The Activity and Stability of p56Lck and TCR Signaling Do Not Depend on the Co-Chaperone Cdc37
Abstract
:1. Introduction
2. Results and Discussion
2.1. Overexpression of Cdc37 Did Not Affect Lck Expression, Lck Activation, and TCR Signaling
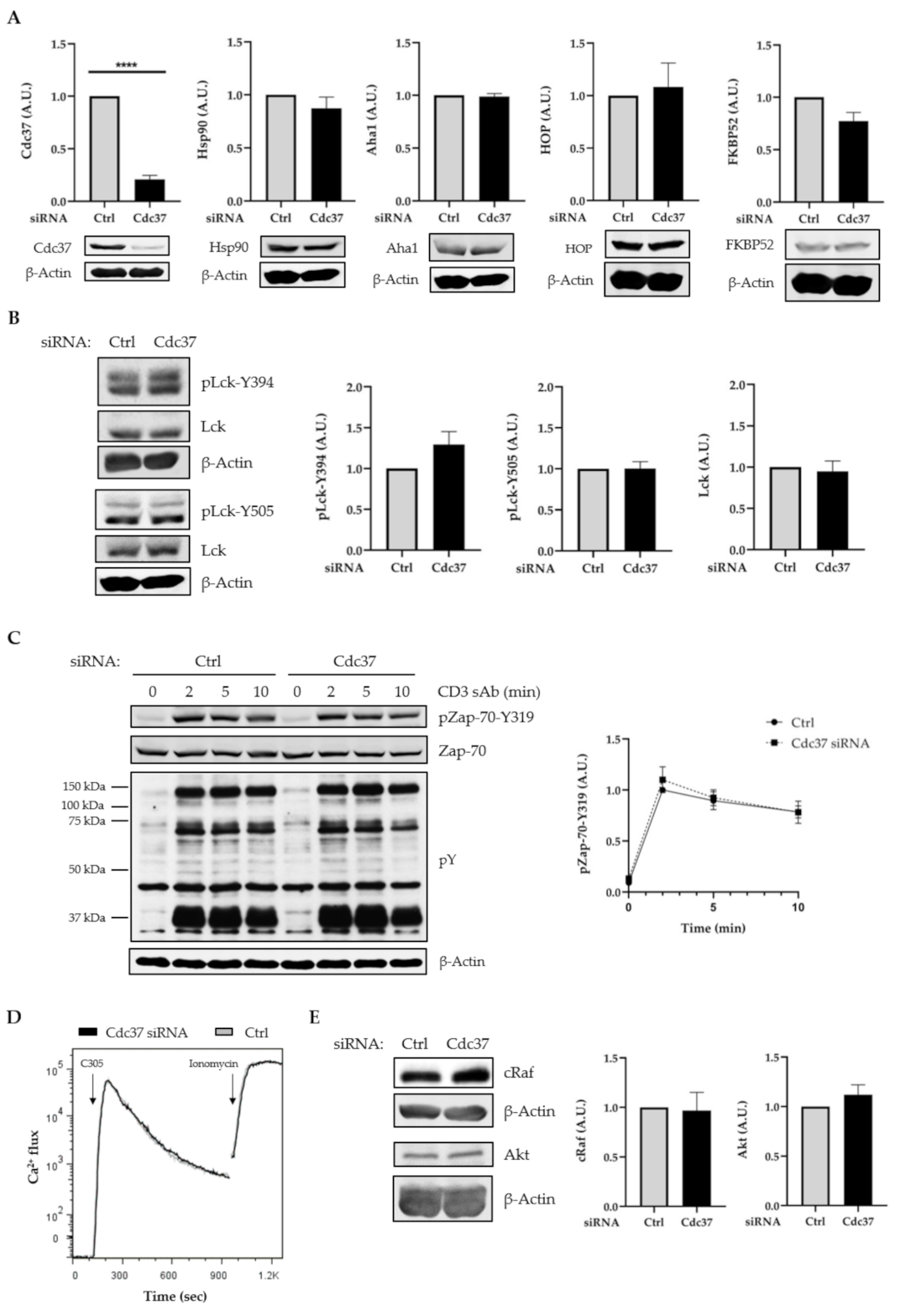
2.2. Suppression of Cdc37 by RNAi Did Not Affect Lck Expression, Lck Activation, and TCR Signaling
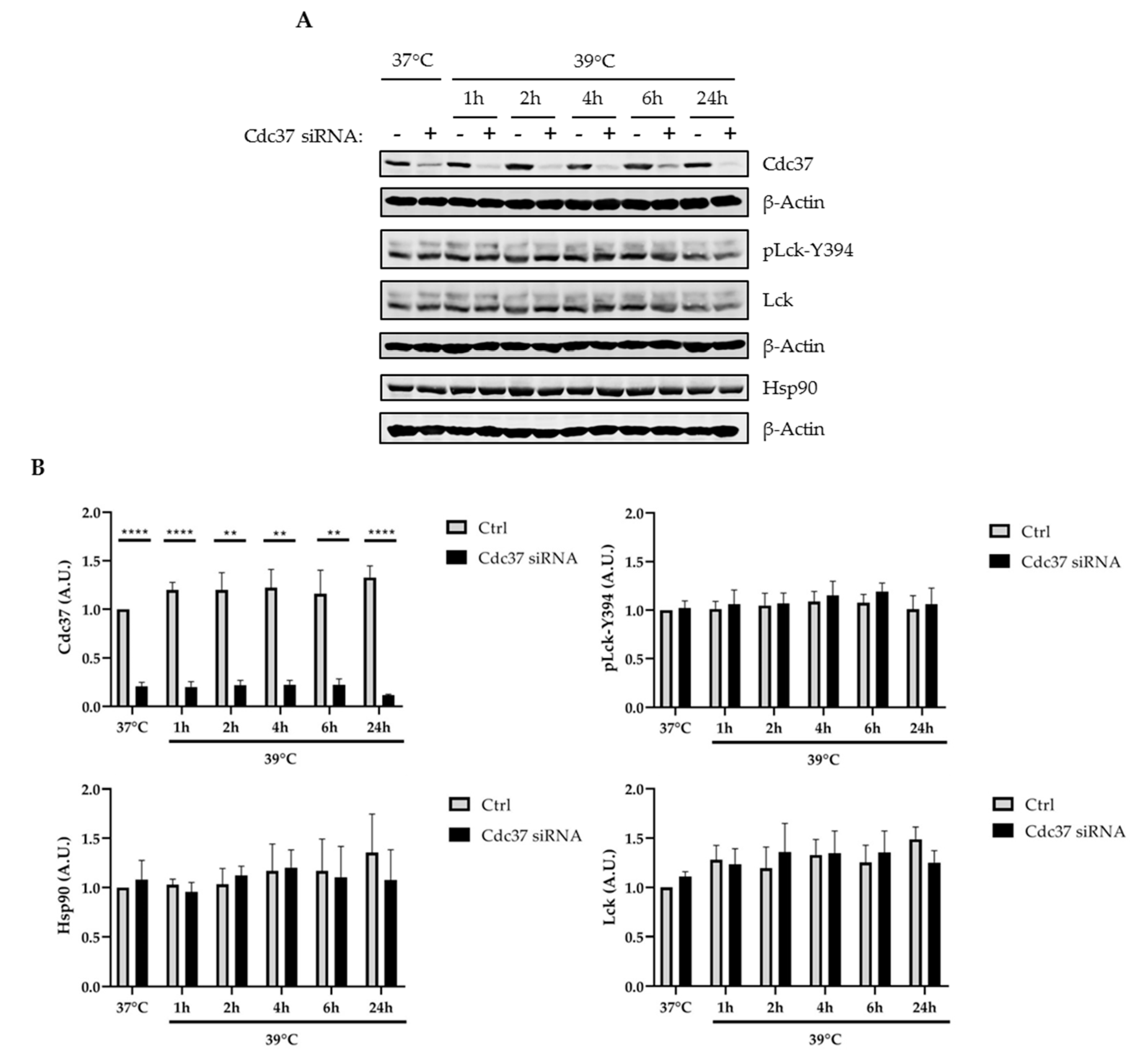
2.3. Cdc37 Was Not Required for the Regulation of Lck under Stress Conditions
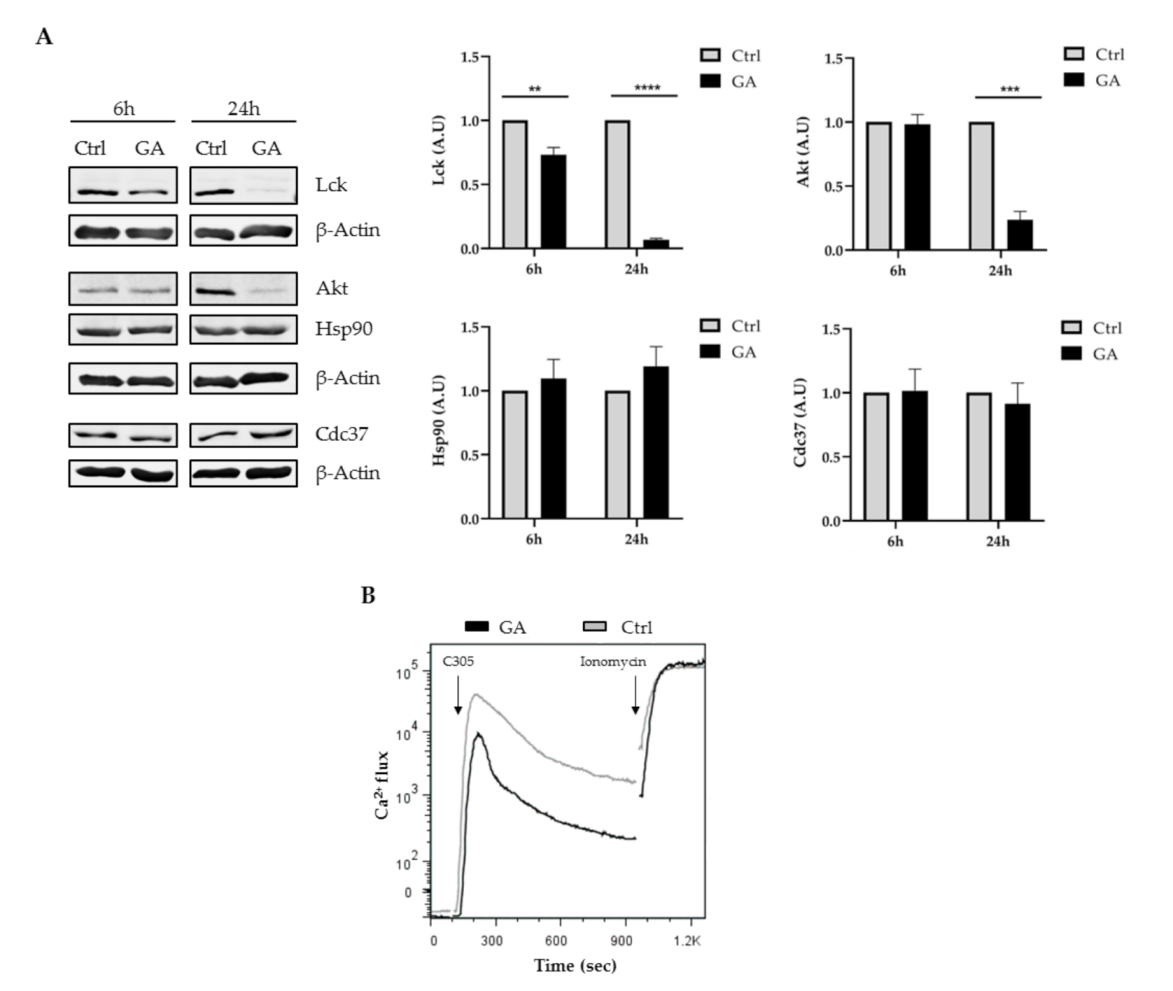
2.4. Inhibition of Hsp90 Affected Lck Expression and Impaired TCR Signaling
3. Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. Overexpression of Cdc37
4.3. Suppression of Cdc37
4.4. Cell Stimulation and Immunoblot Analysis
4.5. Geldanamycin Treatment
4.6. Ca2+ Influx Measurement
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| GA | Geldanaymycin |
| FBS | Fetal bovine serum |
| Hsp90 | Heat shock protein 90 |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| Lck | Lymphocyte-specific protein tyrosine kinase |
| MHC | Major histocompatibility complex |
| RNAi | RNA interference |
| TCR | T cell receptor |
| Y | Tyrosine |
| Zap-70 | Zeta chain of T cell receptor-associated protein kinase 70 |
References
- Bommhardt, U.; Schraven, B.; Simeoni, L. Beyond TCR Signaling: Emerging Functions of Lck in Cancer and Immunotherapy. Int. J. Mol. Sci. 2019, 20, 3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaud, G.; Lesourne, R.; Love, P.E. Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 2018, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Alonso, M.A. The role of membrane rafts in Lck transport, regulation and signalling in T-cells. Biochem. J. 2013, 454, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipsen, L.; Reddycherla, A.V.; Hartig, R.; Gumz, J.; Kastle, M.; Kritikos, A.; Poltorak, M.P.; Prokazov, Y.; Turbin, E.; Weber, A.; et al. De novo phosphorylation and conformational opening of the tyrosine kinase Lck act in concert to initiate T cell receptor signaling. