Therapeutic Effect of Rapamycin on Aortic Dissection in Mice
Abstract
1. Introduction
2. Results
2.1. Effect of Cell Cycle Inhibitors on AD
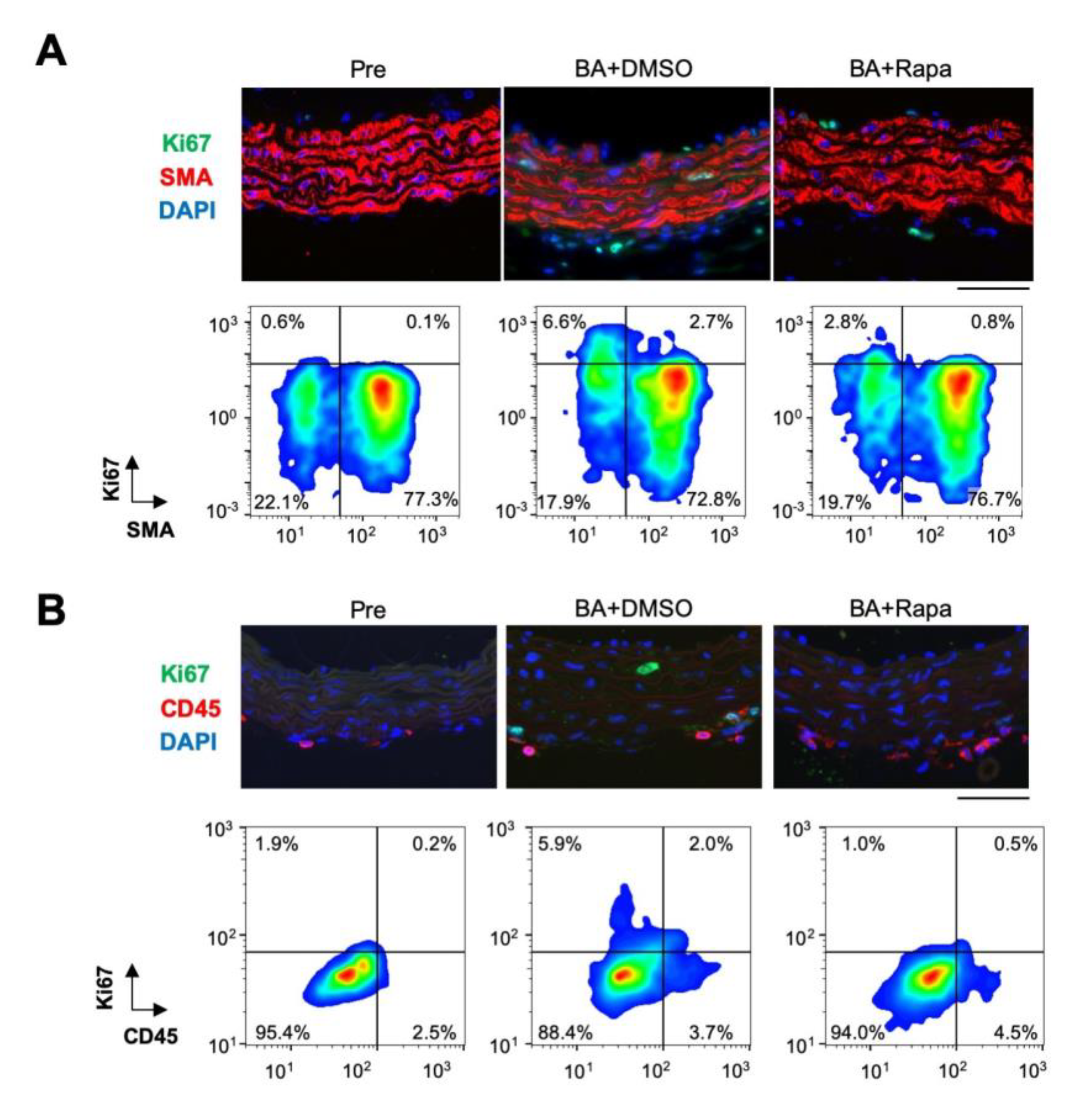
2.2. Rapamycin-Sensitive Cell Types in AD
2.3. AD-Related Gene Expression Network
2.4. Effect of Rapamycin on Protein Expression in the Aorta
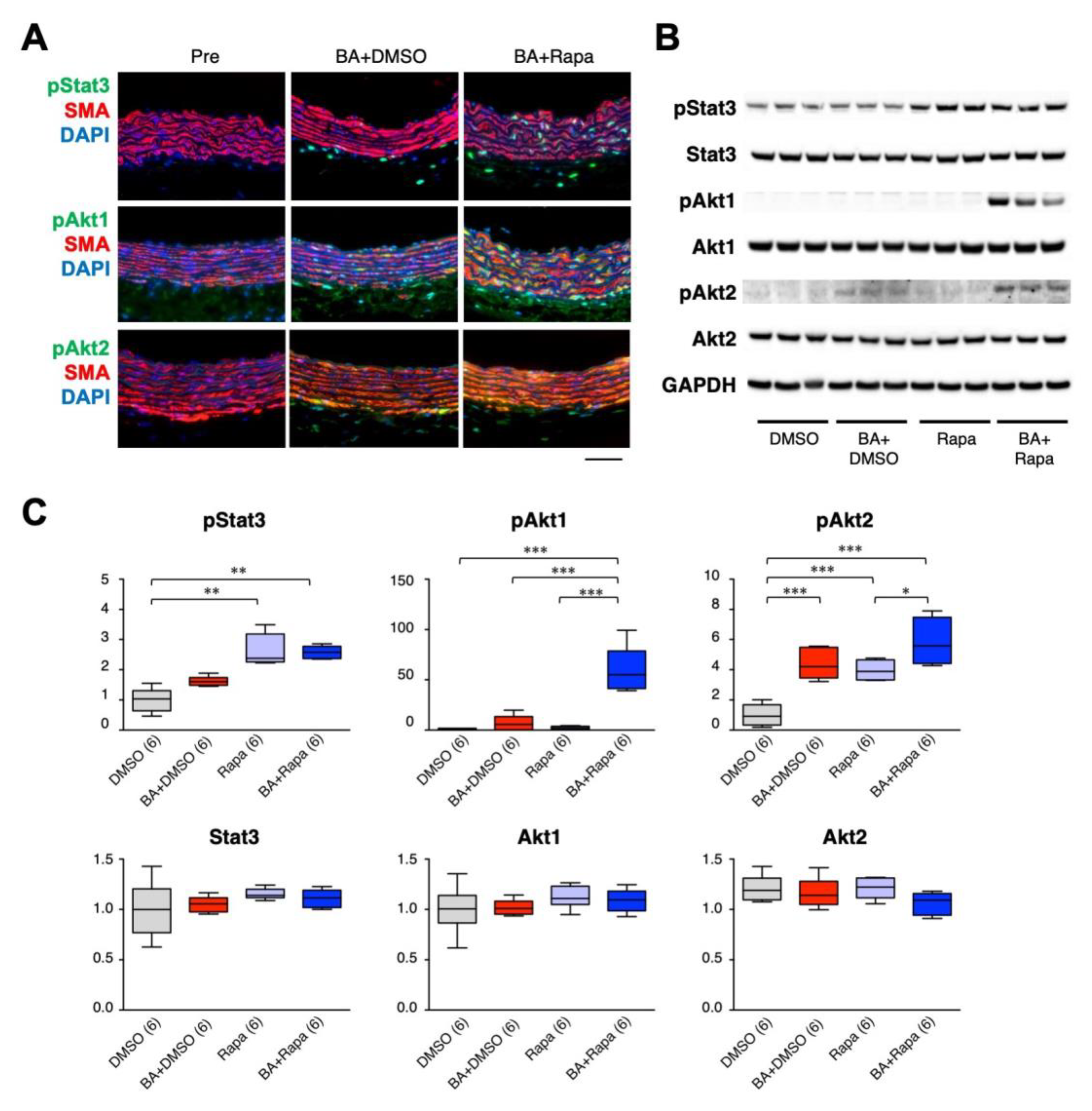
2.5. Effect of Rapamycin on Stat3, Akt1, and Akt2 in SMCs
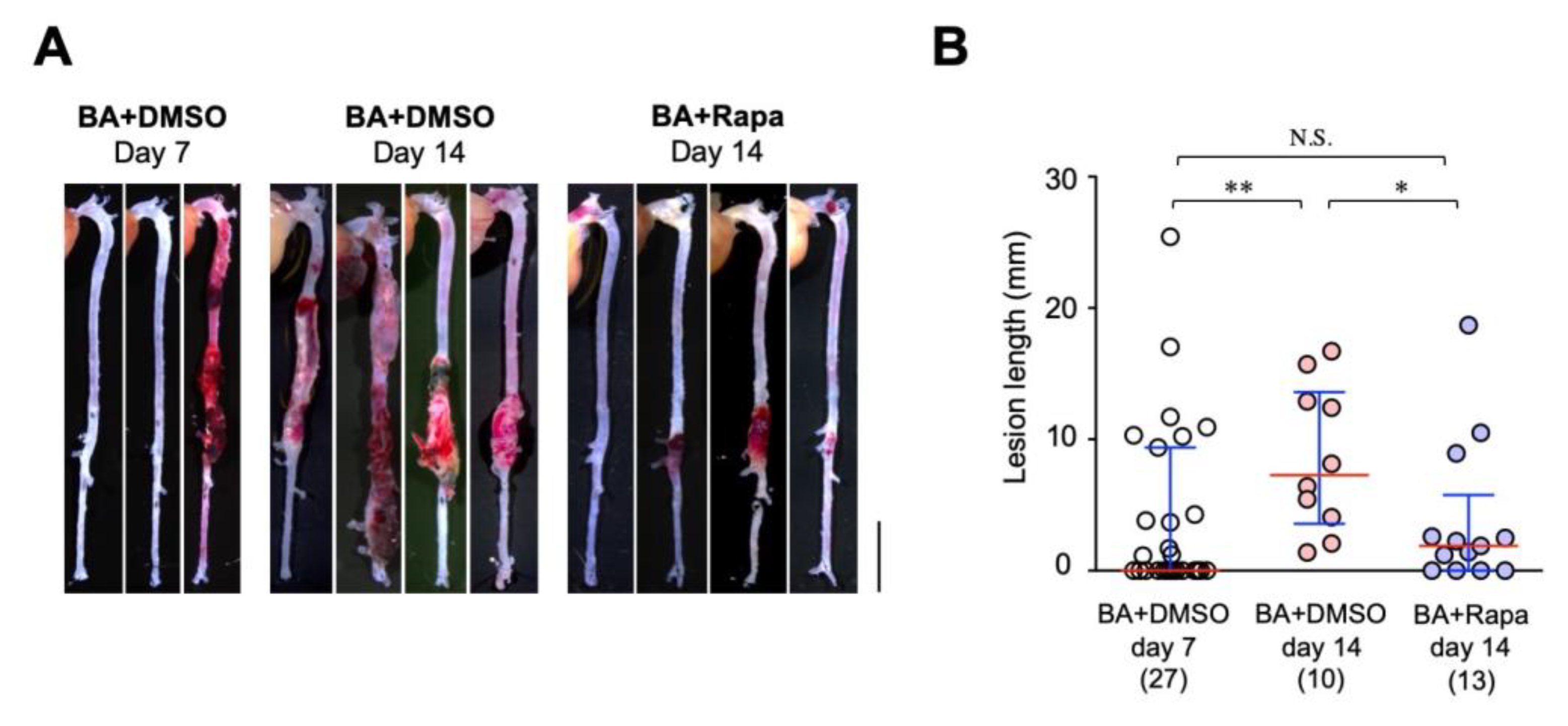
2.6. Effect of Rapamycin on AD Progression
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Histological Analysis
4.3. Expression Analysis
4.4. Gene Expression Network Analysis
4.5. Cell Culture Experiments
4.6. Data Acquisition and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | aortic dissection |
| AngII | angiotensin II |
| BAPN | β-aminopropionitrile |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DMSO | dimethylsulfoxide |
| EGFR | epidermal growth factor receptor |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| IGF-1 | insulin-like growth factor-1 |
| mTOR | mechanistic target of rapamycin |
| PI3-K | phosphatidylinositol 3-kinase |
| S6K | p70 S6 kinase |
| Smad | Sma- and Mad-related protein |
| SMC | smooth muscle cell |
| Stat | signal transducer and activator of transcription |
| Tgfbr | transforming growth factor β receptor |
| Tnc | tenascin C |
References
- Cronenwett, J.L.; Johnston, K.W. Vascular Surgery, 8th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014. [Google Scholar]
- Chen, K.; Varon, J.; Wenker, O.C.; Judge, D.K.; Fromm, R.E., Jr.; Sternbach, G.L. Acute thoracic aortic dissection: The basics. J. Emerg. Med. 1997, 15, 859–867. [Google Scholar] [CrossRef]
- Krol, E.; Panneton, J.M. Uncomplicated Acute Type B Aortic Dissection: Selection Guidelines for TEVAR. Ann. Vasc. Dis. 2017, 10. [Google Scholar] [CrossRef]
- Tsai, T.T.; Evangelista, A.; Nienaber, C.A.; Trimarchi, S.; Sechtem, U.; Fattori, R.; Myrmel, T.; Pape, L.; Cooper, J.V.; Smith, D.E.; et al. Long-term survival in patients presenting with type A acute aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006, 114, I350–I356. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Clough, R.E. Management of acute aortic dissection. Lancet 2015, 385, 800–811. [Google Scholar] [CrossRef]
