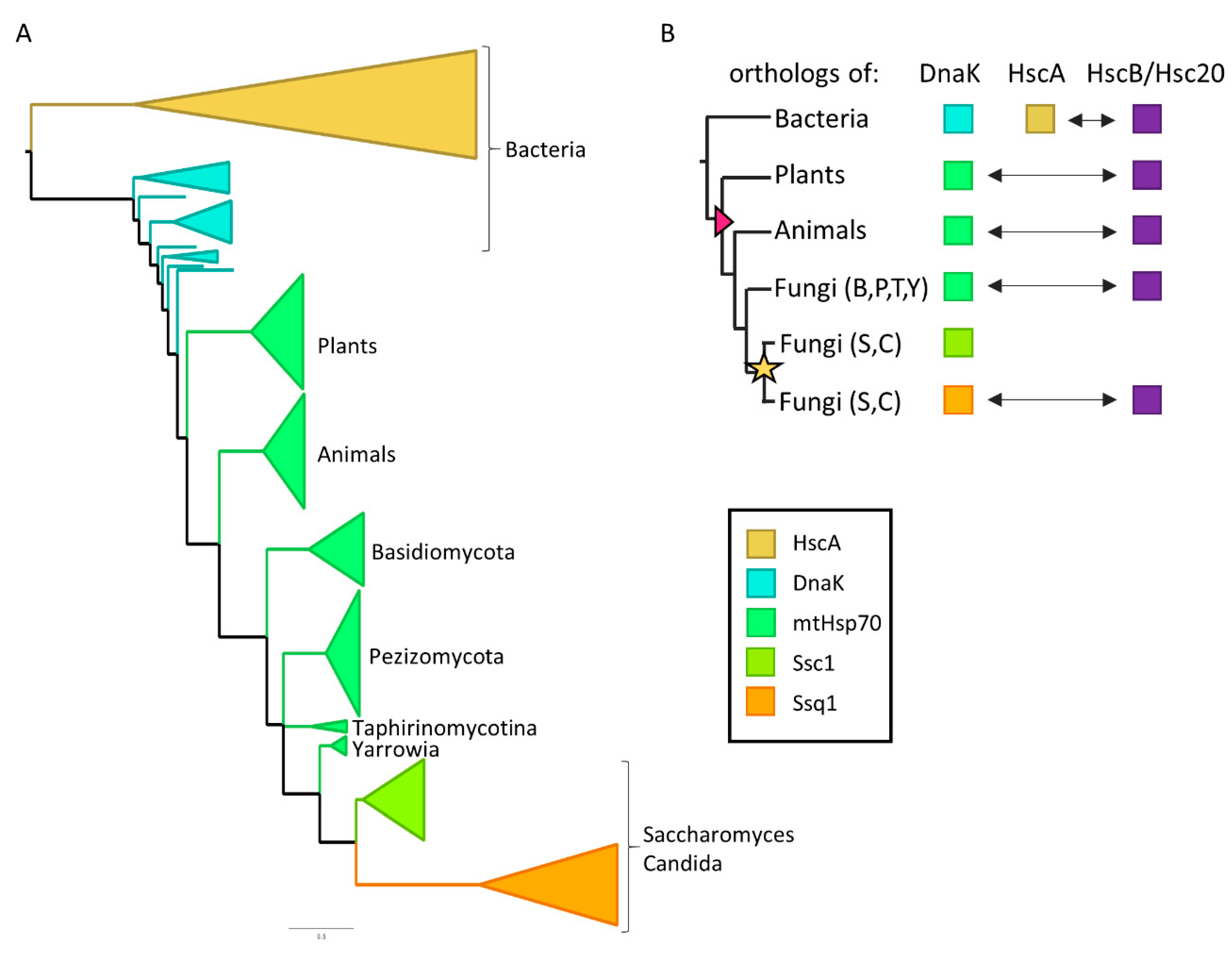
2.1. HscA Is Distantly Related to Ssq1 and Not Present in Eukaryotic Proteomes
2.2. HscA and Ssq1 Have Comparable Maximally Stimulated ATPase Rates
2.3. Ssq1 Has Higher Affinity for Protein Substrate Than HscA
2.4. Ssq1 Has Higher Affinity for JDP Cochaperone Than HscA
3. Discussion
4. Materials and Methods
4.1. Evolutionary Analyses
4.2. Protein Purification
4.3. ATPase Activity of Hsp70
4.3.1. Radioactive ATPase Assay
4.3.2. Enzyme-Coupled Spectrophotometric Assay
4.3.3. EnzChek Phosphate Assay
4.4. GST- Pull-Down Assay
4.5. Circular Dichroism
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lill, R.; Dutkiewicz, R.; Freibert, S.A.; Heidenreich, T.; Mascarenhas, J.; Netz, D.J.; Paul, V.D.; Pierik, A.J.; Richter, N.; Stümpfig, M.; et al. The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron–sulfur proteins. Eur. J. Cell Biol. 2015, 94, 280–291. [Google Scholar] [CrossRef]
- Roche, B.; Aussel, L.; Ezraty, B.; Mandin, P.; Py, B.; Barras, F. Iron/sulfur proteins biogenesis in prokaryotes: Formation, regulation and diversity. BBA-Bioenerg. 2013, 1827, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Dutkiewicz, R.; Nowak, M. Molecular chaperones involved in mitochondrial iron-sulfur protein biogenesis. J. Biol. Inorg. Chem. 2018, 23, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Maio, N.; Rouault, T.A. Iron-sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta 2015, 1853, 1493–1512. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Bothe, J.R.; Alderson, T.R.; Markley, J.L. Tangled web of interactions among proteins involved in iron-sulfur cluster assembly as unraveled by NMR, SAXS, chemical crosslinking, and functional studies. Biochim. Biophys. Acta 2015, 1853, 1416–1428. [Google Scholar] [CrossRef] [Green Version]
- Vickery, L.E.; Cupp-Vickery, J.R. Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 95–111. [Google Scholar] [CrossRef]
- Craig, E.A.; Marszalek, J. Hsp70 Chaperones; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, C.; Zhou, L. Structural and functional analysis of the Hsp70/Hsp40 chaperone system. Protein Sci. 2019, 29, 378–390. [Google Scholar] [CrossRef]
- Clerico, E.M.; Meng, W.; Pozhidaeva, A.; Bhasne, K.; Petridis, C.; Gierasch, L.M. Hsp70 molecular chaperones: Multifunctional allosteric holding and unfolding machines. Biochem. J. 2019, 476, 1653–1677. [Google Scholar] [CrossRef]
- Mayer, M.P.; Gierasch, L.M. Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J. Biol. Chem. 2019, 294, 2085–2097. [Google Scholar] [CrossRef] [Green Version]
- Bonomi, F.; Iametti, S.; Morleo, A.; Ta, D.; Vickery, L.E. Facilitated transfer of IscU–[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry 2011, 50, 9641–9650. [Google Scholar] [CrossRef]