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Liaunardy-Jopeace, A.; Murton, B.L.; Mahesh, M.; Chin, J.W.; James, J.R. Encoding optical control in LCK kinase to quantitatively investigate its activity in live cells. Nat. Struct. Mol. Biol. 2017, 24, 1155–1163. [Google Scholar] [CrossRef]
- Marth, J.D.; Cooper, J.A.; King, C.S.; Ziegler, S.F.; Tinker, D.A.; Overell, R.W.; Krebs, E.G.; Perlmutter, R.M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol. Cell Biol. 1988, 8, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Amrein, K.E.; Sefton, B.M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc. Natl. Acad. Sci. USA 1988, 85, 4247–4251. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Harrison, S.C.; Eck, M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 1997, 385, 595–602. [Google Scholar] [CrossRef]
- Stirnweiss, A.; Hartig, R.; Gieseler, S.; Lindquist, J.A.; Reichardt, P.; Philipsen, L.; Simeoni, L.; Poltorak, M.; Merten, C.; Zuschratter, W.; et al. T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Sci. Signal. 2013, 6, ra13. [Google Scholar] [CrossRef] [Green Version]
- Courtney, A.H.; Lo, W.L.; Weiss, A. TCR Signaling: Mechanisms of Initiation and Propagation. Trends Biochem. Sci. 2018, 43, 108–123. [Google Scholar] [CrossRef]
- Hwang, J.R.; Byeon, Y.; Kim, D.; Park, S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Straus, D.B.; Weiss, A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 1992, 70, 585–593. [Google Scholar] [CrossRef]