- Michel, J.B.; Jondeau, G.; Milewicz, D.M. From genetics to response to injury: Vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovasc. Res. 2018, 114, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Shiraishi, K.; Furusho, A.; Ito, S.; Hirakata, S.; Nishida, N.; Yoshimura, K.; Imanaka-Yoshida, K.; Yoshida, T.; Ikeda, Y.; et al. Tenascin C protects aorta from acute dissection in mice. Sci. Rep. 2014, 4, 4051. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, S.; Aoki, H.; Ohno-Urabe, S.; Nishihara, M.; Furusho, A.; Nishida, N.; Ito, S.; Hayashi, M.; Yasukawa, H.; Imaizumi, T.; et al. Genetic Deletion of Socs3 in Smooth Muscle Cells Ameliorates Aortic Dissection in Mice. JACC Basic Transl. Sci. 2020, 5, 126–144. [Google Scholar] [CrossRef]
- Shen, Y.H.; Zhang, L.; Ren, P.; Nguyen, M.T.; Zou, S.; Wu, D.; Wang, X.L.; Coselli, J.S.; LeMaire, S.A. AKT2 confers protection against aortic aneurysms and dissections. Circ. Res. 2013, 112, 618–632. [Google Scholar] [CrossRef]
- Fry, J.L.; Shiraishi, Y.; Turcotte, R.; Yu, X.; Gao, Y.Z.; Akiki, R.; Bachschmid, M.; Zhang, Y.; Morgan, K.G.; Cohen, R.A.; et al. Vascular Smooth Muscle Sirtuin-1 Protects Against Aortic Dissection During Angiotensin II-Induced Hypertension. J. Am. Heart Assoc. 2015, 4, e002384. [Google Scholar] [CrossRef]
- Ohno-Urabe, S.; Aoki, H.; Nishihara, M.; Furusho, A.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Yasukawa, H.; Imaizumi, T.; et al. Role of Macrophage Socs3 in the Pathogenesis of Aortic Dissection. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Son, B.K.; Sawaki, D.; Tomida, S.; Fujita, D.; Aizawa, K.; Aoki, H.; Akishita, M.; Manabe, I.; Komuro, I.; Friedman, S.L.; et al. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat. Commun. 2015, 6, 6994. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Shimoda, M.; Endo, J.; Kohno, T.; Katsumata, Y.; Matsuhashi, T.; Yamamoto, T.; Ito, K.; Yan, X.; Shirakawa, K.; et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ. Res. 2015, 116, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Tieu, B.C.; Lee, C.; Sun, H.; Lejeune, W.; Recinos, A., III; Ju, X.; Spratt, H.; Guo, D.C.; Milewicz, D.; Tilton, R.G.; et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 2009, 119, 3637–3651. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Chappell, J.; Raffort, J.; Lareyre, F.; Vandestienne, M.; Taylor, A.L.; Finigan, A.; Harrison, J.; Bennett, M.R.; Bruneval, P.; et al. Vascular Smooth Muscle Cell Plasticity and Autophagy in Dissecting Aortic Aneurysms. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1149–1159. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Fu, W.; Guo, D.; Jiang, J.; Wang, Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J. Vasc. Surg. 2012, 56, 1698–1709, 1709 e1691. [Google Scholar] [CrossRef]
- Ferruzzi, J.; Murtada, S.I.; Li, G.; Jiao, Y.; Uman, S.; Ting, M.Y.; Tellides, G.; Humphrey, J.D. Pharmacologically Improved Contractility Protects Against Aortic Dissection in Mice With Disrupted Transforming Growth Factor-beta Signaling Despite Compromised Extracellular Matrix Properties. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 919–927. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.M.; Zhang, G.X.; Yang, F.; Yan, Y.; Liu, S.X.; Li, S.H.; Wang, G.K.; Xu, Z.Y. Dynamic autophagic activity affected the development of thoracic aortic dissection by regulating functional properties of smooth muscle cells. Biochem. Biophys. Res. Commun. 2016, 479, 358–364. [Google Scholar] [CrossRef]
- Muller, B.T.; Modlich, O.; Prisack, H.B.; Bojar, H.; Schipke, J.D.; Goecke, T.; Feindt, P.; Petzold, T.; Gams, E.; Muller, W.; et al. Gene expression profiles in the acutely dissected human aorta. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 356–364. [Google Scholar] [CrossRef]
- Hu, J.H.; Wei, H.; Jaffe, M.; Airhart, N.; Du, L.; Angelov, S.N.; Yan, J.; Allen, J.K.; Kang, I.; Wight, T.N.; et al. Postnatal Deletion of the Type II Transforming Growth Factor-beta Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2647–2656. [Google Scholar] [CrossRef]
- Nishida, N.; Aoki, H.; Ohno-Urabe, S.; Nishihara, M.; Furusho, A.; Hirakata, S.; Hayashi, M.; Ito, S.; Yamada, H.; Hirata, Y.; et al. High Salt Intake Worsens Aortic Dissection in Mice: Involvement of IL (Interleukin)-17A-Dependent ECM (Extracellular Matrix) Metabolism. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 189–205. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, L.; Qiu, Z.; Huang, Q.; Chen, Y.; Chen, L. Mechanical stretch aggravates aortic dissection by regulating MAPK pathway and the expression of MMP-9 and inflammation factors. Biomed. Pharmacother. 