- Bonomi, F.; Iametti, S.; Morleo, A.; Ta, D.; Vickery, L.E. Studies on the Mechanism of Catalysis of Iron−Sulfur Cluster Transfer from IscU [2 Fe2S] by HscA/HscB Chaperones. Biochemistry 2008, 47, 12795–12801. [Google Scholar] [CrossRef]
- Chandramouli, K.; Johnson, M.K. HscA and HscB Stimulate [2Fe-2S] Cluster Transfer from IscU to Apoferredoxin in an ATP-Dependent Reaction. Biochemistry 2006, 45, 11087–11095. [Google Scholar] [CrossRef] [Green Version]
- Bracher, A.; Verghese, J. The nucleotide exchange factors of Hsp70 molecular chaperones. Front. Mol. Biosci. 2015, 2, 10. [Google Scholar] [CrossRef]
- Huynen, M.A.; Snel, B.; Bork, P.; Gibson, T.J. The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum. Mol. Genet. 2001, 10, 2463–2468. [Google Scholar] [CrossRef] [Green Version]
- Schilke, B.; Williams, B.; Knieszner, H.; Pukszta, S.; Silva, P.; Craig, E.A.; Marszalek, J. Evolution of Mitochondrial Chaperones Utilized in Fe-S Cluster Biogenesis. Curr. Biol. 2006, 16, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Pukszta, S.; Schilke, B.; Dutkiewicz, R.; Kominek, J.; Moczulska, K.; Stepien, B.; Reitenga, K.G.; Bujnicki, J.M.; Williams, B.; Craig, E.A.; et al. Co-evolution-driven switch of J-protein specificity towards an Hsp70 partner. EMBO Rep. 2010, 11, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Delewski, W.; Paterkiewicz, B.; Manicki, M.; Schilke, B.; Tomiczek, B.; Ciesielski, S.J.; Nierzwicki, L.; Czub, J.; Dutkiewicz, R.; Craig, E.A.; et al. Iron–Sulfur Cluster Biogenesis Chaperones: Evidence for Emergence of Mutational Robustness of a Highly Specific Protein–Protein Interaction. Mol. Biol. Evol. 2016, 33, 643–656. [Google Scholar] [CrossRef] [Green Version]
- Hoff, K.G.; Silberg, J.J.; Vickery, L.E. Interaction of the iron-sulfur cluster assembly protein IscU with the Hsc66/Hsc20 molecular chaperone system of Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 7790–7795. [Google Scholar] [CrossRef] [Green Version]
- Hoff, K.G.; Ta, D.T.; Tapley, T.L.; Silberg, J.J.; Vickery, L.E. Hsc66 substrate specificity is directed toward a discrete region of the iron-sulfur cluster template protein IscU. J. Biol. Chem. 2002, 277, 27353–27359. [Google Scholar] [CrossRef] [Green Version]
- Silberg, J.J.; Hoff, K.G.; Vickery, L.E. The Hsc66-Hsc20 chaperone system in Escherichia coli: Chaperone activity and interactions with the DnaK-DnaJ-grpE system. J. Bacteriol. 1998, 180, 6617–6624. [Google Scholar] [CrossRef] [Green Version]
- Dutkiewicz, R.; Schilke, B.; Knieszner, H.; Walter, W.; Craig, E.A.; Marszalek, J. Ssq1, a mitochondrial Hsp70 involved in iron-sulfur (Fe/S) center biogenesis. Similarities to and differences from its bacterial counterpart. J. Biol. Chem. 2003, 278, 29719–29727. [Google Scholar] [CrossRef] [Green Version]