- Lovatt, M.; Filby, A.; Parravicini, V.; Werlen, G.; Palmer, E.; Zamoyska, R. Lck regulates the threshold of activation in primary T cells, while both Lck and Fyn contribute to the magnitude of the extracellular signal-related kinase response. Mol. Cell Biol. 2006, 26, 8655–8665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, T.J.; Kishihara, K.; Siderovski, D.P.; van Ewijk, W.; Narendran, A.; Timms, E.; Wakeham, A.; Paige, C.J.; Hartmann, K.U.; Veillette, A.; et al. Profound block in thymocyte development in mice lacking p56lck. Nature 1992, 357, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Legname, G.; Tomlinson, P.; Zamoyska, R. Long-term survival but impaired homeostatic proliferation of Naive T cells in the absence of p56lck. Science 2000, 290, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Bozso, S.J.; Kang, J.J.H.; Nagendran, J. The role of competing mechanisms on Lck regulation. Immunol. Res. 2020, 68, 289–295. [Google Scholar] [CrossRef]
- Bijlmakers, M.J.; Marsh, M. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol. Biol. Cell 2000, 11, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Giannini, A.; Bijlmakers, M.J. Regulation of the Src family kinase Lck by Hsp90 and ubiquitination. Mol. Cell Biol. 2004, 24, 5667–5676. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K.; Okoshi, R.; Wakabayashi, M.; Ishikawa, A.; Sato, Y.; Kizaki, H. Geldanamycin, a heat-shock protein 90-binding agent, induces thymocyte apoptosis through destabilization of Lck in presence of 12-O-tetradecanoylphorbol 13-acetate. Biomed. Res. 2007, 28, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Nika, K.; Soldani, C.; Salek, M.; Paster, W.; Gray, A.; Etzensperger, R.; Fugger, L.; Polzella, P.; Cerundolo, V.; Dushek, O.; et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 2010, 32, 766–777. [Google Scholar] [CrossRef] [Green Version]
- Pearl, L.H. Review: The HSP90 molecular chaperone-an enigmatic ATPase. Biopolymers 2016, 105, 594–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, T.; Poon, R.Y. Cdc37: A protein kinase chaperone? Trends Cell Biol. 1997, 7, 157–161. [Google Scholar] [CrossRef]