2018, 108, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Q.; Jiao, Y.; Qin, L.; Ali, R.; Zhou, J.; Ferruzzi, J.; Kim, R.W.; Geirsson, A.; Dietz, H.C.; et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J. Clin. Invest. 2014, 124, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Y.; Zhou, Z.; Huang, J.; Chen, S.; Zhou, W.; Yang, Q.; Bai, H.; Zhang, H.; Ben, J.; et al. Scavenger receptor A1 attenuates aortic dissection via promoting efferocytosis in macrophages. Biochem. Pharmacol. 2019, 168, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, W.; Zhao, G.; Yu, B.; Ma, B.; Liu, Z.; Xie, N.; Fu, Y.; Gong, Z.; Dai, R.; et al. Rapamycin prevents thoracic aortic aneurysm and dissection in mice. J. Vasc. Surg. 2019, 69, 921–932 e923. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Singh, R.S.; Franklin, D.P.; Carey, D.J.; Elmore, J.R. Rapamycin suppresses experimental aortic aneurysm growth. J. Vasc. Surg. 2004, 40, 334–338. [Google Scholar] [CrossRef]
- Li, F.F.; Shang, X.K.; Du, X.L.; Chen, S. Rapamycin Treatment Attenuates Angiotensin II -induced Abdominal Aortic Aneurysm Formation via VSMC Phenotypic Modulation and Down-regulation of ERK1/2 Activity. Curr. Med. Sci. 2018, 38, 93–100. [Google Scholar] [CrossRef]
- Moran, C.S.; Jose, R.J.; Moxon, J.V.; Roomberg, A.; Norman, P.E.; Rush, C.; Korner, H.; Golledge, J. Everolimus limits aortic aneurysm in the apolipoprotein E-deficient mouse by downregulating C-C chemokine receptor 2 positive monocytes. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 814–821. [Google Scholar] [CrossRef]
- Li, G.; Qin, L.; Wang, L.; Li, X.; Caulk, A.W.; Zhang, J.; Chen, P.Y.; Xin, S. Inhibition of the mTOR pathway in abdominal aortic aneurysm: Implications of smooth muscle cell contractile phenotype, inflammation, and aneurysm expansion. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1110–H1119. [Google Scholar] [CrossRef]
- Jiao, J.; Xiong, W.; Wang, L.; Yang, J.; Qiu, P.; Hirai, H.; Shao, L.; Milewicz, D.; Chen, Y.E.; Yang, B. Differentiation defect in neural crest-derived smooth muscle cells in patients with aortopathy associated with bicuspid aortic valves. EBioMedicine 2016, 10, 282–290. [Google Scholar] [CrossRef]