- Voisine, C.; Schilke, B.; Ohlson, M.; Beinert, H.; Marszalek, J.; Craig, E.A. Role of the mitochondrial Hsp70s, Ssc1 and Ssq1, in the maturation of Yfh1. Mol. Cell. Biol. 2000, 20, 3677–3684. [Google Scholar] [CrossRef]
- Schilke, B.; Forster, J.; Davis, J.; James, P.; Walter, W.; Laloraya, S.; Johnson, J.; Miao, B.; Craig, E. The cold sensitivity of a mutant of Saccharomyces cerevisiae lacking a mitochondrial heat shock protein 70 is suppressed by loss of mitochondrial DNA. J. Cell Biol. 1996, 134, 603–613. [Google Scholar] [CrossRef]
- Laloraya, S.; Dekker, P.J.; Voos, W.; Craig, E.A.; Pfanner, N. Mitochondrial GrpE modulates the function of matrix Hsp70 in translocation and maturation of preproteins. Mol. Cell. Biol. 1995, 15, 7098–7105. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012, 4, a011403. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.; Hartich, D.; Seifert, U.; Rios, P.D.L. Thermodynamic Bounds on the Ultra- and Infra-affinity of Hsp70 for Its Substrates. Biophys. J. 2017, 113, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Barducci, A.; De Los Rios, P. Non-equilibrium conformational dynamics in the function of molecular chaperones. Curr. Opin. Struct. Biol. 2015, 30, 161–169. [Google Scholar] [CrossRef]
- Silberg, J.J.; Tapley, T.L.; Hoff, K.G.; Vickery, L.E. Regulation of the HscA ATPase reaction cycle by the co-chaperone HscB and the iron-sulfur cluster assembly protein IscU. J. Biol. Chem. 2004, 279, 53924–53931. [Google Scholar] [CrossRef] [Green Version]
- Webert, H.; Freibert, S.-A.; Gallo, A.; Heidenreich, T.; Linne, U.; Amlacher, S.; Hurt, E.; Mühlenhoff, U.; Banci, L.; Lill, R. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 2014, 5, 5013. [Google Scholar] [CrossRef] [Green Version]
- Dutkiewicz, R.; Nowak, M.; Craig, E.A.; Marszalek, J. Fe-S Cluster Hsp70 Chaperones: The ATPase Cycle and Protein Interactions. Methods Enzymol. 2017, 595, 161–184. [Google Scholar] [PubMed]
- Kampinga, H.H.; Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christin, P.-A.; Weinreich, D.M.; Besnard, G. Causes and evolutionary significance of genetic convergence. Trends Genet. 2010, 26, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Koonin, E.V. Divergence and Convergence in Enzyme Evolution. J. Biol. Chem. 2011, 287, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Storz, J.F. Causes of molecular convergence and parallelism in protein evolution. Nat. Rev. Genet. 2016, 17, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Voisine, C.; Cheng, Y.C.; Ohlson, M.; Schilke, B.; Hoff, K.; Beinert, H.; Marszalek, J.; Craig, E.A. Jac1, a mitochondrial J-type chaperone, is involved in the biogenesis of Fe/S clusters in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2001, 98, 1483–1488. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Ding, J.S.