- Pearl, L.H. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr. Opin. Genet. Dev. 2005, 15, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Karnitz, L.M.; Felts, S.J. Cdc37 regulation of the kinome: When to hold ’em and when to fold ’em. Sci. STKE 2007, 2007, pe22. [Google Scholar] [CrossRef]
- Grammatikakis, N.; Lin, J.H.; Grammatikakis, A.; Tsichlis, P.N.; Cochran, B.H. p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol. Cell Biol. 1999, 19, 1661–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Grammatikakis, N.; Scroggins, B.T.; Uma, S.; Huang, W.; Chen, J.J.; Hartson, S.D.; Matts, R.L. Hsp90 regulates p50(cdc37) function during the biogenesis of the activeconformation of the heme-regulated eIF2 alpha kinase. J. Biol. Chem. 2001, 276, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Siligardi, G.; Panaretou, B.; Meyer, P.; Singh, S.; Woolfson, D.N.; Piper, P.W.; Pearl, L.H.; Prodromou, C. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 2002, 277, 20151–20159. [Google Scholar] [CrossRef] [Green Version]
- Siligardi, G.; Hu, B.; Panaretou, B.; Piper, P.W.; Pearl, L.H.; Prodromou, C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J. Biol. Chem. 2004, 279, 51989–51998. [Google Scholar] [CrossRef] [Green Version]
- Roe, S.M.; Ali, M.M.; Meyer, P.; Vaughan, C.K.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. The Mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 2004, 116, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.R.; Workman, P. Targeting CDC37: An alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 2009, 8, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Prince, T.; Matts, R.L. Definition of protein kinase sequence motifs that trigger high affinity binding of Hsp90 and Cdc37. J. Biol. Chem. 2004, 279, 