- Hirata, Y.; Aoki, H.; Shojima, T.; Takagi, K.; Takaseya, T.; Akasu, K.; Tobinaga, S.; Fukumoto, Y.; Tanaka, H. Activation of the AKT Pathway in the Ascending Aorta With Bicuspid Aortic Valve. Circ. J. 2018, 82, 2485–2492. [Google Scholar] [CrossRef]
- Hayashi, K.; Takahashi, M.; Kimura, K.; Nishida, W.; Saga, H.; Sobue, K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J. Cell Biol. 1999, 145, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Merenick, B.L.; Ding, M.; Fetalvero, K.M.; Rzucidlo, E.M.; Kozul, C.D.; Brown, D.J.; Chiu, H.Y.; Shyu, M.; Drapeau, B.L.; et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J. Biol. Chem. 2007, 282, 36112–36120. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Kai, H.; Seki, Y.; Kato, S.; Wada, Y.; Hanakawa, Y.; Hashimoto, K.; Yoshimura, A.; Imaizumi, T. Inhibition of STAT3 prevents neointima formation by inhibiting proliferation and promoting apoptosis of neointimal smooth muscle cells. Hum. Gene. Ther. 2003, 14, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Oh, S.P.; Kasahara, H.; Wagner, K.U.; Sayeski, P.P. Vascular smooth muscle Jak2 deletion prevents angiotensin II-mediated neointima formation following injury in mice. J. Mol. Cell Cardiol. 2011, 50, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.H.; Wang, N.; Zhao, D.W.; Zheng, D.L.; Zheng, L.; Xing, W.J.; Ma, W.J.; Bao, L.Y.; Dong, J.; Zhang, T.C. STAT3 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin. J. Biol. Chem. 2015, 290, 19641–19652. [Google Scholar] [CrossRef]
- Lucchesi, P.A. Rapamycin plays a new role as differentiator of vascular smooth muscle phenotype. focus on “The mTOR/p70 S6K1 pathway regulates vascular smooth muscle differentiation”. Am. J. Physiol. Cell Physiol. 2004, 286, C480–C481. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. mTOR signalling: The molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes. Rev. 2012, 13 (Suppl. 2), 58–68. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Fattori, R.; Mehta, R.H.; Richartz, B.M.; Evangelista, A.; Petzsch, M.; Cooper, J.V.; Januzzi, J.L.; Ince, H.; Sechtem, U.; et al. Gender-related differences in acute aortic dissection. Circulation 2004, 109, 3014–3021. [Google Scholar] [CrossRef]