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 1–6. [Google Scholar] [CrossRef]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, G.K.; Romagné, F.; Castellano, S. Distinct Patterns of Selection in Selenium-Dependent Genes between Land and Aquatic Vertebrates. Mol. Biol. Evol. 2018, 35, 1744–1756. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 46, W537–W545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, G.; Bradnam, K.; Korf, I. CEGMA: A pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 2007, 23, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Vickery, L.E.; Silberg, J.J.; Ta, D.T. Hsc66 and Hsc20, a new heat shock cognate molecular chaperone system from Escherichia coli. Protein Sci. 1997, 6, 1047–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicki, M.; Majewska, J.; Ciesielski, S.; Schilke, B.; Blenska, A.; Kominek, J.; Marszalek, J.; Craig, E.A.; Dutkiewicz, R. Overlapping Binding Sites of the Frataxin Homologue Assembly Factor and the Heat Shock Protein 70 Transfer Factor on the Isu Iron-Sulfur Cluster Scaffold Protein. J. Biol. Chem. 2014, 289, 30268–30278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielski, S.J.; Schilke, B.A.; Osipiuk, J.; Bigelow, L.; Mulligan, R.; Majewska, J.; Joachimiak, A.; Marszalek, J.; Craig, E.A.; Dutkiewicz, R. Interaction of J-Protein Co-Chaperone Jac1 with Fe–S Scaffold Isu Is Indispensable In Vivo and Conserved in Evolution. J. Mol. Biol. 2012, 417, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørby, J.G. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988, 156, 116–119. [Google Scholar]
- Webb, M.R. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc. Natl. Acad. Sci. USA 1992, 89, 4884–4887. [Google Scholar] [CrossRef] [Green Version]
- Gerber, J.; Mühlenhoff, U.; Lill, R. An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 2003, 4, 906–911. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleczewska, M.; Grabinska, A.; Jelen, M.; Stolarska, M.; Schilke, B.; Marszalek, J.; Craig, E.A.; Dutkiewicz, R. Biochemical Convergence of Mitochondrial Hsp70 System Specialized in Iron–Sulfur Cluster Biogenesis. Int. J. Mol. Sci. 2020, 21, 3326. https://doi.org/10.3390/ijms21093326
Kleczewska M, Grabinska A, Jelen M, Stolarska M, Schilke B, Marszalek J, Craig EA, Dutkiewicz R. Biochemical Convergence of Mitochondrial Hsp70 System Specialized in Iron–Sulfur Cluster Biogenesis. International Journal of Molecular Sciences. 2020; 21(9):3326. https://doi.org/10.3390/ijms21093326
Chicago/Turabian StyleKleczewska, Malgorzata, Aneta Grabinska, Marcin Jelen, Milena Stolarska, Brenda Schilke, Jaroslaw Marszalek, Elizabeth A. Craig, and Rafal Dutkiewicz. 2020. "Biochemical Convergence of Mitochondrial Hsp70 System Specialized in Iron–Sulfur Cluster Biogenesis" International Journal of Molecular Sciences 21, no. 9: 3326. https://doi.org/10.3390/ijms21093326