39975–39981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, J.; Wesselschmidt, R.L.; Lu, W. Cdc37 regulates Ryk signaling by stabilizing the cleaved Ryk intracellular domain. J. Biol. Chem. 2009, 284, 12940–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nardo, D.; Masendycz, P.; Ho, S.; Cross, M.; Fleetwood, A.J.; Reynolds, E.C.; Hamilton, J.A.; Scholz, G.M. A central role for the Hsp90.Cdc37 molecular chaperone module in interleukin-1 receptor-associated-kinase-dependent signaling by toll-like receptors. J. Biol. Chem. 2005, 280, 9813–9822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarze, S.R.; Fu, V.X.; Jarrard, D.F. Cdc37 enhances proliferation and is necessary for normal human prostate epithelial cell survival. Cancer Res. 2003, 63, 4614–4619. [Google Scholar] [PubMed]
- Liu, Y.; Wang, S.; Ding, D.; Yu, Z.; Sun, W.; Wang, Y. Up-Regulation of Cdc37 Contributes to Schwann Cell Proliferation and Migration After Sciatic Nerve Crush. Neurochem. Res. 2018, 43, 1182–1190. [Google Scholar] [CrossRef]
- Kim, H.; Abd Elmageed, Z.Y.; Davis, C.; El-Bahrawy, A.H.; Naura, A.S.; Ekaidi, I.; Abdel-Mageed, A.B.; Boulares, A.H. Correlation between PDZK1, Cdc37, Akt and breast cancer malignancy: The role of PDZK1 in cell growth through Akt stabilization by increasing and interacting with Cdc37. Mol. Med. 2014, 20, 270–279. [Google Scholar] [CrossRef]
- Smith, J.R.; Clarke, P.A.; de Billy, E.; Workman, P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene 2009, 28, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wei, W.; Sun, C.K.; Chua, M.S.; So, S. Suppressing the CDC37 cochaperone in hepatocellular carcinoma cells inhibits cell cycle progression and cell growth. Liver Int. 2015, 35, 1403–1415. [Google Scholar] [CrossRef]
- Gray, P.J., Jr.; Stevenson, M.A.; Calderwood, S.K. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Res. 2007, 67, 11942–11950. [Google Scholar] [CrossRef] [Green Version]