- Zhao, S.; Kuge, Y.; Zhao, Y.; Takeuchi, S.; Hirata, K.; Takei, T.; Shiga, T.; Dosaka-Akita, H.; Tamaki, N. Assessment of early changes in 3H-fluorothymidine uptake after treatment with gefitinib in human tumor xenograft in comparison with Ki-67 and phospho-EGFR expression. BMC Cancer 2013, 13, 525. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]



| AD Phenotype | BA + DMSO | BA + Gef | BA + Rapa |
|---|---|---|---|
| AD + | 11 | 9 | 2 |
| AD – | 3 | 0 | 6 |
| Total | 14 | 9 | 8 |
| Incidence (%) | 78.6 | 100.0 | 25.0 |
| Subnetwork #1 | |||
| Annotation cluster 1 (Enrichment score: 11.77) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0007049, cell cycle | 49 | <0.001 |
| GOTERM_BP | GO:0022402, cell cycle process | 42 | <0.001 |
| GOTERM_BP | GO:0000278, mitotic cell cycle | 36 | <0.001 |
| Annotation cluster 2 (Enrichment score: 10.65) | |||
| Category | Term | Gene count | p value |
| GOTERM_CC | GO:0044427, chromosomal part | 37 | <0.001 |
| GOTERM_CC | GO:0005694, chromosome | 38 | <0.001 |
| GOTERM_CC | GO:0000228, nuclear chromosome | 24 | <0.001 |
| Annotation cluster 3 (Enrichment score: 5.91) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0007067, mitotic nuclear division | 26 | <0.001 |
| GOTERM_BP | GO:0051301, cell division | 28 | <0.001 |
| GOTERM_BP | GO:0000226, microtubule cytoskeleton organization | 17 | <0.001 |
| Subnetwork #2 | |||
| Annotation cluster 1 (Enrichment score: 17.04) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0006955, immune response | 66 | <0.001 |
| GOTERM_BP | GO:0006952, defense response | 66 | <0.001 |
| GOTERM_BP | GO:0002684, positive regulation of immune system process | 41 | <0.001 |
| Annotation cluster 2 (Enrichment score: 17.03) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0006952, defense response | 66 | <0.001 |
| GOTERM_BP | GO:0009605, response to external stimulus | 81 | <0.001 |
| GOTERM_BP | GO:0051707, response to other organism | 43 | <0.001 |
| Annotation cluster 3 (Enrichment score: 9.43) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0050729, positive regulation of inflammatory response | 18 | <0.001 |
| GOTERM_BP | GO:0050727, regulation of inflammatory response | 21 | <0.001 |
| GOTERM_BP | GO:0002675, positive regulation of acute inflammatory response | 8 | <0.001 |
| Subnetwork #3 | |||
| Annotation cluster 1 (Enrichment Score: 2.40) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0090066, regulation of anatomical structure size | 23 | <0.001 |
| GOTERM_BP | GO:0032535, regulation of cellular component size | 16 | <0.005 |
| GOTERM_BP | GO:0008361, regulation of cell size | 9 | <0.05 |
| Annotation cluster 2 (Enrichment Score: 2.06) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0055001, muscle cell development | 11 | <0.005 |
| GOTERM_CC | GO:0044449, contractile fiber part | 12 | <0.005 |
| GOTERM_BP | GO:0030239, myofibril assembly | 6 | <0.005 |
| Annotation cluster 3 (Enrichment Score: 2.06) | |||
| Category | Term | Gene count | p value |
| GOTERM_BP | GO:0007584, response to nutrient | 11 | <0.005 |
| GOTERM_BP | GO:0031667, response to nutrient levels | 18 | <0.005 |
| GOTERM_BP | GO:0009991, response to extracellular stimulus | 18 | <0.01 |
| AD Phenotype | BA+DMSO Day 7 | BA+DMSO Day 14 | BA+Rapa Day 14 |
|---|---|---|---|
| AD + | 13 | 10 | 9 |
| AD – | 14 | 0 | 4 |
| Total | 27 | 10 | 13 |
| Incidence (%) | 48.1 | 100.0 | 69.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi-Hori, M.; Aoki, H.; Matsukuma, M.; Majima, R.; Hashimoto, Y.; Ito, S.; Hirakata, S.; Nishida, N.; Furusho, A.; Ohno-Urabe, S.; et al. Therapeutic Effect of Rapamycin on Aortic Dissection in Mice. Int. J. Mol. Sci. 2020, 21, 3341. https://doi.org/10.3390/ijms21093341
Hayashi-Hori M, Aoki H, Matsukuma M, Majima R, Hashimoto Y, Ito S, Hirakata S, Nishida N, Furusho A, Ohno-Urabe S, et al. Therapeutic Effect of Rapamycin on Aortic Dissection in Mice. International Journal of Molecular Sciences. 2020; 21(9):3341. https://doi.org/10.3390/ijms21093341
Chicago/Turabian StyleHayashi-Hori, Makiko, Hiroki Aoki, Miho Matsukuma, Ryohei Majima, Yohei Hashimoto, Sohei Ito, Saki Hirakata, Norifumi Nishida, Aya Furusho, Satoko Ohno-Urabe, and et al. 2020. "Therapeutic Effect of Rapamycin on Aortic Dissection in Mice" International Journal of Molecular Sciences 21, no. 9: 3341. https://doi.org/10.3390/ijms21093341
APA StyleHayashi-Hori, M., Aoki, H., Matsukuma, M., Majima, R., Hashimoto, Y., Ito, S., Hirakata, S., Nishida, N., Furusho, A., Ohno-Urabe, S., & Fukumoto, Y. (2020). Therapeutic Effect of Rapamycin on Aortic Dissection in Mice. International Journal of Molecular Sciences, 21(9), 3341. https://doi.org/10.3390/ijms21093341