- Thurm, C.; Poltorak, M.P.; Reimer, E.; Brinkmann, M.M.; Leichert, L.; Schraven, B.; Simeoni, L. A highly conserved redox-active Mx(2)CWx(6)R motif regulates Zap70 stability and activity. Oncotarget 2017, 8, 30805–30816. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Chaperone-assisted protein folding: The path to discovery from a personal perspective. Nat. Med. 2011, 17, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnecke, N.; Poltorak, M.; Kowtharapu, B.S.; Arndt, B.; Stone, J.C.; Schraven, B.; Simeoni, L. TCR-mediated Erk activation does not depend on Sos and Grb2 in peripheral human T cells. EMBO Rep. 2012, 13, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowallik, S.; Kritikos, A.; Kästle, M.; Thurm, C.; Schraven, B.; Simeoni, L. The Activity and Stability of p56Lck and TCR Signaling Do Not Depend on the Co-Chaperone Cdc37. Int. J. Mol. Sci. 2021, 22, 126. https://doi.org/10.3390/ijms22010126
Kowallik S, Kritikos A, Kästle M, Thurm C, Schraven B, Simeoni L. The Activity and Stability of p56Lck and TCR Signaling Do Not Depend on the Co-Chaperone Cdc37. International Journal of Molecular Sciences. 2021; 22(1):126. https://doi.org/10.3390/ijms22010126
Chicago/Turabian StyleKowallik, Sarah, Andreas Kritikos, Matthias Kästle, Christoph Thurm, Burkhart Schraven, and Luca Simeoni. 2021. "The Activity and Stability of p56Lck and TCR Signaling Do Not Depend on the Co-Chaperone Cdc37" International Journal of Molecular Sciences 22, no. 1: 126. https://doi.org/10.3390/ijms22010126
APA StyleKowallik, S., Kritikos, A., Kästle, M., Thurm, C., Schraven, B., & Simeoni, L. (2021). The Activity and Stability of p56Lck and TCR Signaling Do Not Depend on the Co-Chaperone Cdc37. International Journal of Molecular Sciences, 22(1), 126. https://doi.org/10.3390/ijms22010126



